Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Łukasz Szeleszczuk | -- | 4623 | 2023-06-08 11:00:06 | | | |
| 2 | Sirius Huang | + 1 word(s) | 4624 | 2023-06-09 02:52:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Araj, S.K.; Szeleszczuk, �. Cyclodextrins/Estrogens Inclusion Complexes. Encyclopedia. Available online: https://encyclopedia.pub/entry/45326 (accessed on 07 February 2026).
Araj SK, Szeleszczuk �. Cyclodextrins/Estrogens Inclusion Complexes. Encyclopedia. Available at: https://encyclopedia.pub/entry/45326. Accessed February 07, 2026.
Araj, Szymon Kamil, Łukasz Szeleszczuk. "Cyclodextrins/Estrogens Inclusion Complexes" Encyclopedia, https://encyclopedia.pub/entry/45326 (accessed February 07, 2026).
Araj, S.K., & Szeleszczuk, �. (2023, June 08). Cyclodextrins/Estrogens Inclusion Complexes. In Encyclopedia. https://encyclopedia.pub/entry/45326
Araj, Szymon Kamil and Łukasz Szeleszczuk. "Cyclodextrins/Estrogens Inclusion Complexes." Encyclopedia. Web. 08 June, 2023.
Copy Citation
In the pharmaceutical industry, cyclodextrins (CDs) are frequently used to improve the aqueous solubility, stability, and bioavailability of medications. Because estrogens have a low polarity, they can interact with some cyclodextrins’ hydrophobic cavities to create inclusion complexes, if their geometric properties are compatible. Estrogen-CD complexes have been widely applied in several fields for various objectives.
cyclodextrin
inclusion complex
estrogen
CD
host-guest complexes
1. Introduction
The group of molecules known as steroids are characterized by the presence of a gonane (cyclopentaperhydrophenanthrene) unit in their structures (Figure 1). Many substituents may be added to the gonane unit, which consists of four fused rings made up of three cyclohexanes (A, B, and C rings) and one cyclopentane (D ring). This results in hundreds of different molecules that are present in fungi, animals, and plants. Figure 1 illustrates the first steroid to be complexed by cyclodextrins: ergosterol. Seven Nobel Prizes were given for works on the structure, isolation, synthesis, and biological effects of steroids from 1927 by H.O. Wieland [1] through to 1975 by V. Prelog [2], demonstrating the diversity of steroids and the complexity of their biological roles.

Figure 1. Chemical structure of: (a) a gonane unit and (b) ergosterol.
Steroids serve a variety of biological purposes, including, among others: constructing the cell membrane (cholesterol and phytosterols) [3], breaking down fats [4], controlling spermatogenesis [5][6] and secondary gender features (male hormones), increasing muscle mass [7], controlling various reproductive processes [8], contraception [9], and bone density (estrone, estradiol, and estriol) [10][11]. The last three compounds belong to the group of endogenous estrogens; however, the list of compounds with estrogen-like properties is much wider, as will be presented in the following sections.
In the pharmaceutical industry, cyclodextrins (CDs) are frequently used to improve the aqueous solubility, stability, and bioavailability of medications [12][13][14][15]. Water-insoluble medicines, such as estrogens, may insert part or all of their structure into the cavity of the CD because of its hydrophobic interior, which can lead to the development of host-guest association complexes. In these compounds, there are no covalent interactions between the host CD and the guest molecule, unlike many other complexes. Hydrophobic desolvation, hydrogen bonding, and van der Waals interactions are some of the most significant driving forces for CD complexation [16][17][18], even though the dimensional fit between the host cavity and the guest molecule is crucial for the complex’s formation [19].
2. Estrogens
2.1. Chemical Structures of Different Estrogens
As previously outlined in the introduction, the core element of the chemical structure of most estrogens is the gonane (cyclopentaperhydrophenanthrene) unit (Figure 2). However, as has already been proven, this polycyclic system is not necessarily needed for the compound to exhibit the estrogenic activity. Examples of estrogens that do not possess a gonane unit are dienestrol, diethylstilbestrol fosfestrol, hexestrol, zeranol, chlorotrianisene, and methallenestril (Figure 3). The steroid estrogens differ in the number of carbonyl and hydroxyl groups as well as in terms of their location. It should also be noted that some of the steroid estrogens exhibit stereoisomerism and the enantiomers usually significantly differ in their biological activities, such as in the case of 17-α and 17-β estradiol.
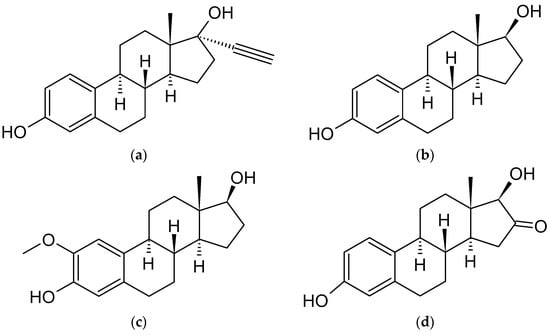
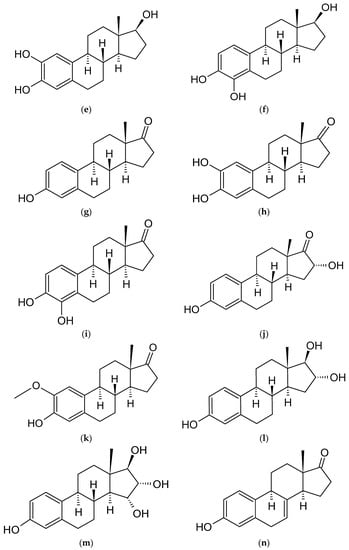
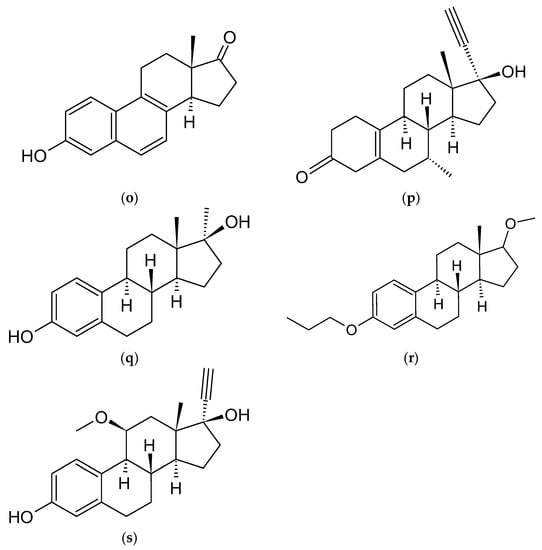
Figure 2. Chemical structures of steroidal estrogen molecules, which form host-guest complexes with CDs: (a) ethinylestradiol (EE2), (b) estradiol (E2), (c) 2-methoxyestradiol (2ME2), (d) 16-keto-17β-estradiol (16KE2), (e) 2-hydroxyestradiol (2HE2), (f) 4-hydroxyestradiol (4HE2), (g) estrone (E1), (h) 2-hydroxyestrone (2HE1), (i) 4-hydroxyestrone (4HE1), (j) 16α-hydroxyestrone (16HE1), (k) 2-metoxyestrone (2ME1), (l) estriol (E3), (m) estetrol (E4), (n) equilin, (o) eqilenin, (p) tibolone (T), and chemical structures of steroidal estrogen molecules, which have no evidence of forming host-guest complexes with CDs: (q) methylestradiol, (r) promestriene, (s) moxestrol.
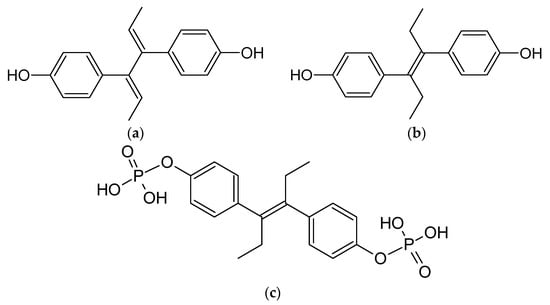

Figure 3. Chemical structures of non-steroidal estrogen molecules, which do not form host-guest complexes with CDs: (a) diensterol, (b) diethylstilbestrol, and chemical structures of estrogen molecules, which have no evidence of forming host-guest complexes with CDs: (c) fosfestrol, (d) hexestrol, (e) zeranol, (f) chlorotrianisene, (g) methallenestril.
2.2. Biological Functions and Applications of Estrogens as Therapeutic Agents
Estrogens are a class of steroid hormone that have a wide range of effects on human physiology, including the control of male and female reproductive processes, the development of various tissues, bone integrity, the cardiovascular system, the immune system, and the brain system. These hormones are also linked to the onset or advancement of several cancers [20], osteoporosis [21], neurological [22] and cardiovascular disorders [23], insulin resistance [24], lupus erythematosus [25], endometriosis [26], and obesity [27], as unfavorable consequences.
The three primary endogenous estrogens are 17-β-estradiol (E2), estriol (E3), and estrone (E1), with E2 being the strongest and most prevalent in humans. Another type of estrogen called estetrol (E4) is only produced during pregnancy [28]. The enzyme aromatase converts androgens, particularly testosterone and androstenedione, into all of the abovementioned estrogens, and the liver is where all of these changes mostly take place [29].
The main route of action of estrogens is through the estrogen receptor, a dimeric nuclear protein that possess affinity to DNA, and therefore modulates the expression of genes [30]. Similar to the other steroids, estrogens passively enter into the cell due to their lipophilic nature and bind to the estrogen receptor, causing its activation. As the estrogens can enter all cells, their actions depend on the presence and concentration of the estrogen receptor in the specific cell [31].
Medical applications of estrogens include, among others, hormonal contraception, hormone replacement therapy, and the treatment of gender dysphoria in transgender women as a part of feminizing hormone therapy [32]. The synthetic estrogen derivative of E2, known as 17-α-ethinylestradiol (EE2), is utilized in practically all current formulations of combination oral contraceptive tablets. Other conditions such as menopausal symptoms, breast cancer, and prostate cancer are also treated with it. Many research findings have supported the idea that EE2 might have certain negative consequences, including headaches, breast tenderness, nausea, dizziness, and weight gain. Therefore, EE2 is often advised for oral administration at a lower doses to avoid its side effects [33][34].
EE2, like most estrogens, belongs to the class II of the Biopharmaceutical Classification System (BCS). Active pharmaceutical ingredients (APIs) from this group are characterized by high permeability, but also low solubility. Therefore, the bioavailability of those products is limited by their dissolution rate. According to previous studies, the bioavailability of EE2 is directly proportional to the water solubility of the drug [35][36]. Therefore, various methods such as cocrystals formation or the application of nanocarriers are utilized to boost the EE2 dissolution rate [37][38]. Moreover, the clearance of EE2 measured after intravenous administration is weaker than that measured after oral administration of the same dose of EE2, and the absorption value of EE2 after injectable administration is higher than that of oral administration [39]. Complexes of various CDs with EE2 are used to obtain drugs in various dosage forms.
2.3. Endocrine Disrupting Chemicals (EDCs)
Both endogenous estrogens, as well as the artificial ones from contraceptives and animal growth agents, enter into the environment through the metabolism and excretion of humans and animals. Once in the environment, estrogens contribute, amongst other compounds such as bisphenol, octyl phenol, or nonylphenol, to a group of endocrine disrupting chemicals (EDCs). These compounds are typical pollutants in water systems, exhibiting strong estrogenic potencies and eco-toxicity [40][41][42][43].
Even at very low concentrations, EDCs can cause serious endocrine disorders. For example, in water environments, EDCs have been attributed to reproductive disturbances in wildlife, such as the feminization of male fish [44][45]. Due to their physicochemical properties, such as lipophilicity and neutral character, estrogen pollutants are difficult to remove and they gradually accumulate in water systems, posing a great risk to ecological security [46]. Furthermore, freshwater worms can bioaccumulate EDCs, making a transfer to benthivores possible and the subsequent secondary poisoning of predators. In addition, synergistic effects can be observed between some EDCs, inducing greater harm and increasing the risk of poisoning [47]. In this regard, the purification and sanitation of estrogen pollutants is a critical issue worldwide. As will be shown in the following sections, CDs can be of particular help regarding this topic.
3. Estrogens That form Host-Guest Complexes with Cyclodextrins
3.1. Ethinylestradiol (EE2)
3.1.1. Complex Preparation Methods
The preparation of EE2 (Figure 2) and cyclodextrin complexes usually occurs in a methanol/water environment. It is important to note that the ratio between MeOH and water causes marked association constant values differences. Studies have shown that association constants in water are higher than in methanol [48]. Because of the high logKow = 4.12 of EE2, the usage of surfactants (sodium dodecyl sulfate, SDS) and buffering agents to maintain pH = 11.5 is helpful in this case [49]. The reactants for the complexation reaction usually include water/methanol solutions of EE2 and CDs. The solution composed of methanol, water, and EE2 should be stored at 6.5 °C, with the exclusion of the evaporation of methanol and without the presence of light before the reaction. The procedure for preparing the CD solution for the reaction is quite similar; CD should also be dissolved in water with methanol (usually at a 45:55 ratio). There are known cases of using ethanol instead of methanol; i.e., 1 mL of pure EtOH to 10 mL of deionized water. The reaction takes place by mixing the two reagents and stirring them [50]. There was no information found regarding the preparation of high concentration complexes. The stoichiometry of the reaction shows that the formation of complexes can occur, depending on the CD used, at a ratio of 1:1 [48][51][52], 2:1 [53], 1:2 (only for γ-CD, because it has the largest cavity) [54], or a mix of these values.
Studies have shown that the usage of β-CD to form complexes with EE2 can cause difficulties because its cavity may be too small to fully bind with the hydrophobic elements of this steroid. That is why the usage of γ-CD or 2HP-γ-CD is more effective, due to their larger sizes. The association constant of EE2 with γ-CD in methanol-water (45:55, v/v) at 35 °C is about twice as high as with β-CD [55] (or even more, as shown in other studies [49]). The association constants in an acetonitrile-water environment are lower than in methanol-water solutions [56]. Even while working with γ-CD, one can encounter obstacles. According to the comparison of EE2 with γ-CD and 2HP-γ-CD complexes by Shakalisava and Regan [49], despite the application of CDs with larger cavities, the steroid can still have difficulties in forming hydrogen bonds between substituted hydroxyl groups and itself. The same can occur in cases of other estrogens, such as equilin, E3, E2, and E1 [49].
3.1.2. Complex Structure Analysis
To study their structure and properties, the obtained complexes have been analyzed using many methods. While association constants have been usually determined using HPLC, one paper also showed association constants of EE2/β-CD and EE2/γ-CD fluorometrically measured in methanol/water (20/80 v/v) at 35 °C [57]. Then, the values were compared to corresponding ones obtained from an another HPLC study. The values from fluorometric determination were found to be lower, which was, however, not fully explained by the authors [58]. The solubility of pure EE2 in water is very low, approximately 11.3 mg L−1. Gong et al. showed that complexation with diethylenetriamine-β-cyclodextrin (DETA-β-CD) increases the solubility more than forty times to approximately 496 mg L−1, which was the highest value among the analyzed types of CD in this study [50]. The surface morphology and composition of complexes have been analyzed using scanning electron microscopy (SEM). It confirmed the difference between the EE2 and CD physical mixture and the complex, showing a homogeneous and compact plate-like structure crystal [50]. To prove that van der Waals interactions occur in the cavity of CD, UV spectroscopy has been applied [50].
3.2. Estradiol (E2)
3.2.1. Complex Preparation Methods
The process of the formulation of E2/CD complexes is similar to EE2/CD. It can be achieved by adding an excess amount of E2 (Figure 2) to a CD solution. To allow the complexes to be formed, the solution should be stirred for 24 h [59]. Storing the complex for further use should be carried out at low temperature; for example, at 4 °C [60]. The current state of knowledge regarding the stoichiometry of the complexes indicates a 1:1 [60][61][62] value for HP-β-CD and β-CD or a 2:1 [61][62] for β-CD in the form of polymer-based materials. The 1:1 stoichiometry with β-CD was also shown to be stable in a molecular modeling study [63]. Both native and modified β-CDs are more effective in forming complexes with E2 in aqueous solutions than α-CD or γ-CD [64].
3.2.2. Complex Structure Analysis
The structure of the complexes of E2 with various CDs has been extensively studied. It has been generally approved that the complex is formed by the penetrating of the cyclodextrin’s cavity by the estradiol molecule. In a recent work [62], the authors claimed to successfully solve the crystal structure of the β-CD/E2 complex. However, after a closer look, researchers found that, in this study, it was only possible to determine the positions of the CD’s atoms, while the orientations of E2 molecules were not established due to the high level of structural disorder. In the same article, the structure and stability of the β-CD/E2 complex have been studied using many different methods, such as DSC, NMR, thermogravimetric analysis, and hot stage microscopy [62].
In another theoretical work, the representative geometries of β-CD/E2 and HP-βCD/E2 were obtained using molecular dynamics simulations. The in silico study showed that the aromatic ring was located closer to the wider rim and the cyclopentyl to the narrow rim of the CD. The association constants of β-CD/E2 and HP-β-CD/E2 were established at 298 K [63]. Another paper also showed the association constants of E2/β-CD and E2/γ-CD fluorometrically measured in methanol/water (20/80 v/v) at 35 °C. Then, the values were compared to values from an HPLC study. The values from fluorometric determination were found to be lower than those obtained by the chromatographic method [58]. In another HPLC study, the values of retention factors were determined for the complexes formed between the E2 and α-, β-, and γ-CDs, in their native and hydroxypropyl derivatives forms at temperatures from 0 to 60 °C. The retention factor of the complex decreases with the increase in the number of the sugar molecules in the cyclodextrin ring. The same study pointed out that the high retention factor reflects the strength of the host-guest interactions and highlights the importance of temperature in chromatographic complex separations [65]. The presence of β-CD makes it easier to separate E2 from other estrogens using HPLC at specific temperatures. This is caused by shorter retention times for this estrogen [66]. When E1, E2 (both stereoisomers), E3, and E4 have to be separated using HPLC, the addition of β-CD and a temperature at exactly 26 or 47 °C enables excellent separation [67]. Another chromatographic method—micellar electrokinetic chromatography (MEKC)—also showed an enhancing effect on estrogen separations using β-CD and γ-CD as additives. Thanks to the addition of CDs, E2 was more easily separated from another nine different estrogens. A separation using α-CD was also performed, but the results showed no successful separation in that case. This can be explained by the cavity of α-CD not being large enough to successfully bind with the estrogens [68]. DSC experiments showed that the characteristic endothermic curve peaks of E2 at 179.60 °C disappear from the curve after complexation with β-CD [61]. The thermodynamic parameters of the complex formation were also measured and analyzed. This determined that the dominating interaction forces are the hydrophobic and hydrogen-bonding interactions, which stabilize the β-CD/E2 complex, as well as the hydrogen bonding interaction and van der Waals forces for the HP-β-CD/E2 complex [63].
3.3. Estradiol Derivatives
2ME2 (Figure 2) forms stable complexes with CDs, usually in a 1:1 stoichiometry. A UV spectroscopy method was used to study the stability of the 2ME2 complexes with DM-β-CD and TM-β-CD. In these cases, the inclusion occurs by the penetration of the D ring of 2ME2 to form the secondary side of the CD while the A and part of the B ring are protruding from the secondary side of the CD. These complexes have been extensively studied using many methods, including DSC, HSM, TGA, FTIR-spectroscopy, UV-spectroscopy, X-ray, and molecular modelling. While DM-β-CD/2ME2 forms monoclinic crystals and TM-β-CD/2ME2 triclinic ones, both complexes showed an enhancement of the water solubility of 2ME. The solubility enhancement factors were compared within a group of 10 cyclodextrins. It was shown that TM-β-CD/2ME2 had the highest solubility enhancement factor value, nearly 25 higher than β-CD [69].
SBE-β-CD/2ME2 is a CD complex that has been studied in silico using the PM3 semi-empirical method. The SBE-β-CD was determined as a CD derivative with either two or four sulfobuthyl ether groups. In the case of four groups, their orientation can be different and have an significant impact on the complexation. These groups can be connected to the CD in two orientations—“up” or “sideways”. The variant with no groups in the “up” orientation forms the most stable complex. Other variants are unfavorable for the complexation. The heat of formation, dipole moments, stabilization energies, and the migration time (capillary electrophoresis) for the PM3-optimized geometry of the 2-hydroxyestrone inclusion complexes were calculated. A correlation between migration time and the total heat of formation occurred. It suggests that more stable complexes have a longer migration time in capillary electrophoresis. Furthermore, the theoretical in silica calculations suggest that a β-CD/2ME2 complex may exist with the A or D ring in the cavity while migrating [70].
The MEKC separation of ten different estrogens, including 2HE2, 4HE2, and 16KE2, was performed in the presence of α-, β- and γ-CD. The addition of α-CD did not improve the separation. However, the β-CD and γ-CD made the separation more effective. Furthermore, 16KE2 was one of the two studied estrogens (the other was 16HE1), for which the addition of CD impacted in a reversion of the migration order. The addition of γ-CD showed the best results, and it is highly recommended to add the γ-CD to MEKC separations of various estrogen mixtures [68].
In addition, 4HE2 and 2HE2 can be obtained as products of an E2 bioconversion using phenoloxidase in mucuna pruriens cell cultures. An E2/β-CD complex was used to enhance the concentration of E2 in water before the reaction. The bioconversion was successfully performed. The fact that E2 was inside the CD cavity during the reaction did not affect the structure of the products. The products of the reaction, 2HE2 and 4HE2 (with the majority of 4HE2), were still complexed with CD after the reaction was completed. This was confirmed using DSC [71][72].
3.4. Estrone (E1)
3.4.1. Structure of the Complexes and Complex Formation Mechanism
Estrone (Figure 2) is a steroidal estrogen and, like most of the other compounds from this group, it forms stable complexes with various CDs, including both native α, β, and γ CDs as well as their functionalized derivatives. These host molecules significantly differ not only in terms of the size of their cavities, but also in their solubilities, lipophilicity, and their abilities to form the inter- and intramolecular forces. The stoichiometry of such complexes is usually determined to be 1:1 [51][55]. The stoichiometry of 1:1 with six various cyclodextrins was confirmed during an estimation of their association constant values using a relationship analogous to the Benesi–Hildebrand equation [57]. Another study confirmed the 1:1 stoichiometry using the Hummel-Dreyer method [55].
E1 can enter the cavity of β-CD by either the A-ring or the D-ring. This is possible because of the weak steric hindrance of the molecule with the CD. Such conclusions were obtained from the molecular docking results. In addition, the calculations allowed for an estimation of the stability constants of the E1 complex with β-CD and γ-CD in two possible orientations, the A-ring or D-ring in the cavity. The calculated KA−up was greater the larger the KD−up, which indicates that the A-ring penetrating the cavity is more likely to occur [73]. The association constant of E1/β-CD was measured using HPLC in methanol/H2O 45:55 v/v solution at 35 °C. It was shown that the effect of adding the CD to increase the solubility is much weaker for E1 than it was for E2, EE2, or E3 [51]. What was unique for E1, in comparison with other estrogens, was that γ-CD/E1 was characterized by a higher association constant than β-CD/E1 [55]. This was also confirmed in another study with γ-CD in the native form and using its derivatives [49]. The complexation constant was determined using the spectrofluorimetric method for five other CD complexes with E1 in water and other solvents as well [57].
3.4.2. Methods of Complex Preparation
It has been shown multiple times that the solvent has an major influence on the association constant value for E1/CD complexes. For example, in MeCN/water solutions, the value of the complexation constant is nearly three times lower than in the MeOH/water environment [56]. In another study, the fluorescence spectra of complexes formed between E1 and various CDs were measured in aqueous solution at neutral pH. The results confirmed the formation of inclusion complexes between E1 and all of the studied CD derivatives, except for α-CD and S-β-CD [57]. The enthalpy and entropy of formation values for γ-CD/E1 and β-CD/E1 were determined in two different environments [56]. The retention factors for α-, β-, and γ-CD/E3 complexes and their HP-CD derivatives were measured using HPLC at 0 and 60 °C in MeCN/water solution (35% v/v). The differences between the retention factors point out the importance of temperature control in the HPLC analysis of those complexes. γ-CD/E3 and its HP-derivative were the most effective host-guest complexation [65].
3.5. Estrone Derivatives
SBE-β-CD/2HE1 has only been studied by means of molecular modeling methods so far. Quantum-mechanical studies calculations have been used to determine the heat of formation, dipole moments, stabilization energies, and the capillary electrophoresis migration time for PM3-optimized geometry of this complex. A correlation between migration time and the total heat of formation occurred. It suggested that more stable complexes have a longer migration time in capillary electrophoresis. Furthermore, the theoretical calculations suggest that a CD/2HE1 complex may exist with either the A or D ring in the cavity while migrating [70].
The separation of estrogens may be very difficult. To facilitate this task, the influence of β-CD, DM-β-CD, and HE-β-CD on the separation of 2HE1, 4HE1, or 16HE1 using HPLC was studied. The addition of CD was found to make the separation much more effective. The influence of concentration on the efficiency of this process was also studied. β-CD derivatives are especially recommended for successful HPLC separation [74]. The MEKC separation of ten different estrogens, including 4HE1, 16HE1, and 2ME1, was performed in the presence of α-, β-, and γ-CD. The addition of α-CD did not improve the separation. However, the β-CD and γ-CD made the separation more effective. Furthermore, 16HE1 was one of two studied estrogens (the other was 16KE2) where the addition of CD impacted in reversion of the migration order. The addition of γ-CD showed the best results, and it is highly recommended to add the γ-CD to MEKC separations in various estrogens mixtures [68].
3.6. Estriol (E3)
3.6.1. Complex Preparation and Structural Studies
Estriol (E3) (Figure 2) is another example of steroidal estrogen. Like other compounds from this group, the most stable complexes of E3 are those with β-CD and its derivatives. The stoichiometry of such complexes is usually determined as 1:1 [51][55]. The stoichiometry of 1:1 with six various cyclodextrins was confirmed during an estimation of their association constant values using a relationship analogous to the Benesi–Hildebrand equation [57]. Another study confirmed the 1:1 stoichiometry by using the Hummel-Dreyer method [55].
Estriol strongly binds with the β-CD by fully penetrating its cavity, which was studied using the solution 1H NMR technique [75]. Furthermore, as shown by the molecular docking calculations, E3 can enter the cavity of β-CD either by the A ring or the D ring, resulting in both orientations of host-guest complexes present in the solution. This is possible because of the weak steric hindrance of the molecule in the CD, which is also true for E1. The calculations also estimated the stability constants of the E3 complexes with β-CD and γ-CD in two possible orientations, with either the A ring or the D ring penetrating the cavity. ln KA−up was found to be larger than ln KD−up, which indicates that the A-ring penetrating the cavity is more likely to occur [73]. The association constant (Ka) of E3/β-CD was measured using HPLC in methanol/H2O 45:55 v/v solution at 35 °C. The complex had a much lower value of Ka than E2. For E1 and EE2, Ka was at a very similar level [51]. The Hummel-Dreyer method was also used to estimate the Ka in methanol/water solution. It showed major differences of this constant value among three studied estrogens, with the E3 possessing the highest one. γ-CD/E3 had a higher association constant than β-CD/E3. This was also confirmed in another study also on γ-CD derivatives [49]. The same constant was estimated using a spectrofluorimetric study for five other CD complexes with E3 in water, as well as in many other solvents [57]. Another study also showed the association constants of E3/β-CD and E3/γ-CD fluorometrically measured in methanol/water (20/80 v/v) at 35 °C. Then, the values were compared to corresponding ones from an HPLC study. The values from fluorometric determination were lower [58]. The solvent has an major influence on the Ka value. For example, in MeCN/water solutions, this value is nearly three times smaller than in a MeOH/water environment [56].
The fluorescence spectra of complexes formed with various CDs were recorded in aqueous solution at neutral pH. The results confirmed the formation of inclusion complexes with all studied CD derivatives, except for α-CD and S-β-CD [57]. The enthalpy and entropy of the formation of γ-CD/E3 and β-CD/E3 were determined in two different environments [56]. The retention factor for α-, β-, and γ-CD/E3 complexes and their HP-CD derivatives were measured using HPLC at 0 and 60 °C in MeCN/water (35% v/v). The differences of the retention factors point out the importance of the temperature in the HPLC studies. γ-CD/E3 and its HP-derivative have been shown to be the most effective CDs for the host-guest complexation [65].
3.7. Estetrol (E4)
A highly interesting in vivo study of E4/CD complexes has been recently reported. In this work, E4 (Figure 2) was complexed with HP-β-CD and encapsuled in liposomes. The solubility of E4 linearly increased with the CD concentration until 50 mM, which indicated the formation of a 1:1 molar ratio complex. Such a formulation increased the capability of passing the brain-blood barrier by E4. This has a major effect for dealing with the brain problems of some premature babies mentioned in the paper. The complex showed no cellular toxicity and had good physicochemical properties—suitable for future pharmacological usage. Simultaneously, this work provides evidence of the existence of E4/β-CD complexes [76].
E4 was also used in an HPLC study, where the influence of temperature and the addition of β-CD on the efficiency of the separation was analyzed. The temperature had an major effect when estrogens in the presence of β-CD were separated. Temperatures of 47 and 26 °C showed the best selectivity of the process and enabled excellent separation [67].
3.8. Equilin and Equilenin
Equilin and equilenin (Figure 2) are horse estrogens, which are not present in human organisms under physiological conditions. Despite that, they have found application as APIs; for example, as one of the ingredients of conjugated estrogens (CEEs) mixtures, which are used in replacement hormone therapies for menopausal symptoms [77]. The influence of β-CD on the chromatographic estrogen separation of mixtures containing equilin and equilenin has been studied. It has been shown that, at subambient temperatures, the selectivity of the chromatographic system was greatly enhanced by the addition of CD [66]. A similar study was performed for equilin, this time using β-CD derivatives such as DM-β-CD and HE-β-CD. The results were similar to the previous work. The influence of CD concentration has also been extensively studied. It was concluded that β-CD, both in a native form but especially its derivatives, is very useful in equilin and E3 chromatographic separations [74]. In a study with five other estrogens, it was shown that the best temperature for equilin separation in the presence of β-CD was either 47 or 26 °C [67].
References
- Percec, V.; Xiao, Q. From organic chemistry to chemical biology via macromolecules with Hermann Staudinger. Giant 2020, 4, 100036.
- Shampo, M.A.; Kyle, R.A.; Steensma, D.P. Vladimir Prelog—Nobel Prize for Work in Stereochemistry. Mayo Clin. Proc. 2008, 83, 391.
- Atkovska, K.; Klingler, J.; Oberwinkler, J.; Keller, S.; Hub, J.S. Rationalizing Steroid Interactions with Lipid Membranes: Conformations, Partitioning, and Kinetics. ACS Cent. Sci. 2018, 9, 1155–1165.
- Krawczyńska, A.; Herman, A.P.; Antushevich, H.; Bochenek, J.; Dziendzikowska, K.; Gajewska, A.; Gromadzka-Ostrowska, J. Modifications of Western-type diet regarding protein, fat and sucrose levels as modulators of steroid metabolism and activity in liver. J. Steroid Biochem. Mol. Biol. 2017, 165, 331–341.
- Zhang, Z.; Cheng, Q.; Liu, Y.; Peng, C.; Wang, Z.; Ma, H.; Liu, D.; Wang, L.; Wang, C. Zinc-Enriched Yeast May Improve Spermatogenesis by Regulating Steroid Production and Antioxidant Levels in Mice. Biol. Trace Elem. Res. 2022, 200, 3712–3722.
- Desai, A.; Yassin, M.; Cayetano, A.; Tharakan, T.; Jayasena, C.N.; Minhas, S. Understanding and managing the suppression of spermatogenesis caused by testosterone replacement therapy (TRT) and anabolic-androgenic steroids (AAS). Ther. Adv. Urol. 2022, 14, 17562872221105017.
- Bochud, M.; Ponte, B.; Pruijm, M.; Ackermann, D.; Guessous, I.; Ehret, G.; Escher, G.; Groessl, M.; Estoppey Younes, S.; d’Uscio, C.H.; et al. Urinary Sex Steroid and Glucocorticoid Hormones Are Associated with Muscle Mass and Strength in Healthy Adults. J. Clin. Endocrinol. Metab. 2019, 104, 2195–2215.
- Esposito, M.; Salerno, M.; Calvano, G.; Agliozzo, R.; Ficarra, V.; Sessa, F.; Favilla, V.; Cimino, S.; Pomara, C. Impact of anabolic androgenic steroids on male sexual and reproductive function: A systematic review. Panminerva Med. 2023, 65, 43–50.
- Sech, L.A.; Mishell, D.R., Jr. Oral steroid contraception. Womens Health 2015, 11, 743–748.
- Nunes, E.; Gallardo, E.; Morgado-Nunes, S.; Fonseca-Moutinho, J. Steroid hormone levels and bone mineral density in women over 65 years of age. Sci. Rep. 2023, 13, 4925.
- Kuniyil, A.; Pal, S.; Sachdev, N.; Yadav, T.P. Effect of 2–6 weeks of systemic steroids on bone mineral density in children. Clin. Exp. Pediatr. 2022, 65, 254–261.
- Kovacs, T.; Nagy, P.; Panyi, G.; Szente, L.; Varga, Z.; Zakany, F. Cyclodextrins: Only Pharmaceutical Excipients or Full-Fledged Drug Candidates? Pharmaceutics 2022, 14, 2559.
- Paiva-Santos, A.C.; Ferreira, L.; Peixoto, D.; Silva, F.; Soares, M.J.; Zeinali, M.; Zafar, H.; Mascarenhas-Melo, F.; Raza, F.; Mazzola, P.G.; et al. Cyclodextrins as an encapsulation molecular strategy for volatile organic compounds- Pharmaceutical applications. Colloids Surf. B Biointerfaces 2022, 218, 112758.
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389.
- Zaghloul, N.; El Hoffy, N.M.; Mahmoud, A.A.; Elkasabgy, N.A. Cyclodextrin Stabilized Freeze-Dried Silica/Chitosan Nanoparticles for Improved Terconazole Ocular Bioavailability. Pharmaceutics 2022, 14, 470.
- Zhao, Q.; Ye, Z.; Su, Y.; Ouyang, D. Predicting complexation performance between cyclodextrins and guest molecules by integrated machine learning and molecular modeling techniques. Acta Pharm. Sin. B 2019, 9, 1241–1252.
- Rahim, M.; Madi, F.; Nouar, L.; Bouhadiba, A.; Haiahem, S.; Khatmi, D.E.; Belhocine, Y. Driving forces and electronic structure in β-cyclodextrin/3,3′-diaminodiphenylsulphone complex. J. Mol. Liq. 2014, 199, 501–510.
- Liu, L.; Guo, Q.X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14.
- Farcas, A.; Resmerita, A.-M.; Balan-Porcarasu, M.; Cojocaru, C.; Peptu, C.; Sava, I. Inclusion Complexes of 3,4-Ethylenedioxythiophene with Per-Modified β- and γ-Cyclodextrins. Molecules 2023, 28, 3404.
- Miedl, H.; Oswald, D.; Haslinger, I.; Gstoettner, M.; Wenzl, R.; Proestling, K.; Schneeberger, C.; Yotova, I.; Schreiber, M. Association of the Estrogen Receptor 1 Polymorphisms rs2046210 and rs9383590 with the Risk, Age at Onset and Prognosis of Breast Cancer. Cells 2023, 12, 515.
- Khosla, S.; Pacifici, R. Estrogen deficiency and the pathogenesis of osteoporosis. In Marcus and Feldman’s Osteoporosis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 773–797.
- Brann, D.W.; Lu, Y.; Wang, J.; Sareddy, G.R.; Pratap, U.P.; Zhang, Q.; Tekmal, R.R.; Vadlamudi, R.K. Brain-Derived Estrogen and Neurological Disorders. Biology 2022, 11, 1698.
- Rytz, C.L.; Turino Miranda, K.; Ronksley, P.E.; Dumanski, S.M.; Saad, N.; Raj, S.R.; Somayaji, R.; Ganshorn, H.; Newbert, A.M.; Peace, L.; et al. Serum oestradiol levels and risk of adverse cardiovascular events associated with gender-affirming oestrogen therapy: A protocol for a systematic review and meta-analysis. BMJ Open 2022, 12, e064961.
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498.
- Liu, H.; Peng, L.; Ma, J.; He, L.; Long, K.; Ouyang, X.; Wu, C.; Xie, M.; Dai, L.; Cai, X. Low expression of estrogen receptor β in renal tubular epithelial cells may cause hyperuricemia in premenopausal patients with systemic lupus erythematosus. Lupus 2021, 30, 560–567.
- Guimarães Ferreira, L.R.; Cavalcanti, T.; Zomer, M.; Kondo, W.; Araujo Júnior, E.; Kulak Junior, J. Estrogen and progesterone receptors in endometriosis. Minerva Obstet. Gynecol. 2022, 74, 330–336.
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690.
- Thomas, M.P.; Potter, B.V. The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol. 2013, 137, 27–49.
- Chan, H.J.; Petrossian, K.; Chen, S. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and -resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2016, 161, 73–83.
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170.
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genom. 2006, 7, 497–508.
- Pofi, R.; Feliciano, C.; Sbardella, E.; Argese, N.; Woods, C.P.; Grossman, A.B.; Jafar-Mohammadi, B.; Gleeson, H.; Lenzi, A.; Isidori, A.M.; et al. The Short Synacthen (Corticotropin) Test Can Be Used to Predict Recovery of Hypothalamo-Pituitary-Adrenal Axis Function. J. Clin. Endocrinol. Metab. 2018, 103, 3050–3059.
- Hasegawa, Y.; Itonaga, T.; Ikegawa, K.; Nishigaki, S.; Kawai, M.; Koga, E.; Sakakibara, H.; Ross, J.L. Ultra-low-dose estrogen therapy for female hypogonadism. Clin. Pediatr. Endocrinol. 2020, 29, 49–53.
- Hilakivi-Clarke, L.; Wärri, A.; Bouker, K.B.; Zhang, X.; Cook, K.L.; Jin, L.; Zwart, A.; Nguyen, N.; Hu, R.; Cruz, M.I.; et al. Effects of In Utero Exposure to Ethinyl Estradiol on Tamoxifen Resistance and Breast Cancer Recurrence in a Preclinical Model. J. Natl. Cancer Inst. 2016, 109, djw188.
- Alsop, D.; Wilson, J.Y. Waterborne pharmaceutical uptake and toxicity is modified by pH and dissolved organic carbon in zebrafish. Aquat. Toxicol. 2019, 210, 11–18.
- Juárez-Niño, E.D.; Moreno-Rodríguez, A.; Juárez-Chávez, L.; Santillan, R.; Ochoa, M.A.; Argueta-Figueroa, L.; Torres-Rosas, R.; Domínguez-Diaz, L.R.; Soto-Castro, D. Synthesis of acetylenic 17α-ethynylestradiol derivatives as potential trypanocidal oral drugs: In vitro and in silico evaluation. J. Mol. Struct. 2023, 1274, 134431.
- Bisesi, J.H., Jr.; Robinson, S.E.; Lavelle, C.M.; Ngo, T.; Castillo, B.; Crosby, H.; Liu, K.; Das, D.; Plazas-Tuttle, J.; Saleh, N.B.; et al. Influence of the Gastrointestinal Environment on the Bioavailability of Ethinyl Estradiol Sorbed to Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2017, 51, 948–957.
- Du, R.; Xu, J.; Zhang, L.; Ning, L.; Li, S. Ethinyl estradiol cocrystals assembled by chain structures: Improvement in stability and solubility. New J. Chem. 2019, 43, 16889–16897.
- Shafik, A. Contraceptive efficacy of polyester-induced azoospermia in normal men. Contraception 1992, 45, 439–451.
- Alva-Gallegos, R.; Carazo, A.; Mladěnka, P. Toxicity overview of endocrine disrupting chemicals interacting in vitro with the oestrogen receptor. Environ. Toxicol. Pharmacol. 2023, 99, 104089.
- Zhu, L.; Hajeb, P.; Fauser, P.; Vorkamp, K. Endocrine disrupting chemicals in indoor dust: A review of temporal and spatial trends, and human exposure. Sci. Total Environ. 2023, 874, 162374.
- Modica, R.; Benevento, E.; Colao, A. Endocrine-disrupting chemicals (EDCs) and cancer: New perspectives on an old relationship. J. Endocrinol. Investig. 2023, 46, 667–677.
- Ahn, C.; Jeung, E.-B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342.
- Delbes, G.; Blázquez, M.; Fernandino, J.I.; Grigorova, P.; Hales, B.F.; Metcalfe, C.; Navarro-Martín, L.; Parent, L.; Robaire, B.; Rwigemera, A.; et al. Effects of endocrine disrupting chemicals on gonad development: Mechanistic insights from fish and mammals. Environ. Res. 2022, 204, 112040.
- Gross-Sorokin, M.Y.; Brighty, G.; Roast, S. Assessment of Feminization of Male Fish in English Rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 2005, 114, 147–151.
- Rhind, S.; Kyle, C.; Mackie, C.; McDonald, L. Accumulation of endocrine disrupting compounds (EDCs) in sheep fetal and maternal liver tissue following exposure to pastures treated with sewage sludge. J. Environ. Monit. 2009, 11, 1469–1476.
- Zhou, R.; Cheng, W.; Feng, Y.; Wei, H.; Liang, F.; Wang, Y. Interactions between three typical endocrine-disrupting chemicals (EDCs) in binary mixtures exposure on myocardial differentiation of mouse embryonic stem cell. Chemosphere 2017, 178, 378–383.
- Wang, H.; Duan, A.; Dahlgren, R.A.; Li, Y.; Li, C.; Wang, W.; Zeng, A.; Wang, X. The joint effects of room temperature ionic liquids and ordered media on fluorescence characteristics of estrogens in water and methanol. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014, 128, 497–507.
- Shakalisava, Y.; Regan, F. Determination of association constants of inclusion complexes of steroid hormones and cyclodextrins from their electrophoretic mobility. Electrophoresis 2006, 27, 3048–3056.
- Gong, F.; Lv, R.; Ma, J.; Wang, X.; Qu, Y.; Zhang, C.; Xu, J.; Wang, T. Synthesis and Characterization of Water Soluble Diethylenetriamine-β-Cyclodextrin/Ethinylestradiol Inclusion Complex. Chem. Eur. 2022, 7, 37.
- Sadlej-Sosnowska, N. Molecular complexation: β-cyclodextrin and steroid hormones inclusion complexes studied by high performance liquid chromatography. Eur. J. Pharm. Sci. 1995, 3, 1–5.
- Lin, Z.Y.; Liu, Y.X.; Kou, S.B.; Wang, B.L.; Shi, J.H. Characterization of the inclusion interaction of ethinyloestradiol with β-cyclodextrin and hydroxypropyl-β-cyclodextrin: Multi-spectroscopic and molecular modeling methods. J. Mol. Liq. 2020, 311, 113290.
- Fenyvesi, É.; Puskás, I.; Szente, L. Chapter 2 Cyclodextrin-Steroid Interactions and Applications to Pharmaceuticals, Food, Biotechnology and Environment. In Cyclodextrin Applications in Medicine, Food, Environment and Liquid Crystals; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 19–57.
- Tang, P.; Sun, Q.; Suo, Z.; Zhao, L.; Yang, H.; Xiong, X.; Pu, H.; Gan, N.; Li, H. Rapid and efficient removal of estrogenic pollutants from water by using beta- and gamma-cyclodextrin polymers. J. Chem. Eng. 2018, 344, 514–523.
- Sadlej-Sosnowska, N. Inclusion complexes of steroid hormones with cyclodextrins studied by the Hummel-Dreyer method using reversed-phase liquid chromatography. J. Pharm. Biomed. Anal. 1995, 13, 701–704.
- Sadlej-Sosnowska, N. Thermodynamic parameters of the formation of a complex between cyclodextrins and steroid hormones. J. Chromatogr. 1996, 728, 89–95.
- Perez, R.L.; Escandar, G.M. Spectrofluorimetric study of estrogen–cyclodextrin inclusion complexes in aqueous systems. Analyst 2013, 138, 1239.
- Sadlej-Sosnowska, N. Fluorometric Determination of Association Constants of Three Estrogens with Cyclodextrins. J. Flouresc. 1997, 7, 195–200.
- Gallez, A.; Palazzo, C.; Blacher, S.; Tskitishvili, E.; Noël, A.; Foidart, J.-M.; Evrard, B.; Pequeux, C.; Piel, G. Liposomes and drug-in-cyclodextrin-in-liposomes formulations encapsulating 17β-estradiol: An innovative drug delivery system that prevents the activation of the membrane-initiated steroid signaling (MISS) of estrogen receptor α. Int. J. Pharm. 2019, 573, 118861.
- Silva, M.C.G.D.; Silva, J.F.D.; Santos, T.P.; Silva, N.P.C.D.; Santos, A.R.D.; Andrade, A.L.C.; Souza, E.H.L.D.S.; Sales Cadena, M.R.; Sá, F.B.; Silva Junior, V.A.D.; et al. The complexation of steroid hormones into cyclodextrin alters the toxic effects on the biological parameters of zebrafish (Danio rerio). Chemosphere 2019, 214, 330–340.
- Haimhoffer, Á.; Vas, A.; Árvai, G.; Fenyvesi, É.; Jicsinszky, L.; Budai, I.; Bényei, A.; Regdon, G., Jr.; Rusznyák, Á.; Vasvári, G.; et al. Investigation of the Drug Carrier Properties of Insoluble Cyclodextrin Polymer Microspheres. Biomolecules 2022, 12, 931.
- Vicatos, A.I.; Hoossen, Z.; Caira, M.R. Inclusion complexes of the steroid hormones 17β-estradiol and progesterone with β- and γ-cyclodextrin hosts: Syntheses, X-ray structures, thermal analyses and API solubility enhancements. Beilstein J. Org. Chem. 2022, 18, 1749–1762.
- Lin, Z.Y.; Wang, X.X.; Kou, S.B.; Shi, J.H. Exploring the inclusion interaction of estradiol with β-CD and HP-β-CD with the help of molecular dynamics simulation as well as multi-spectroscopic approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 269, 120764.
- Oishi, K.; Toyao, K.; Kawano, Y. Suppression of estrogenic activity of 17 β-estradiol by β-cyclodextrin. Chemosphere 2008, 73, 1788–1792.
- Zarzycki, P.K.; Ohta, H.; Saito, Y.; Jinno, K. Interaction of native α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin and their hydroxypropyl derivatives with selected organic low molecular mass compounds at elevated and subambient temperature under RP-HPLC conditions. Anal. Bioanal. Chem. 2008, 391, 2793–2801.
- Zarzycki, P.K.; Wierzbowska, M.; Lamparczyk, H. The influence of temperature on the multiple separation of estrogenic steroids using mobile phases modified with beta-cyclodextrin in high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1997, 15, 1281–1287.
- Zarzycki, P.K.; Smith, R. Separation of steroids using temperature-dependent inclusion chromatography. J. Chromatogr. A 2001, 912, 45–52.
- Chan, K.C.; Muschik, G.M.; Issaq, H.J.; Siiteri, P.K. Separation of estrogens by micellar electrokinetic chromatography. J. Chromatogr. A 1995, 690, 149–154.
- Caira, M.R.; Bourne, S.A.; Samsodien, H.; Smith, V.J. Inclusion complexes of 2-methoxyestradiol with dimethylated and permethylated β-cyclodextrins: Models for cyclodextrin-steroid interaction. Beilstein J. Org. Chem. 2015, 11, 2616–2630.
- Deng, Y.; Huang, M. Capillary Electrophoretic Separation and Theoretical Study of Inclusion Complexes of Sulfobutyl Ether β-Cyclodextrin with Estrogens. Int. J. Quantum Chem. 2004, 100, 746–752.
- Woerdenbag, H.J.; Pras, N.; Frijlink, H.W.; Lerk, C.F.; Malingré, T.M. Cyclodextrin-facilitated bioconversion of 17 beta-estradiol by a phenoloxidase from Mucuna pruriens cell cultures. Phytochemistry 1990, 29, 1551–1554.
- Van Uden, W.; Woerdenbag, H.J.; Pras, N. Cyclodextrins as a useful tool for bioconversions in plant cell biotechnology. Plant Cell Tissue Organ Cult. 1994, 38, 103–113.
- Wensheng, C.; Xuexia, Y.; Xueguang, S.; Zhongxiao, P. Bimodal Complexations of Steroids with Cyclodextrins by a Flexible Docking Algorithm. J. Incl. Phenom. Macrocycl. Chem. 2005, 51, 41–51.
- Spencer, B.J.; William, C. Purdy, High-Performance Liquid Chromatographic Separation of Equilin, Estrone, and Estrone Derivatives with Cyclodextrins as Mobile Phase Additives. J. Liq. Chromat. 2006, 450, 414–419.
- Bednarek, E.; Bocian, W.; Poznański, J.; Sitkowski, J.; Sadlej-Sosnowska, N.; Kozerski, L. Complexation of steroid hor-mones: Prednisolone, ethinyloestradiol and estriol with B-cyclodextrin. An aqueous 1 H NMR study. J. Chem. Soc. Perkin Trans. 2002, 2, 999–1004.
- Palazzo, C.; Laloy, J.; Delvigne, A.S.; Nys, G.; Fillet, M.; Dogne, J.M.; Pequeux, C.; Foidart, J.M.; Evrard, B.; Piel, G. Development of injectable liposomes and drug-in-cyclodextrin-in-liposome formulations encapsulating estetrol to prevent cerebral ischemia of premature babies. Eur. J. Pharm. Sci. 2019, 127, 52–59.
- Bhavnani, B.R.; Stanczyk, F.Z. Pharmacology of conjugated equine estrogens: Efficacy, safety and mechanism of action. J. Steroid Biochem. Mol. Biol. 2014, 142, 16–29.
More
Information
Subjects:
Chemistry, Medicinal; Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
880
Revisions:
2 times
(View History)
Update Date:
09 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




