Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dimitra Dimopoulou | -- | 1166 | 2023-06-08 09:02:54 | | | |
| 2 | Fanny Huang | Meta information modification | 1166 | 2023-06-08 13:07:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moschopoulos, C.D.; Dimopoulou, D.; Dimopoulou, A.; Dimopoulou, K.; Protopapas, K.; Zavras, N.; Tsiodras, S.; Kotanidou, A.; Fragkou, P.C. Fluid Resuscitation in Sepsis. Encyclopedia. Available online: https://encyclopedia.pub/entry/45314 (accessed on 07 March 2026).
Moschopoulos CD, Dimopoulou D, Dimopoulou A, Dimopoulou K, Protopapas K, Zavras N, et al. Fluid Resuscitation in Sepsis. Encyclopedia. Available at: https://encyclopedia.pub/entry/45314. Accessed March 07, 2026.
Moschopoulos, Charalampos D., Dimitra Dimopoulou, Anastasia Dimopoulou, Konstantina Dimopoulou, Konstantinos Protopapas, Nikolaos Zavras, Sotirios Tsiodras, Anastasia Kotanidou, Paraskevi C. Fragkou. "Fluid Resuscitation in Sepsis" Encyclopedia, https://encyclopedia.pub/entry/45314 (accessed March 07, 2026).
Moschopoulos, C.D., Dimopoulou, D., Dimopoulou, A., Dimopoulou, K., Protopapas, K., Zavras, N., Tsiodras, S., Kotanidou, A., & Fragkou, P.C. (2023, June 08). Fluid Resuscitation in Sepsis. In Encyclopedia. https://encyclopedia.pub/entry/45314
Moschopoulos, Charalampos D., et al. "Fluid Resuscitation in Sepsis." Encyclopedia. Web. 08 June, 2023.
Copy Citation
The importance of fluid resuscitation therapy during the early stages of sepsis management is a well-established principle. Current Surviving Sepsis Campaign (SSC) guidelines recommend the early administration of intravenous crystalloid fluids for sepsis-related hypotension or hyperlactatemia due to tissue hypoperfusion, within the first 3 h of resuscitation and suggest using balanced solutions (BSs) instead of normal saline (NS) for the management of patients with sepsis or septic shock.
fluids
resuscitation
sepsis
1. Introduction
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection, whereas septic shock is a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a higher risk of mortality than with sepsis alone [1]. Although the exact worldwide burden of sepsis is difficult to ascertain, it certainly represents a major global health issue. In 2017, there was an estimate of 48.9 million cases of sepsis; during the same year, 11 million sepsis-related deaths were reported worldwide, representing almost 20% of global deaths [2]. Between 1990 and 2017, age-standardized sepsis incidence fell by 37% and mortality decreased by 52.8% [2]. Despite these trends, sepsis still remains a major cause of death worldwide. Interestingly, there are significant regional disparities in sepsis-related incidence and mortality, with approximately 85% of sepsis cases and sepsis-related deaths occurring in low- and middle-income countries [2].
The management of sepsis has not significantly changed over the past 40 years. Current guidelines recommend the early administration of antibiotics and intravenous (IV) fluids, in addition to source control and the judicious use of vasopressors [3]. Fluid resuscitation therapy represents one of the cornerstones of sepsis management [3]. Understanding the pathophysiology of sepsis is crucial in order to determine the role of intensive fluid administration in the initial phase of septic shock.
Although there is a consensus on the need for adequate fluid therapy in sepsis and despite the multiple recent clinical trials examining fluid management in sepsis, the ideal fluid management strategy is still controversial and elusive, as there are no clear guidelines about the optimal fluid resuscitation in critically ill patients with sepsis.
2. Fluid Resuscitation in Sepsis
Despite the scientific advances of the last 20 years, sepsis management has not changed drastically, apart from the introduction of the bundles, which designate multiple interventions that should be completed within a specific time frame. After initial airway and respiratory stabilization, sepsis bundle should be performed within the first 3 h of presentation. The SSC 2021 bundle includes fluid resuscitation, antibiotic administration, lactate measurement and obtainment of cultures [3]. Vasopressors should be initiated if the patient remains hypotensive despite adequate fluid resuscitation [3]. However, a group of 34 European Society of Intensive Care Medicine (ESICM) experts recently suggested to start vasopressors early, before full completion of fluid resuscitation [4]. In the revision of the Surviving Sepsis Campaign (SSC) guidelines in 2018, the 3 and 6 h bundles were combined into a single “1-h bundle” where fluid resuscitation is required in all patients without exception [5]. The implementation of these sepsis protocols in clinical practice have led to decreased sepsis mortality [6].
Fluid resuscitation remains an integral part of sepsis management, since it was first employed during the European cholera epidemic as early as 1830 [7]. The following years, fluid resuscitation was used to treat hypovolemia and restore tissue perfusion pressure in order to improve oxygen transport to cells [8]. Previous versions of SSC guidelines recommended a quantitative resuscitation protocol, that was based entirely on the early goal-directed therapy (EGDT) study [9]. This landmark study showed the benefit of early and aggressive fluid resuscitation in the mortality and the maintenance of a CVP of 8–12 mmHg and a central venous oxygen saturation (SCVO2) of at least 70% [9]. The era of a time-sensitive bundled care was then introduced in sepsis. However, subsequent multi-center randomized controlled trials (RCTs) failed to reproduce the benefits observed in the EGDT trial [10].
There is a growing scepticism regarding aggressive fluid resuscitation, since this approach may lead to massive fluid overload and, inevitably, to adverse outcomes [11]. An increasing number of studies have associated fluid overload to worse outcomes and increased mortality in septic patients [12][13][14]. Current SSC guidelines recommend the early administration of 30 mL/kg of IV fluids for sepsis-related hypotension or a lactate ≥ 4 mmol/L, within the first 3 h of resuscitation [3]. This recommendation remains weak, as it is based on low-quality evidence. Infusing an initial 1 L bolus over the first 30 min and administrating the remainder volume of fluid resuscitation with repeated bolus infusions is an acceptable approach [15]. A proposed algorithm about fluid resuscitation in patients with sepsis is shown in Figure 1 [3][16][17]. Four distinct phases of IV fluid therapy have been proposed: resuscitation, optimization, stabilization, and evacuation (ROSE), which are all crucial steps in sepsis management (Figure 2) [18]. In addition, specific strategies for fluid minimization and de-escalation or de-resuscitation have been reported, demonstrating that fluid restriction is associated with improved outcomes [19][20].
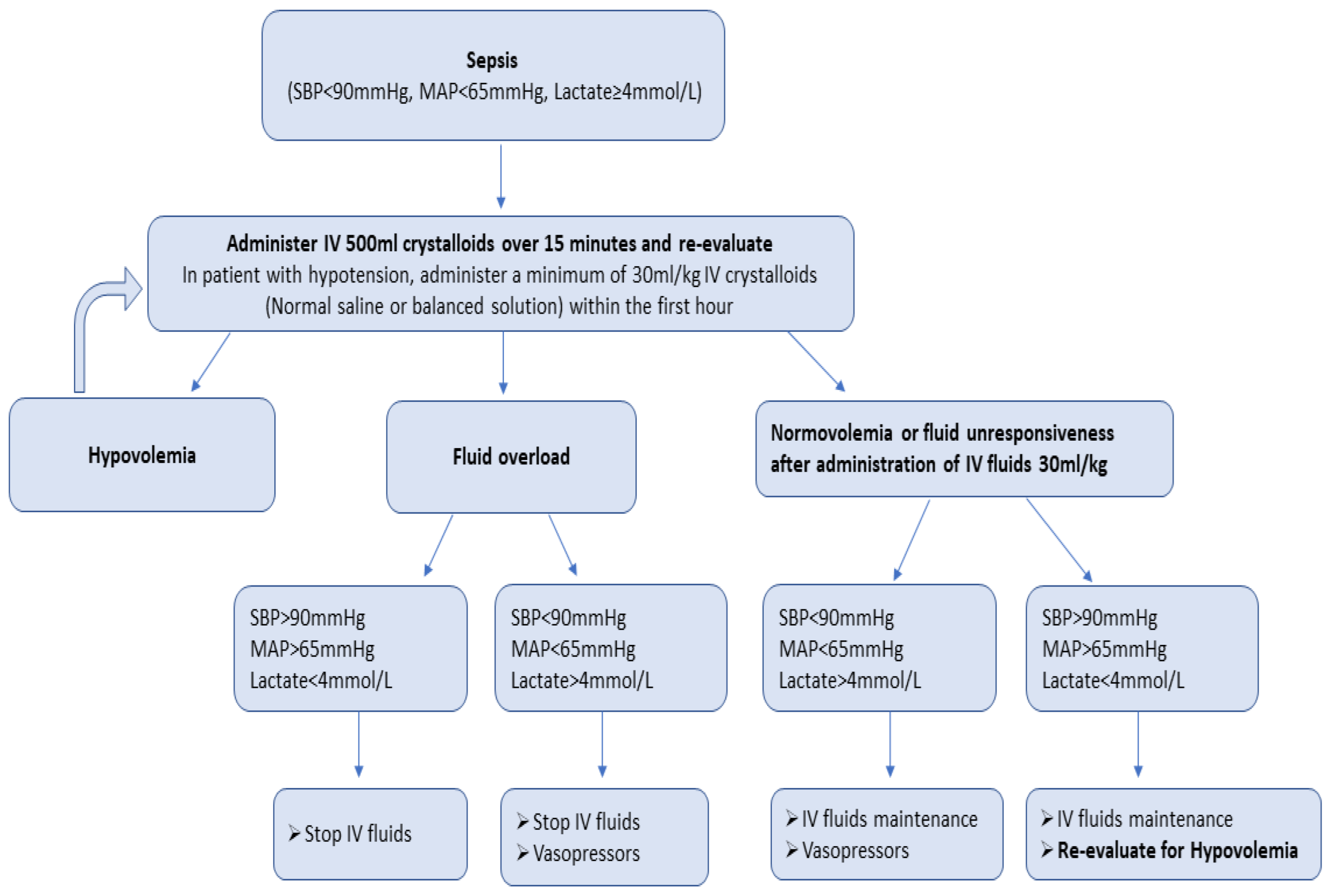
Figure 1. Proposed algorithm of fluid resuscitation in patients with sepsis.
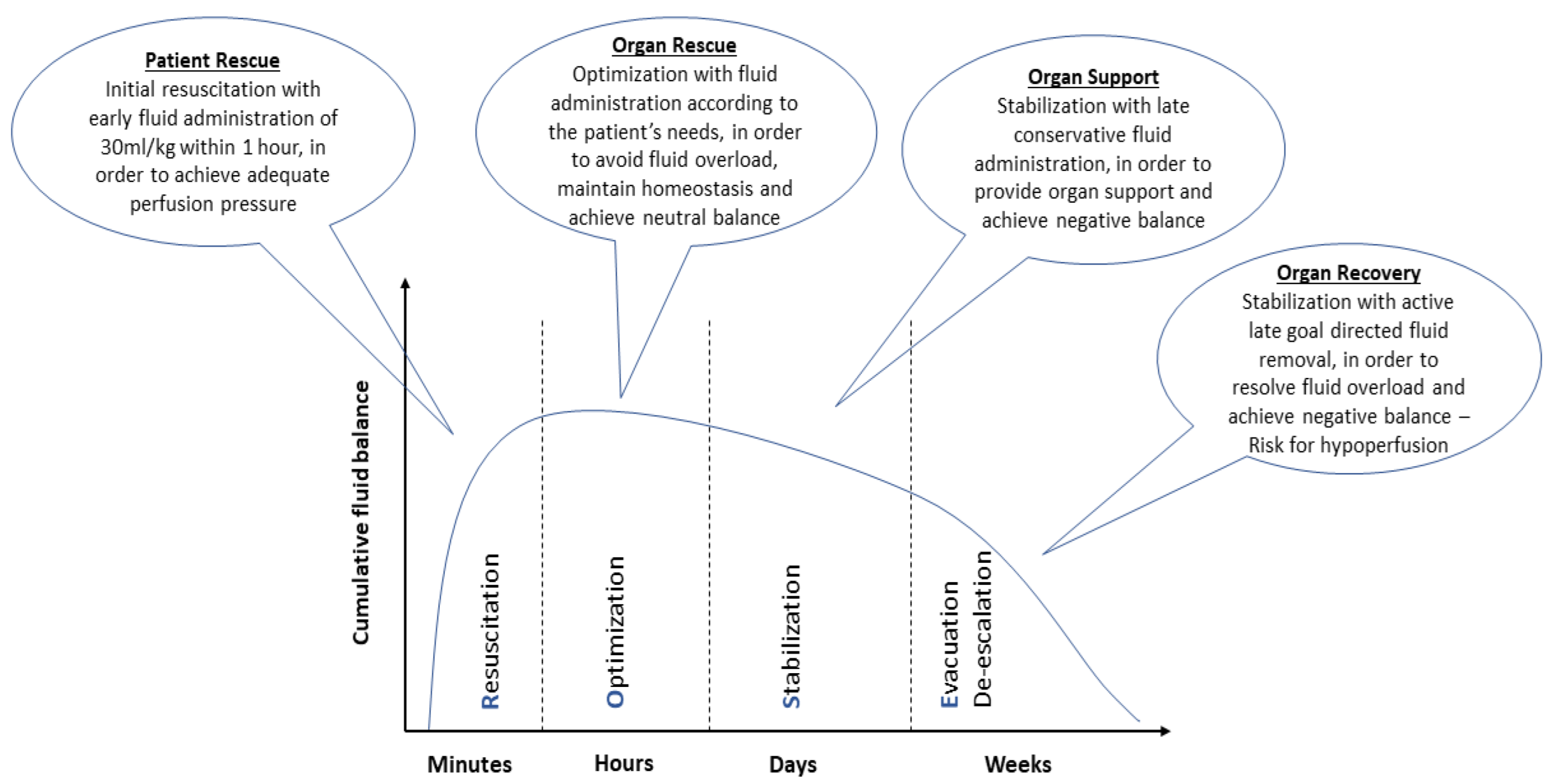
Figure 2. Characteristics of the four distinct phases of intravenous fluid therapy: resuscitation, optimization, stabilization, and evacuation (ROSE).
The 2021 SSC guidelines suggest the use of crystalloid fluids [3]. However, different types of fluids have been proposed. Colloids, including albumin and semisynthetic colloids, such as hydroxyethyl starch (HES), dextrans, and gelatins, were commonly used in the past. Several studies which examined their use in septic patients recommend against the administration of HES and other semisynthetic colloids [21][22][23][24][25]. HES use has been associated with acute kidney injury and the need for renal replacement therapy, as well as with increased mortality [25]. Gelatins have been found to increase anaphylaxis, renal failure, bleeding, and mortality [26]. Hence, the side effects of semisynthetic colloids far outweigh any potential benefits and, according to the SSC guidelines, their use should be avoided in sepsis management [3].
Current SSC guidelines suggest using albumin in septic patients who received large volumes of crystalloids over using crystalloids alone [3]. Albumin is not recommended as the first-line fluid for resuscitation in sepsis due to the lack of proven benefit and its higher cost compared to crystalloids [3]. However, two RCTs, the Saline versus Albumin Fluid Evaluation (SAFE) and the Albumin Italian Outcome Sepsis (ALBIOS) study, as well as a meta-analysis of randomized clinical trials, compared the effect of albumin and crystalloid use in patients with sepsis or septic shock, and showed a trend towards reduced mortality and improved outcomes in the albumin group, without observing serious side effects [27][28][29].
In septic patients, human albumin solution can be given for two indications: to restore or expand intravascular volume and to supplement serum albumin in the septic patients with hypoalbuminemia [30]. In addition, human albumin acts as the most significant modulator of plasma oncotic pressure, which is typically in the 25–30 mmHg range. This is a major endogenous antioxidant agent and a major binding protein of several endogenous compounds and drugs [30]. Albumin appears to have important immunomodulatory effects that likely impact the host inflammatory response in critical illness [30]. The time, dose, and concentration of the albumin, as well as the determination of a specific target for serum albumin level remains controversial. Of note, in the ALBIOS trial, albumin was administered as a 20% solution, with a treatment goal of a serum albumin concentration of 30 g/L until intensive care unit (ICU) discharge or 28 days [28].
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211.
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247.
- Scheeren, T.W.L.; Bakker, J.; De Backer, D.; Annane, D.; Asfar, P.; Boerma, E.C.; Cecconi, M.; Dubin, A.; Dünser, M.W.; Duranteau, J.; et al. Current use of vasopressors in septic shock. Ann. Intensiv. Care 2019, 9, 20.
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensiv. Care Med. 2018, 44, 925–928.
- Mukherjee, V.; Evans, L. Implementation of the Surviving Sepsis Campaign guidelines. Curr. Opin. Crit. Care 2017, 23, 412–416.
- Cosnett, J. The Origins of Intravenous Fluid Therapy. Lancet 1989, 333, 768–771.
- Daniels, R. Surviving the first hours in sepsis: Getting the basics right (an intensivist’s perspective). J. Antimicrob. Chemother. 2011, 66 (Suppl. 2), ii11–ii23.
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377.
- Angus, D.C.; Barnato, A.E.; Bell, D.; Bellomo, R.; Chong, C.-R.; Coats, T.J.; Davies, A.; Delaney, A.; Harrison, D.A.; Holdgate, A.; et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensiv. Care Med. 2015, 41, 1549–1560.
- Ehrman, R.R.; Gallien, J.Z.; Smith, R.K.; Akers, K.; Malik, A.N.; Harrison, N.; Welch, R.D.; Levy, P.D.; Sherwin, R.L. Resuscitation Guided by Volume Responsiveness Does Not Reduce Mortality in Sepsis: A Meta-Analysis. Crit. Care Explor. 2019, 1, e0015.
- Samoni, S.; Vigo, V.; Reséndiz, L.I.B.; Villa, G.; De Rosa, S.; Nalesso, F.; Ferrari, F.; Meola, M.; Brendolan, A.; Malacarne, P.; et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: Comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit. Care 2016, 20, 95.
- Sadaka, F.; Juarez, M.; Naydenov, S.; O’brien, J. Fluid resuscitation in septic shock: The effect of increasing fluid balance on mortality. J. Intensiv. Care Med. 2013, 29, 213–217.
- Smith, S.H.; Perner, A. Higher vs. lower fluid volume for septic shock: Clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit. Care 2012, 16, R76.
- McIntyre, L.; Rowe, B.H.; Walsh, T.S.; Gray, A.; Arabi, Y.; Perner, A.; Gordon, A.; Marshall, J.; Cook, D.; Fox-Robichaud, A.; et al. Multicountry survey of emergency and critical care medicine physicians’ fluid resuscitation practices for adult patients with early septic shock. BMJ Open 2016, 6, e010041.
- Gavelli, F.; Castello, L.M.; Avanzi, G.C. Management of sepsis and septic shock in the emergency department. Intern. Emerg. Med. 2021, 16, 1649–1661.
- Macdonald, S. Fluid Resuscitation in Patients Presenting with Sepsis: Current Insights. Open Access Emerg. Med. 2022, 14, 633–638.
- Hoste, E.A.; Maitland, K.; Brudney, C.S.; Mehta, R.; Vincent, J.-L.; Yates, D.; Kellum, J.A.; Mythen, M.G.; Shaw, A.D. Four phases of intravenous fluid therapy: A conceptual model. Br. J. Anaesth. 2014, 113, 740–747.
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensiv. Ther. 2014, 46, 361–380.
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Owczuk, R. It is time to consider the four D’s of fluid management. Anaesthesiol. Intensiv. Ther. 2015, 47, s1–s5.
- Brunkhorst, F.M.; Engel, C.; Bloos, F.; Meier-Hellmann, A.; Ragaller, M.; Weiler, N.; Moerer, O.; Gruendling, M.; Oppert, M.; Grond, S.; et al. Intensive Insulin Therapy and Pentastarch Resuscitation in Severe Sepsis. N. Engl. J. Med. 2008, 358, 125–139.
- Guidet, B.; Martinet, O.; Boulain, T.; Philippart, F.; Poussel, J.; Maizel, J.; Forceville, X.; Feissel, M.; Hasselmann, M.; Heininger, A.; et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit. Care 2012, 16, R94.
- Perner, A.; Haase, N.; Guttormsen, A.B.; Tenhunen, J.; Klemenzson, G.; Åneman, A.; Madsen, K.R.; Møller, M.H.; Elkjær, J.M.; Poulsen, L.M.; et al. Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N. Engl. J. Med. 2012, 367, 124–134.
- Myburgh, J.A.; Finfer, S.; Bellomo, R.; Billot, L.; Cass, A.; Gattas, D.; Glass, P.; Lipman, J.; Liu, B.; McArthur, C.; et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N. Engl. J. Med. 2012, 367, 1901–1911.
- Zarychanski, R.; Abou-Setta, A.M.; Turgeon, A.F.; Houston, B.; McIntyre, L.; Marshall, J.C.; Fergusson, D. Association of Hydroxyethyl Starch Administration With Mortality and Acute Kidney Injury in Critically Ill Patients Requiring Volume Resuscitation: A systematic review and meta-analysis. JAMA 2013, 309, 678–688.
- Moeller, C.; Fleischmann, C.; Thomas-Rueddel, D.; Vlasakov, V.; Rochwerg, B.; Theurer, P.; Gattinoni, L.; Reinhart, K.; Hartog, C.S. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J. Crit. Care 2016, 35, 75–83.
- Finfer, S.; McEvoy, S.; Bellomo, R.; McArthur, C.; Myburgh, J.; Norton, R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensiv. Care Med. 2011, 37, 86–96.
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421.
- Xu, J.-Y.; Chen, Q.-H.; Xie, J.-F.; Pan, C.; Liu, S.-Q.; Huang, L.-W.; Yang, C.-S.; Liu, L.; Huang, Y.-Z.; Guo, F.-M.; et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: A meta-analysis of randomized clinical trials. Crit. Care 2014, 18, 702.
- Chien, S.-C.; Chen, C.-Y.; Lin, C.-F.; Yeh, H.-I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31.
More
Information
Subjects:
Critical Care Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
08 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





