Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David R Dolbow | -- | 2387 | 2023-06-08 00:00:17 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dolbow, D.R.; Gorgey, A.S.; Johnston, T.E.; Bersch, I. Electrical Stimulation Exercise for People with SCI. Encyclopedia. Available online: https://encyclopedia.pub/entry/45307 (accessed on 07 February 2026).
Dolbow DR, Gorgey AS, Johnston TE, Bersch I. Electrical Stimulation Exercise for People with SCI. Encyclopedia. Available at: https://encyclopedia.pub/entry/45307. Accessed February 07, 2026.
Dolbow, David R., Ashraf S. Gorgey, Therese E. Johnston, Ines Bersch. "Electrical Stimulation Exercise for People with SCI" Encyclopedia, https://encyclopedia.pub/entry/45307 (accessed February 07, 2026).
Dolbow, D.R., Gorgey, A.S., Johnston, T.E., & Bersch, I. (2023, June 07). Electrical Stimulation Exercise for People with SCI. In Encyclopedia. https://encyclopedia.pub/entry/45307
Dolbow, David R., et al. "Electrical Stimulation Exercise for People with SCI." Encyclopedia. Web. 07 June, 2023.
Copy Citation
Electrical stimulation exercise has become an important modality to help improve the mobility and health of individuals with spinal cord injury (SCI). Electrical stimulation is used to stimulate peripheral nerves in the extremities to assist with muscle strengthening or functional activities such as cycling, rowing, and walking. Electrical stimulation of the peripheral nerves in the upper extremities has become a valuable tool for predicting the risk of hand deformities and rehabilitating functional grasping activities.
peripheral nerve stimulation
functional electrical stimulation
neuromuscular electrical stimulation
spinal cord injury
1. Introduction
Spinal cord injury (SCI) disrupts efferent and afferent pathways, including the descending pathways from the motor cortex to the spinal motor neurons which activate muscle activity [1]. Electrical stimulation can be used to bypass spinal disruption and elicit muscle contractions for rehabilitation purposes [1]. Electrical stimulation exercise has become an important modality for improving the health and mobility of individuals with SCI [2][3]. The two primary types of electrical stimulation producing exercise are (1) peripheral nerve stimulation (PNS), which is the electrical stimulation of the peripheral nerves, usually in the extremities, and (2) spinal cord stimulation (SCS), which is the electrical stimulation of the spinal nerves at the spinal cord [4]. PNS is further classified into neuromuscular electrical stimulation (NMES) and functional electrical stimulation (FES). While some people use the two terms interchangeably, the two modalities are commonly separated into two categories. NMES is defined as electrically induced muscle contractions, which includes resistance training [5]. FES is defined as electrically induced functional activities, including FES cycling, FES rowing, FES walking, and FES-assisted grasping activities [6][7][8][9]. Similarly, SCS is further divided into transcutaneous spinal stimulation and epidural electrical stimulation [3][9][10]. Transcutaneous spinal stimulation provides electrical input via surface electrodes aligned along the external surface of the spine, while epidural stimulation is derived from electrodes surgically implanted in the epidural spaces of the spinal cord [10][11][12]. Three primary electrical stimulation parameters are adjusted to optimize the activity of interest: pulse duration (time duration for a single pulse), frequency (pulses produced per second), and amplitude (strength of the current) that is often referred to as stimulation intensity [13].
The potential benefits of electrical stimulation in individuals with SCI may include changes in body composition, such as increasing muscle mass and bone mass while decreasing fat mass; improving cardiovascular and metabolism efficiency; decreasing spasticity; and improving functional mobility [2][8][14][15][16]. In addition, electrical stimulation can be used as a diagnostic tool for determining lower motor neuron damage caused by cervical SCI which may affect the risk of developing grasping anomalies [16]. Most recently, electrical stimulation has been employed to guide clinicians and researchers in the estimation of the quantities of muscle and bone. During NMES, Gorgey and colleagues used the amplitude of the current (<100 mA) and the number of leg extension repetitions (>70) as cut-offs to provide both diagnostic and prognostic assessments of the muscle cross-sectional area and knee bone mineral densities in persons with SCI [17].
Electrical stimulation activities have been shown to be safe with proper supervision and instruction by healthcare professionals. Telehealth monitoring has also been successfully used for the application of home-based NMES. Participants were educated to use surface NMES to induce resistance training exercises of the knee extensors and were monitored over an eight-week period via telehealth [18]. However, some important contraindications and precautions should be recognized, including very low bone density; a history of bone fractures; uncontrolled autonomic dysreflexia; uncontrolled hyper/hypotension; open pressure wounds; thrombosis; pregnancy; cancer; pacemaker and defibrillator, depending on the distance from the implant; and orthopedic problems that preclude the selected activity [19].
2. Body Composition Assessment
Along with optimizing functional mobility, healthcare providers use FES activities to decrease the risk of secondary conditions after SCI, including cardiometabolic diseases which is two to three times greater than in the able-bodied population [20]. Two recent systematic literature reviews concluded that NMES and FES activities provided positive results on muscle mass after eight to sixteen weeks of training [21][22]. Atkins and Bickel [21] reported increases in muscle volume that range from 20–72% with an average increase of 26% among NMES and FES studies. Similarly, Bekhet et al. [22] reported skeletal muscle increases in cross-section areas (CSA) from 5.7% to 75% via NMES and FES, with NMES resistance training typically providing greater increases in muscle than FES cycling.
Johnston et al. [6] demonstrated that there is a significant correlation between accumulated torque and muscle volume. In a comparison between low cadence/high torque and high cadence/low torque FES cycling groups, 17 adults with chronic C4-T6 motor complete SCI all increased in muscle volume, but the low cadence/high torque group had a 9% greater increase (Figure 1). Participants cycled three times per week for six months. In a study of ten adults with chronic SCI, Dolbow et al. [23] found a 5.7% increase in lean leg mass and a 2.4% decrease in body fat percentage when combining resistance-guided high-intensity interval FES cycling three times per week for eight weeks with once weekly nutritional counseling.
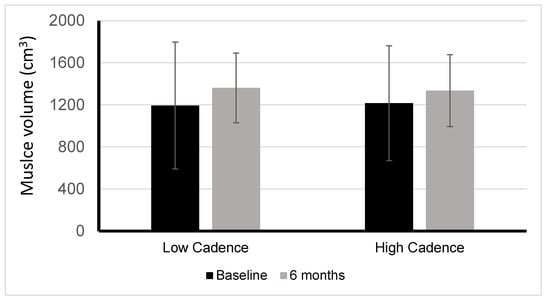
Figure 1. Muscle volume changes between low versus high cadence FES-LEC. Changes in muscle volume after six months of low cadence/high torque cycling (LOW) or high cadence/low torque cycling (HIGH). Between groups p = 0.318; within groups LOW p = 0.014 and HIGH p = 0.049.
A nutritional counseling-only group displayed no changes in body composition. Similarly, Gorgey et al. [24] compared NMES resistance training plus nutritional counseling twice a week for twelve weeks to a control group that received nutritional counseling only. The results showed skeletal muscle CSA increases of 28% for the whole thigh, 35% for the knee extensors, and 16% for the knee flexors for those that received NMES and nutritional counseling. Additionally, there was a 25% increase in insulin-like growth factor 1 (IGF-1) which is associated with muscle hypertrophy. Interestingly, there was a concomitant 25% reduction in visceral adipose tissue (VAT) CSA in the L5-S3 region. Gorgey and Shepard [25] published a unilateral leg case report using NMES resistance training twice a week for twelve weeks on an individual with chronic cervical SCI. The results revealed a 72% increase in CSA of the whole thigh and a 53% decrease in intramuscular fat. This initial report was later confirmed in a randomized clinical trial that demonstrated the efficacy of utilizing NMES-RT with and without androgen replacement therapy to evoke muscle hypertrophy and provide other favorable outcomes similar to an increase in basal metabolic rate and enhancing carbohydrate profile in persons with SCI [24][26].
3. Cardiovascular and Metabolism
In a systematic review of the literature, van der Scheer et al. [27] found that 16 out of 21 selected studies using electrical stimulation exercise for individuals with SCI showed improvements in cardiovascular and metabolic outcomes. The most common outcomes measured were power output and peak oxygen volume (VO2peak). These studies provide consistent evidence that FES cycling can improve aerobic fitness and has the potential to reduce the risk of cardiovascular and metabolic conditions after SCI. On the contrary, Hamzaid and Davis [28] concluded that the lack of consistency in the various studies resulted in insufficient evidence to determine if health and fitness benefits could be derived from FES exercise in individuals with SCI. They suggested that the VO2peak utilized may be more limited due to the reduced active muscle mass and peripheral blood flow than due to central cardiac reserve. Figoni and Dolbow [29] studied the possible benefits of aerobic exercise on those with tetraplegia and concluded that, while further study is needed, the current evidence suggests that the greatest cardiovascular and metabolic benefits derived from FES cycling are likely to result from thirty or more minutes of moderate-intensity exercise, three or more times per week for at least eight to twelve weeks. Studies investigating training programs using FES cycling and FES rowing for individuals with SCI have reported improved VO2peak, lowered blood glucose levels, and enhanced skeletal muscle glucose uptake [30][31][32]. Akins and Bickel [21] summarized the effects of FES activities on metabolic health by suggesting that FES interventions can help to normalize glucose uptake and metabolism after SCI. FES activities also have been shown to improve energy expenditure, increase cardiac output, reverse myocardial atrophy, increase cardiac protective high-density lipoprotein cholesterol, and assist in the reduction of body fat percentage [32][33][34][35].
4. Muscle Spasticity
Muscle spasticity is a common condition secondary to SCI and can potentially increase the level of disability [36]. Spasticity typically results from upper motor neuron injury in those with injuries above T12/L1 [36]. The SCI reduces or eliminates control of reflexes from the supraspinal level of the central nervous system resulting in spasticity that is characterized by increased muscle tone, hyperreflexia, clonus sign, and muscle spasms [36]. NMES is thought to improve spasticity by eliciting disynaptic reciprocal inhibition of the opposing muscle group Alashram et al. [36] completed a systematic review investigating the changes in lower extremities’ spasticity after FES cycling. The investigation included ten independent studies totaling 161 individuals with SCI. Alashram and associates concluded that more randomized control trials are needed; however, current evidence indicates that FES cycling can reduce lower extremities’ spasticity for individuals at all levels of SCI. It is interesting to note that ankle dorsiflexor and plantarflexor spasticity have been shown to be reduced during FES cycling even though electrical stimulation is provided to the quadriceps, hamstrings, and gluteal muscles [36].
5. Exercise Adherence
A national health survey by the Centers for Disease Control and Prevention in 2020 [37] determined that only 24.2% of adults aged 18 and over met the physical activity guidelines for Americans for both aerobic and muscle-strengthening activities (150 min per week of moderate-intensity aerobic exercise and muscle strengthening exercises to the major muscle groups twice per week) [37]. Individuals with SCI perform only 35–40% as much exercise as the largely sedentary able-bodied population, demonstrating the extreme lack of physical activity in the SCI population [38][39]. Recently, Tui et al. [40] completed a qualitative study concerning the motivations and barriers that limit adherence to exercise programs for individuals with SCI. The common self-reported reasons for poor adherence to exercise guidelines for those with SCI were time constraints (54%), lack of motivation (31%), decreased accessibility (24%), and SCI-specific barriers (23%). The 144 participants in the study reported the possible following solutions: scheduling exercise sessions for time constraints (47.9%); introducing fun during the exercise sessions to increase motivation (21.8%); providing equipment to allow home exercise (30.3%); and locating accessible facilities to resolve accessibility barriers (27.3%). In agreement that access to exercise facilities is a problem, Dolbow and Figoni [41] investigated the accommodation of wheelchair users by community fitness centers and found accommodation lacking, especially regarding access to exercise equipment.
Dolbow et al. [39] investigated exercise adherence in a home-based FES cycling program for 17 chronic SCI adults for two consecutive eight-week exercise periods. Participation during the first eight weeks was incentivized with the knowledge that the rented FES cycle would be purchased for the participants if they maintained good exercise adherence with the requested 30 to 40 min FES cycling sessions three times per week for the eight weeks. The second eight weeks of FES cycling provided no incentive for participation. During the first eight weeks, the adherence rate to the exercise program was 71.7% while, during the second eight weeks, exercise adherence was 63.7%, a nominal but not statistically significant decrease. The main factors involved with higher adherence rates were age (under 50 years of age had a higher adherence rate); self-reported prior history of regular exercise; and having a history of recurrent pain but finding the FES cycling activity to be pain-free. The last factor fostered the development of the Pain-Free Affinity Model which states that “when living with frequent or recurrent pain, there is an increased affinity toward activities that are perceived as pain-free” [39]. The level of injury, time since injury, and history of depression did not significantly affect exercise adherence in the study. Another follow-up study determined the feasibility of a video conferencing approach as a telehealth communication to deliver a home-based NMES-RT program for eight weeks. The authors intentionally performed unilateral training on one leg while the other leg served as the control. The training paradigm was successful in enhancing muscle hypertrophy in the trained leg but not in the control limb [18]. The telehealth paradigms were important because, even with a short period of de-training or dose de-escalation, persons with SCI experience a gradual loss in muscle size and a decline in cardio-metabolic gains after a routine training program [42]. Today, the telehealth home-based training paradigm via video conferencing has been extended to implement 12 months of training for persons with lower motor neuron injury [43].
6. Physical Function
Sadowsky et al. [44] hypothesized that restoring normal activity levels should optimize neural regeneration after SCI. This hypothesis was supported by their retrospective cohort cross-sectional evaluation comparing twenty-five people with chronic SCI who underwent an activity-based restorative exercise program including FES cycling to twenty individuals with SCI that received regular standard of care therapy, including range of motion exercises and stretching of the paralyzed limbs. The participants were matched by age, gender, injury level, the severity of the injury, and duration of the injury. After 29 months, those in the FES cohort demonstrated an 80% increase in neurological function, including motor and sensory advancements as shown on the American Spinal Injury Association Impairment scale. This was a more statistically significant increase than the 40% increase shown by the group receiving the regular standard of care.
The systematic review and meta-analysis performed by Fang et al. [45] included two studies that measured functional walking gains induced by FES cycling in individuals with SCI via the Six Minute Walk Test (6MWT) and the Timed Get Up and Go Test (TUG). The evidence displayed significantly improved scores in the 6MWT and TUG after FES cycling [46][47]. In those two studies, Kuhn et al. [46] investigated the effects of FES cycling for 20-min sessions twice a week for four weeks. In addition, Mazzoleni and colleagues [47] combined 20 sessions of FES cycling followed by 20 training sessions of exoskeleton overground walking.
Transcutaneous spinal cord electrical stimulation can be considered a form of FES walking although the activity is unique due to the noninvasive stimulation of the nerves along the lumbosacral region of the spine. This relatively recent innovation in electrical stimulation therapy allows neuromodulation of the spinal circuitry promoting an effective stepping motion that may potentially fine-tune locomotion for those with SCI [48]. Different leg muscles can be stressed by altering the placement of electrodes along the spine, depending on the needs of the individual. For example, low transcutaneous spinal-cord stimulation intensities at the T10–T11 segment produced a higher magnitude response in the vastus lateralis and rectus femoris with a lesser magnitude in the medial gastrocnemius, soleus, and medial hamstrings muscles. The same intensity stimulation at the T12-L1 segment created the reverse relationship of these muscle groups [48].
References
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2.
- Gater, D.R.; Dolbow, D.; Tsui, B.; Gorgey, A.S. Functional Electrical Stimulation Therapies after Spinal Cord Injury. NeuroRehabilitation 2011, 28, 231–248.
- Dolbow, D.R.; Gorgey, A.S.; Sutor, T.W.; Bochkezanian, V.; Musselman, K. Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury. J. Clin. Med. 2021, 10, 5356.
- Duffell, L.D.; Donaldson, N.N. A Comparison of FES and SCS for Neuroplastic Recovery after SCI: Historical Perspectives and Future Directions. Front. Neurol. 2020, 11, 607.
- Gorgey, A.S.; Khalil, R.E.; Lester, R.M.; Dudley, G.A.; Gater, D.R. Paradigms of Lower Extremity Electrical Stimulation Training after Spinal Cord Injury. J. Vis. Exp. 2018, 1, 57000.
- Johnston, T.E.; Marino, R.J.; Oleson, C.V.; Schmidt-Read, M.; Leiby, B.E.; Sendecki, J.; Singh, H.; Modlesky, C.M. Musculoskeletal Effects of 2 Functional Electrical Stimulation Cycling Paradigms Conducted at Different Cadences for People with Spinal Cord Injury: A Pilot Study. Arch Phys. Med. Rehabil. 2016, 97, 1413–1422.
- Hettinga, D.M.; Andrews, B.J. Oxygen consumption during functional electrical stimulation-assisted exercise in persons with spinal cord injury: Implications for fitness and health. Sports Med. 2008, 38, 825–838.
- Bersch, I.; Koch-Borner, S.; Fridén, J. Motor Point Topography of Fundamental Grip Actuators in Tetraplegia: Implications in Nerve Transfer Surgery. J. Neurotrauma 2020, 37, 441–447.
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J. Neurotrauma 2018, 35, 2145–2158.
- Sutor, T.W.; Ghatas, M.P.; Goetz, L.L.; Lavis, T.D.; Gorgey, A.S. Exoskeleton Training and Trans-Spinal Stimulation for Physical Activity Enhancement after Spinal Cord Injury (EXTra-SCI): An Exploratory Study. Front. Rehabil. Sci. 2022, 2, 789422.
- Hachmann, J.T.; Yousak, A.; Wallner, J.J.; Gad, P.N.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, 126, 1843–1859.
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682.
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215.
- Bekhet, A.H.; Bochkezanian, V.; Saab, I.M.; Gorgey, A.S. The Effects of Electrical Stimulation Parameters in Managing Spasticity after Spinal Cord Injury: A Systematic Review. Am. J. Phys. Med. Rehabil. 2019, 98, 484–499.
- Gorgey, A.S.; Dolbow, D.R.; Dolbow, J.D.; Gater, D.R. The Effects of Electrical Stimulation on Body Composition and Metabolic Profile after Spinal Cord Injury—Part II. J. Spinal Cord Med. 2015, 38, 23–37.
- Dolbow, D.R.; Gorgey, A.S.; Ketchum, J.M.; Gater, D.R. Home-Based Functional Electrical Stimulation Cycling Enhances Quality of Life in Individuals with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2013, 19, 324–329.
- Gorgey, A.S.; Khalil, R.E.; Sutor, T.W.; Goldsmith, J.A.; Cifu, D.X. Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury. J. Clin. Med. 2022, 11, 6681.
- Gorgey, A.S.; Lester, R.M.; Wade, R.C.; Khalil, R.E.; Khan, R.K.; Anderson, M.L.; Castillo, T. A feasibility pilot using telehealth videoconference monitoring of home-based NMES resistance training in persons with spinal cord injury. Spinal Cord Ser. Cases 2017, 3, 17039.
- Martin, R.; Sadowsky, C.; Obst, K.; Meyer, B.; McDonald, J. Functional electrical stimulation in spinal cord injury: From theory to practice. Top. Spinal Cord Inj. Rehabil. 2012, 18, 28–33.
- Dolbow, D.R.; Farkas, G.J.; Berg, A.S.; Welsch, M.A.; Gorgey, A.S.; Gater, D.R. Fat to lean mass ratio in spinal cord injury: Possible interplay of components of body composition that may instigate systemic inflammation and metabolic syndrome. J. Spinal Cord Med. 2022, 45, 833–839.
- Atkins, K.D.; Bickel, C.S. Effects of functional electrical stimulation on muscle health after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 226–231.
- Bekhet, A.H.; Jahan, A.M.; Bochkezanian, V.; Musselman, K.E.; Elsareih, A.A.; Gorgey, A.S. Effects of Electrical Stimulation Training on Body Composition Parameters after Spinal Cord Injury: A Systematic Review. Arch Phys. Med. Rehabil. 2022, 103, 1168–1178.
- Dolbow, D.R.; Credeur, D.; Lemacks, J.L.; Stokic, D.S.; Pattanaik, S.; Corbin, G.N.; Courtner, A.S. Electrically Induced Cycling and Nutritional Counseling for Counteracting Obesity after Spinal Cord Injury: A Pilot Study. J. Spinal Cord Med. 2020, 44, 533–540.
- Gorgey, A.S.; Mather, K.J.; Cupp, H.R.; Gater, D.R. Effects of Resistance Training on Adiposity and Metabolism after Spinal Cord Injury. Med. Sci. Sports Exerc. 2012, 44, 165–174.
- Gorgey, A.S.; Shepherd, C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J. Spinal Cord Med. 2010, 33, 90–95.
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury on Cardio-Metabolic Risk Factors: An Open-Label Randomized Clinical Trial. J. Neurotrauma 2019, 36, 2631–2645.
- van der Scheer, J.W.; Goosey-Tolfrey, V.L.; Valentino, S.E.; Davis, G.M.; Ho, C.H. Functional electrical stimulation cycling exercise after spinal cord injury: A systematic review of health and fitness-related outcomes. J. NeuroEngineering Rehabil. 2021, 18, 99.
- Hamzaid, N.A.; Davis, G. Health and Fitness Benefits of Functional Electrical Stimulation-Evoked Leg Exercise for Spinal Cord–Injured Individuals: A Position Review. Top. Spinal Cord Inj. Rehabil. 2009, 14, 88–121.
- Figoni, S.; Dolbow, D.R.; Crawford, C.; White, M.; Pattanaik, S. Does Aerobic Exercise Benefit Persons with Tetraplegia from Spinal Cord Injury? J. Spinal Cord Med. 2021, 44, 690–703.
- Ye, G.; Grabke, E.P.; Pakosh, M.; Furlan, J.C.; Masani, K. Clinical Benefits and System Design of FES-Rowing Exercise for Rehabilitation of Individuals with Spinal Cord Injury: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1595–1605.
- Jeon, J.Y.; Hettinga, D.; Steadward, R.D.; Wheeler, G.D.; Bell, G.; Harber, V. Reduced plasma glucose and leptin after 12 weeks of functional electrical stimulation–rowing exercise training in spinal cord injury patients. Arch. Phys. Med. Rehabil. 2010, 91, 1957–1959.
- Griffin, L.; Decker, M.J.; Hwang, J.Y.; Wang, B.; Kitchen, K.; Ding, Z.; Ivy, J.L. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J. Electromyogr. Kinesiol. 2009, 19, 614–622.
- Farkas, G.J.; Burton, A.M.; McMillan, D.W.; Sneij, A.; Gater, D.R., Jr. The Diagnosis and Management of Cardiometabolic Risk and Cardiometabolic Syndrome after Spinal Cord Injury. J. Pers. Med. 2022, 12, 1088.
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. Energy Expenditure, Cardiorespiratory Fitness, and Body Composition Following Arm Cycling or Functional Electrical Stimulation Exercises in Spinal Cord Injury: A 16-Week Randomized Controlled Trial. Top. Spinal Cord Inj. Rehabil. 2021, 27, 121–134.
- Nash, M.S.; Bilsker, M.S.; Kearney, H.M.; Ramirez, J.N.; Applegate, B.; Green, B.A. Effects of electrically-stimulated exercise and passive motion on echocardiographically-derived wall motion and cardiodynamic functic in tetraplegic persons. Spinal Cord 1995, 33, 80–89.
- Alashram, A.R.; Annino, G.; Mercuri, N.B. Changes in spasticity following functional electrical stimulation cycling in patients with spinal cord injury: A systematic review. J. Spinal Cord Med. 2022, 45, 10–23.
- Elgaddal, N.; Kramarow, K.A.; Reuben, C. Physical Activity among Adults Aged 18 and over: United States. 2020. Available online: https://www.cdc.gov/nchs/products/databriefs/db443.htm (accessed on 11 November 2022).
- Soriano, J.E.; Squair, J.W.; Cragg, J.J.; Thompson, J.; Sanguinetti, R.; Vaseghi, B.; Emery, C.A.; Grant, C.; Charbonneau, R.; Larkin-Kaiser, K.A.; et al. A national survey of physical activity after spinal cord injury. Sci. Rep. 2022, 12, 4405.
- Dolbow, D.R.; Gorgey, A.S.; Ketchum, J.M.; Moore, J.R.; Hackett, L.A.; Gater, D.R. Exercise Adherence during Home-Based Functional Electrical Stimulation Cycling by Individuals with Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2012, 91, 922–930.
- Tiu, C.; Ochoa, C.; Froehlich-Grobe, K. Qualitative analysis of perceived motivators and barriers to exercise in individuals with spinal cord injury enrolled in an exercise study. Spinal Cord Ser. Cases 2022, 8, 74.
- Dolbow, D.R.; Figoni, S.F. Accommodation of Wheelchair-Reliant Individuals by Community Fitness Facilities. Spinal Cord 2015, 53, 515–519.
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Khan, R.; Adler, R.A. Effects of dose de-escalation following testosterone treatment and evoked resistance exercise on body composition, metabolic profile, and neuromuscular parameters in persons with spinal cord injury. Physiol. Rep. 2021, 9, e15089.
- Gorgey, A.S.; Khalil, R.E.; Alrubaye, M.; Gill, R.; Rivers, J.; Goetz, L.L.; Cifu, D.X.; Castillo, T.; Caruso, D.; Lavis, T.D.; et al. Testosterone and long pulse width stimulation (TLPS) for denervated muscles after spinal cord injury: A study protocol of andomized clinical trial. BMJ Open 2022, 12, e064748.
- Sadowsky, C.L.; Hammond, E.R.; Strohl, A.B.; Commean, P.K.; Eby, S.A.; Damiano, D.L.; Wingert, J.R.; Bae, K.T.; McDonald, J.W., 3rd. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 2013, 36, 623–631.
- Fang, C.Y.; Lien, A.S.; Tsai, J.L.; Yang, H.C.; Chan, H.L.; Chen, R.S.; Chang, Y.J. The Effect and Dose-Response of Functional Electrical Stimulation Cycling Training on Spasticity in Individuals with Spinal Cord Injury: A Systematic Review with Meta-Analysis. Front. Physiol. 2021, 12, 756200.
- Kuhn, D.; Leichtfried, V.; Schobersberger, W. Four weeks of functional electrical stimulated cycling after spinal cord injury: A clinical cohort study. Int. J. Rehabil. Res. 2014, 37, 243–250.
- Mazzoleni, S.; Battini, E.; Rustici, A.; Stampacchia, G. An integrated gait rehabilitation training based on Functional Electrical Stimulation cycling and overground robotic exoskeleton in complete spinal cord injury patients: Preliminary results. IEEE Int. Conf. Rehabil. Robot. 2017, 2017, 289–293.
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231.
More
Information
Subjects:
Rehabilitation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
798
Revision:
1 time
(View History)
Update Date:
07 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




