
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hamid Tebyaniyan | -- | 2442 | 2023-06-06 14:43:15 | | | |
| 2 | Conner Chen | -53 word(s) | 2389 | 2023-06-07 07:25:52 | | |
Video Upload Options
eriodontal diseases and dental caries are the most common infectious oral diseases impacting oral health globally. Oral cavity health is crucial for enhancing life quality since it serves as the entranceway to general health. The oral microbiome and oral infectious diseases are strongly correlated. Gram-negative anaerobic bacteria have been associated with periodontal diseases. Due to the shortcomings of several antimicrobial medications frequently applied in dentistry, the lack of resources in developing countries, the prevalence of oral inflammatory conditions, and the rise in bacterial antibiotic resistance, there is a need for reliable, efficient, and affordable alternative solutions for the prevention and treatment of periodontal diseases. Several accessible chemical agents can alter the oral microbiota, although these substances also have unfavorable symptoms such as vomiting, diarrhea, and tooth discoloration. Natural phytochemicals generated from plants that have historically been used as medicines are categorized as prospective alternatives due to the ongoing quest for substitute products.
1. Acacia arabica (Babul)
2. Acacia nilotica
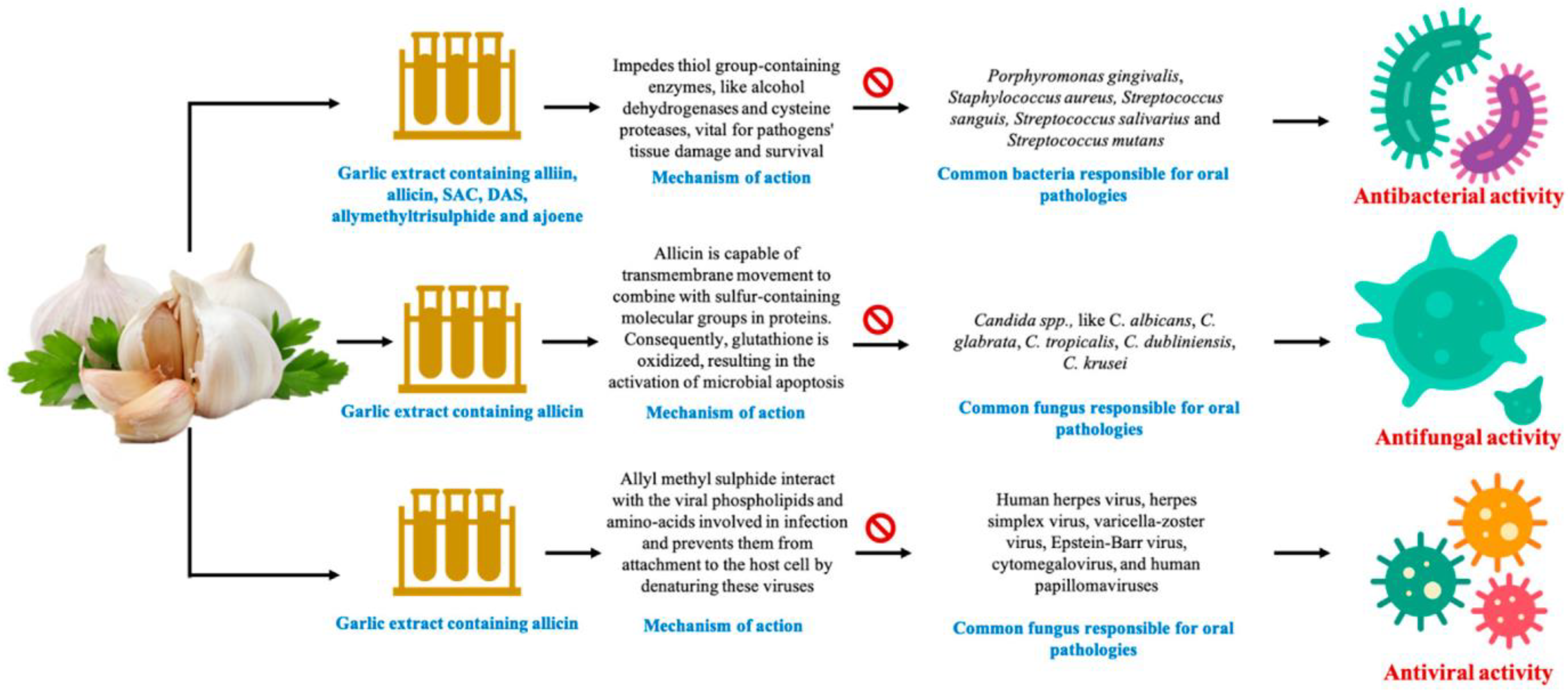
3. Allium sativum (Garlic)
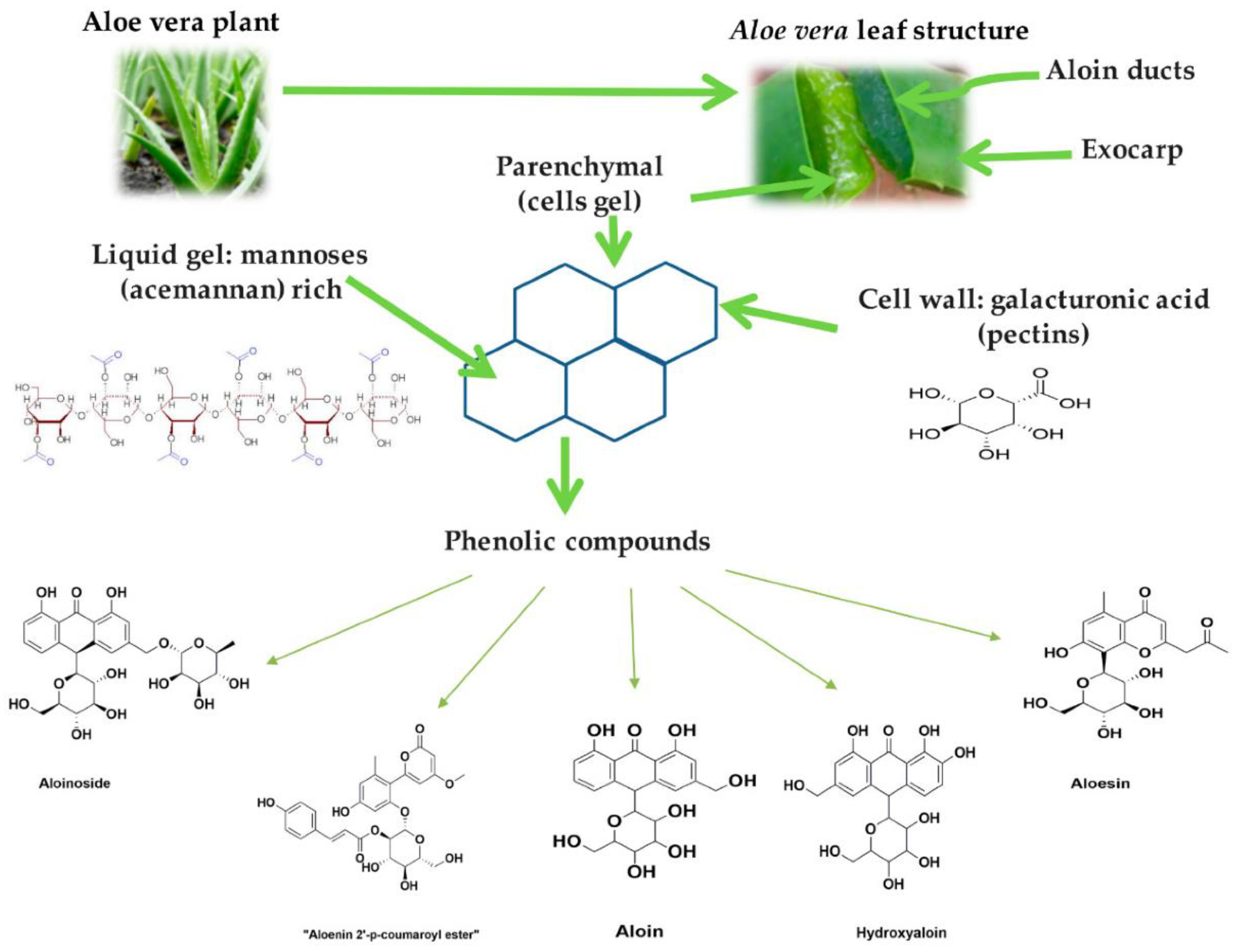
4. Aloe barbadensis Miller (Aloe Vera)
5. Amphipterygium adstringens
6. Azadirachta indica [54]
7. Berberis vulgaris
8. Camellia sinensis (Green Tea)
9. Cinnamomum zeylanicum (Ceylon Cinnamon)
10. Citrus sinensis
11. Coffea canephora (Coffee)
12. Copaifera pubiflora
References
- Singhal, R.; Agarwal, V.; Rastogi, P.; Khanna, R.; Tripathi, S. Efficacy of Acacia arabica gum as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A randomized controlled clinical trial. Saudi Dent. J. 2018, 30, 53–62.
- Kirtikar, K.; Basu, B. Indian medicinal plan Leader road. Allahabad India 1984, 2, 1347–1348.
- Clark, D.; Gazi, M.; Cox, S.; Eley, B.; Tinsley, G. The effects of Acacia arabica gum on the in vitro growth and protease activities of periodontopathic bacteria. J. Clin. Periodontol. 1993, 20, 238–243.
- Pradeep, A.; Agarwal, E.; Bajaj, P.; Naik, S.; Shanbhag, N.; Uma, S. Clinical and microbiologic effects of commercially available gel and powder containing Acacia arabica on gingivitis. Aust. Dent. J. 2012, 57, 312–318.
- Pradeep, A.; Happy, D.; Garg, G. Short-term clinical effects of commercially available gel containing Acacia arabica: A randomized controlled clinical trial. Aust. Dent. J. 2010, 55, 65–69.
- Mnisi, C.M.; Mlambo, V. Influence of harvesting site on chemical composition and potential protein value of Acacia erioloba, A. nilotica and Ziziphus mucronata leaves for ruminants. J. Anim. Physiol. Anim. Nutr. 2017, 101, 994–1003.
- Kaur, K.; Michael, H.; Arora, S.; Härkönen, P.; Kumar, S. In vitro bioactivity-guided fractionation and characterization of polyphenolic inhibitory fractions from Acacia nilotica (L.) Willd. ex Del. J. Ethnopharmacol. 2005, 99, 353–360.
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. In Vitro 2008, 22, 1965–1970.
- Al-Nour, M.Y.; Ibrahim, M.M.; Elsaman, T. Ellagic acid, Kaempferol, and Quercetin from Acacia nilotica: Promising combined drug with multiple mechanisms of action. Curr. Pharmacol. Rep. 2019, 5, 255–280.
- Hussein, G.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T.; Kakiuchi, N.; Shimotohno, K. Inhibitory effects of Sudanese plant extracts on HIV-1 replication and HIV-1 protease. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 31–36.
- Abd El Nabi, O.M.; Reisinger, E.C.; Reinthaler, F.F.; Still, F.; Eibel, U.; Krejs, G.J. Antimicrobial activity of Acacia nilotica (L.) Willd. ex Del. var. nilotica (Mimosaceae). J. Ethnopharmacol. 1992, 37, 77–79.
- Maldini, M.; Montoro, P.; Hamed, A.I.; Mahalel, U.A.; Oleszek, W.; Stochmal, A.; Piacente, S. Strong antioxidant phenolics from Acacia nilotica: Profiling by ESI-MS and qualitative–quantitative determination by LC–ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 228–239.
- Dafallah, A.A.; Al-Mustafa, Z. Investigation of the anti-inflammatory activity of Acacia nilotica and Hibiscus sabdariffa. Am. J. Chin. Med. 1996, 24, 263–269.
- Muddathir, A.M.; Mohieldin, E.A.M.; Mitsunaga, T. In vitro activities of Acacia nilotica (L.) Delile bark fractions against Oral Bacteria, Glucosyltransferase and as antioxidant. BMC Complement. Med. Ther. 2020, 20, 360.
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119.
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129.
- Ceccanti, C.; Rocchetti, G.; Lucini, L.; Giuberti, G.; Landi, M.; Biagiotti, S.; Guidi, L. Comparative phytochemical profile of the elephant garlic (Allium ampeloprasum var. holmense) and the common garlic (Allium sativum) from the Val di Chiana area (Tuscany, Italy) before and after in vitro gastrointestinal digestion. Food Chem. 2021, 338, 128011.
- Harini, K.; Babu, S.; Ajila, V.; Hegde, S. Garlic: It’s role in oral and systemic health. Nitte Univ. J. Health Sci. 2013, 3, 17–22.
- Fenwick, G.R.; Hanley, A.B. The genus Allium—Part 1. Crit. Rev. Food Sci. Nutr. 1985, 22, 199–271.
- Mann, J.; Bernstein, Y.; Findler, M. Periodontal disease and its prevention, by traditional and new avenues (Review). Exp. Ther. Med. 2020, 19, 1504–1506.
- Tsai, C.-W.; Chen, H.-W.; Sheen, L.-Y.; Lii, C.-K. Garlic: Health benefits and actions. BioMedicine 2012, 2, 17–29.
- Ahmad, T.A.; El-Sayed, B.A.; El-Sayed, L.H. Development of immunization trials against Eimeria spp. Trials Vaccinol. 2016, 5, 38–47.
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847.
- Zini, A.; Mann, J.; Mazor, S.; Vered, Y. The Efficacy of Aged Garlic Extract on Gingivitis—A Randomized Clinical Trial. J. Clin. Dent. 2018, 29, 52–56.
- Bin, C.; Al-Dhabi, N.A.; Esmail, G.A.; Arokiyaraj, S.; Arasu, M.V. Potential effect of Allium sativum bulb for the treatment of biofilm forming clinical pathogens recovered from periodontal and dental caries. Saudi J. Biol. Sci. 2020, 27, 1428–1434.
- Muniz, I.A.F.; Campos, D.E.S.; Shinkai, R.S.A.; Trindade, T.G.D.; Cosme-Trindade, D.C. Case report of oral mucosa garlic burn during COVID-19 pandemic outbreak and role of teledentistry to manage oral health in an older adult woman. Spec. Care Dent. 2021, 41, 639–643.
- Ohtani, M.; Nishimura, T. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp. Ther. Med. 2020, 19, 1507–1510.
- Zini, A.; Mann, J.; Mazor, S.; Vered, Y. Beneficial effect of aged garlic extract on periodontitis: A randomized controlled double-blind clinical study. J. Clin. Biochem. Nutr. 2020, 67, 297–301.
- Shetty, S.; Thomas, B.; Shetty, V.; Bhandary, R.; Shetty, R.M. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: A microbiological study. Ayu 2013, 34, 445–451.
- Bhat, G.; Kudva, P.; Dodwad, V. Aloe vera: Nature’s soothing healer to periodontal disease. J. Indian Soc. Periodontol. 2011, 15, 205–209.
- Choi, S.W.; Son, B.W.; Son, Y.S.; Park, Y.I.; Lee, S.K.; Chung, M.H. The wound-healing effect of a glycoprotein fraction isolated from aloe vera. Br. J. Dermatol. 2001, 145, 535–545.
- Reynolds, T.; Dweck, A. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37.
- Gottlieb, K. Aloe Vera Heals: The Scientific Facts; Cancer Book House: Sydney, Australia, 1980.
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index; Merck & Co., Inc.: Merck Rahway, NJ, USA, 1989; Volume 11.
- Vogler, B.K.; Ernst, E. Aloe vera: A systematic review of its clinical effectiveness. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 1999, 49, 823–828.
- Heggers, J.; Pineless, G.; Robson, M. Dermaide aloe aloe vera gel-comparison of the anti-microbial effects. J. Am. Med. Technol. 1979, 41, 293–294.
- Schleifer, K.H.; Kilpper-Bälz, R. Transfer of Streptococcus faecalis and Streptococcus faecium to the Genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Syst. Evol. Microbiol. 1984, 34, 31–34.
- Grindlay, D.; Reynolds, T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 1986, 16, 117–151.
- Saoo, K.; Miki, H.; Ohmori, M.; Winters, W. Antiviral activity of aloe extracts against cytomegalovirus. Phytother. Res. 1996, 10, 348–350.
- Hutter, J.A.; Salman, M.; Stavinoha, W.B.; Satsangi, N.; Williams, R.F.; Streeper, R.T.; Weintraub, S.T. Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J. Nat. Prod. 1996, 59, 541–543.
- Ito, S.; Teradaira, R.; Beppu, H.; Obata, M.; Nagatsu, T.; Fujita, K. Properties and pharmacological activity of carboxypeptidase in Aloe arborescens Mill var. natalensis Berger. Phytother. Res. 1993, 7, S26–S29.
- Tello, C.G.; Ford, P.; Iacopino, A. In vitro evaluation of complex carbohydrate denture adhesive formulations. Quintessence Int. 1998, 29, 585–593.
- Poor, M.R.; Hall, J.E.; Poor, A.S. Reduction in the incidence of alveolar osteitis in patients treated with the SaliCept patch, containing Acemannan hydrogel. J. Oral Maxillofac. Surg. 2002, 60, 374–379.
- Sudworth, R. The Use of Aloe Vera in Dentistry; Positive Health Publications Ltd.: Philadelphia, PA, USA, 2002.
- Chandrahas, B.; Jayakumar, A.; Naveen, A.; Butchibabu, K.; Reddy, P.K.; Muralikrishna, T. A randomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J. Indian Soc. Periodontol. 2012, 16, 543.
- Namiranian, H.; Serino, G. The effect of a toothpaste containing aloe vera on established gingivitis. Swed. Dent. J. 2012, 36, 179–185.
- Tornero-Martínez, A.; Cruz-Ortiz, R.; Jaramillo-Flores, M.E.; Osorio-Díaz, P.; Ávila-Reyes, S.V.; Alvarado-Jasso, G.M.; Mora-Escobedo, R. In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules 2019, 24, 3605.
- Ajmera, N.; Chatterjee, A.; Goyal, V. Aloe vera: It’s effect on gingivitis. J. Indian Soc. Periodontol. 2013, 17, 435–438.
- Cronquist, A.; Takhtadzhian, A.L. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York City, NY, USA, 1981.
- Rivero-Cruz, B.E.; Esturau, N.; Sánchez-Nieto, S.; Romero, I.; Castillo-Juárez, I.; Rivero-Cruz, J.F. Isolation of the new anacardic acid 6--salicylic acid and evaluation of its antimicrobial activity against Streptococcus mutans and Porphyromonas gingivalis. Nat. Prod. Res. 2011, 25, 1282–1287.
- Sung, B.; Pandey, M.K.; Ahn, K.S.; Yi, T.; Chaturvedi, M.M.; Liu, M.; Aggarwal, B.B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood 2008, 111, 4880–4891.
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases signaling pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411.
- Robles-Zepeda, R.E.; Velázquez-Contreras, C.A.; Garibay-Escobar, A.; Gálvez-Ruiz, J.C.; Ruiz-Bustos, E. Antimicrobial activity of Northwestern Mexican plants against Helicobacter pylori. J. Med. Food. 2011, 14, 1280–1283.
- Mandal, A.; Manohar, B.; Shetty, N.; Mathur, A.; Makhijani, B.; Sen, N. A Comparative Evaluation of Anti-Inflammatory and Antiplaque Efficacy of Citrus Sinesis Mouthwash and Chlorhexidine Mouthwash. J. Nepal. Soc. Periodontol. Oral Implantol. 2018, 2, 9–13.
- Brahmachari, G. Neem—An omnipotent plant: A retrospection. Chembiochem 2004, 5, 408–421.
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28.
- Sakagami, H.; Oi, T.; Satoh, K. Prevention of oral diseases by polyphenols. In Vivo 1999, 13, 155–171.
- Alzoreky, N.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230.
- SaiRam, M.; Ilavazhagan, G.; Sharma, S.; Dhanraj, S.; Suresh, B.; Parida, M.; Jana, A.; Devendra, K.; Selvamurthy, W. Anti-microbial activity of a new vaginal contraceptive NIM-76 from neem oil (Azadirachta indica). J. Ethnopharmacol. 2000, 71, 377–382.
- Wolinsky, L.; Mania, S.; Nachnani, S.; Ling, S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J. Dent. Res. 1996, 75, 816–822.
- Vanka, A.; Tandon, S.; Rao, S.; Udupa, N.; Ramkumar, P. The effect of indigenous Neem Azadirachta indica mouth wash on Streptococcus mutans and lactobacilli growth. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2001, 12, 133–144.
- Dasgupta, T.; Banerjee, S.; Yadava, P.; Rao, A. Chemopreventive potential of Azadirachta indica (Neem) leaf extract in murine carcinogenesis model systems. J. Ethnopharmacol. 2004, 92, 23–36.
- Baral, R.; Chattopadhyay, U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 2004, 4, 355–366.
- Raji, Y.; Ogunwande, I.A.; Osadebe, C.A.; John, G. Effects of Azadirachta indica extract on gastric ulceration and acid secretion in rats. J. Ethnopharmacol. 2004, 90, 167–170.
- Bandyopadhyay, U.; Biswas, K.; Sengupta, A.; Moitra, P.; Dutta, P.; Sarkar, D.; Debnath, P.; Ganguly, C.K.; Banerjee, R.K. Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci. 2004, 75, 2867–2878.
- Wu, C.D.; Darout, I.A.; Skaug, N. Chewing sticks: Timeless natural toothbrushes for oral cleansing. J. Periodontal Res. 2001, 36, 275–284.
- Prashant, G.; Chandu, G.; Murulikrishna, K.; Shafiulla, M. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: An in vitro study. Indian J. Dent. Res. 2007, 18, 148.
- Robinson, T. The Organic Constituents of Higher Plants, Diterjemahkan Oleh Padmawinata; Kosasih, P., Ed.; ITB: Bandung, Indonesia, 1995.
- Pai, M.R.; Acharya, L.D.; Udupa, N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel—A 6-week clinical study. J. Ethnopharmacol. 2004, 90, 99–103.
- Schumacher, M.; Cerella, C.; Reuter, S.; Dicato, M.; Diederich, M. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011, 6, 149–160.
- Hu, J.P.; Takahashi, N.; Yamada, T. Coptidis rhizoma inhibits growth and proteases of oral bacteria. Oral Dis. 2000, 6, 297–302.
- Lamont, R.J.; Jenkinson, H.F. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998, 62, 1244–1263.
- Pandit, N.; Changela, R.; Bali, D.; Tikoo, P.; Gugnani, S. Porphyromonas gingivalis: Its virulence and vaccine. J. Int. Clin. Dent. Res. Organ. 2015, 7, 51.
- Moeintaghavi, A.; Shabzendedar, M.; Parissay, I.; Makarem, A.; Orafaei, H.; Hosseinnezhad, M. Berberine gel in periodontal inflammation: Clinical and histological effects. J. Adv. Periodontol. Implant. Dent. 2012, 4, 7–11.
- Strusovskaya, A.; Poroysky, S.; Smirnov, A.; Firsova, I.; Sirotenko, V.; Kirichenko, L.; Strusovskaya, O. A study of the influence of barbaris root (Berberis vulgaris L., Berberidaceae) extract dental gel on the dynamics of the inflammatory process in periodontal tissues of rats on the model of induced gingivitis. In Proceedings of the AIP Conference Proceedings, Yekaterinburg, Russia, 13–16 November 2019.
- Passos, V.F.; Melo, M.A.S.d.; Lima, J.P.M.; Marçal, F.F.; Costa, C.A.G.d.A.; Rodrigues, L.K.A.; Santiago, S.L. Active compounds and derivatives of camellia sinensis responding to erosive attacks on dentin. Braz. Oral Res. 2018, 32, e40.
- Kushiyama, M.; Shimazaki, Y.; Murakami, M.; Yamashita, Y. Relationship between intake of green tea and periodontal disease. J. Periodontol. 2009, 80, 372–377.
- Koyama, Y.; Kuriyama, S.; Aida, J.; Sone, T.; Nakaya, N.; Ohmori-Matsuda, K.; Hozawa, A.; Tsuji, I. Association between green tea consumption and tooth loss: Cross-sectional results from the Ohsaki Cohort 2006 Study. Prev. Med. 2010, 50, 173–179.
- Ide, R.; Fujino, Y.; Hoshiyama, Y.; Mizoue, T.; Kubo, T.; Pham, T.-M.; Shirane, K.; Tokui, N.; Sakata, K.; Tamakoshi, A. A prospective study of green tea consumption and oral cancer incidence in Japan. Ann. Epidemiol. 2007, 17, 821–826.
- Mazur, M.; Ndokaj, A.; Jedlinski, M.; Ardan, R.; Bietolini, S.; Ottolenghi, L. Impact of Green Tea (Camellia Sinensis) on periodontitis and caries. Systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2021, 57, 1–11.
- Wazaify, M.; Afifi, F.U.; El-Khateeb, M.; Ajlouni, K. Complementary and alternative medicine use among Jordanian patients with diabetes. Complement. Ther. Clin. Pract. 2011, 17, 71–75.
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules 2020, 25, 4184.
- Kawatra, P.; Rajagopalan, R. Cinnamon: Mystic powers of a minute ingredient. Pharmacogn. Res. 2015, 7 (Suppl. S1), S1–S6.
- Jayaprakasha, G.K.; Rao, L.J. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011, 51, 547–562.
- Chen, P.; Sun, J.; Ford, P. Differentiation of the four major species of cinnamons (C. burmannii, C. verum, C. cassia, and C. loureiroi) using a flow injection mass spectrometric (FIMS) fingerprinting method. J. Agric. Food Chem. 2014, 62, 2516–2521.
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Oral mucosal lipids are antibacterial against Porphyromonas gingivalis, induce ultrastructural damage, and alter bacterial lipid and protein compositions. Int. J. Oral Sci. 2013, 5, 130–140.
- Mendes, S.J.F.; Sousa, F.I.A.B.; Pereira, D.M.S.; Ferro, T.A.F.; Pereira, I.C.P.; Silva, B.L.R.; Pinheiro, A.J.M.C.R.; Mouchrek, A.Q.S.; Monteiro-Neto, V.; Costa, S.K.P.; et al. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 2016, 34, 60–70.
- Yang, X.Q.; Zheng, H.; Ye, Q.; Li, R.Y.; Chen, Y. Essential oil of Cinnamon exerts anti-cancer activity against head and neck squamous cell carcinoma via attenuating epidermal growth factor receptor—Tyrosine kinase. J. Buon 2015, 20, 1518–1525.
- Wang, Y.; Zhang, Y.; Shi, Y.Q.; Pan, X.H.; Lu, Y.H.; Cao, P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018, 116, 26–32.
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289.
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705.
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506.
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402.
- Bickers, D.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem. Toxicol. 2005, 43, 799–836.
- Cocchiara, J.; Letizia, C.S.; Lalko, J.; Lapczynski, A.; Api, A.M. Fragrance material review on cinnamaldehyde. Food Chem. Toxicol. 2005, 43, 867–923.
- Hussain, K.A.; Tarakji, B.; Kandy, B.P.; John, J.; Mathews, J.; Ramphul, V.; Divakar, D.D. Antimicrobial effects of citrus sinensis peel extracts against periodontopathic bacteria: An in vitro study. Rocz. Panstw. Zakl. Hig. 2015, 66, 173–178.
- Lawal, D.; Bala, J.A.; Aliyu, S.Y.; Huguma, M.A. Phytochemical Screening and In Vitro Anti-Bacterial Studies of the Ethanolic Extract of Citrus Senensis (Linn.) Peel against some Clinical Bacterial Isolates. Int. J. Innov. Appl. Stud. 2013, 2, 138–145.
- Dubey, D.; Balamurugan, K.; Agrawal, R.; Verma, R.K.; Jain, R. Evalution of Antibacterial and Antioxidant Activity of Methanolic and Hydromethanolic Extract of Sweet Orange Peels. Recent Res. Sci. Technol. 2011, 3, 22–25.
- Jabuk, S.; Chabuck, A.; Adil, N.; Chabuck, G. In vitro and in vivo effect of three aqueous plant extract on pathogenicity of Klebsiella pneumonia isolated from patient with urinary tract infection. World J. Pharm. Res. 2014, 5, 160–179.
- Carrol, D.H.; Chassagne, F.; Dettweiler, M.; Quave, C.L. Antibacterial activity of plant species used for oral health against Porphyromonas gingivalis. PLoS ONE 2020, 15, e0239316.
- Joët, T.; Salmona, J.; Laffargue, A.; Descroix, F.; Dussert, S. Use of the growing environment as a source of variation to identify the quantitative trait transcripts and modules of co-expressed genes that determine chlorogenic acid accumulation. Plant Cell Environ. 2010, 33, 1220–1233.
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123.
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74.
- Bogdan, C.; Pop, A.; Iurian, S.M.; Benedec, D.; Moldovan, M.L. Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants 2020, 9, 502.
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84.
- Chaube, S.; Swinyard, C.A. Teratological and toxicological studies of alkaloidal and phenolic compounds from Solanum tuberosum L. Toxicol. Appl. Pharmacol. 1976, 36, 227–237.
- Yadav, M.; Kaushik, M.; Roshni, R.; Reddy, P.; Mehra, N.; Jain, V.; Rana, R. Effect of Green Coffee Bean Extract on Streptococcus mutans Count: A Randomised Control Trial. J. Clin. Diagn. Res. 2017, 11, Zc68–Zc71.
- Tsou, S.-H.; Hu, S.-W.; Yang, J.-J.; Yan, M.; Lin, Y.-Y. Potential Oral Health Care Agent from Coffee Against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235.
- Arruda, C.; Mejía, J.A.A.; Ribeiro, V.P.; Borges, C.H.G.; Martins, C.H.G.; Veneziani, R.C.S.; Ambrosio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed. Pharmacother. 2019, 109, 1–20.
- Bardají, D.K.R.; da Silva, J.J.M.; Bianchi, T.C.; de Souza Eugênio, D.; de Oliveira, P.F.; Leandro, L.F.; Rogez, H.L.G.; Venezianni, R.C.S.; Ambrosio, S.R.; Tavares, D.C. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 2016, 40, 18–27.
- Abrão, F.; de Araújo Costa, L.D.; Alves, J.M.; Senedese, J.M.; de Castro, P.T.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Tavares, D.C.; Martins, C.H.G. Copaifera langsdorffii oleoresin and its isolated compounds: Antibacterial effect and antiproliferative activity in cancer cell lines. BMC Complement. Altern. Med. 2015, 15, 443.
- da S. Moraes, T.; Leandro, L.F.; de O. Silva, L.; Santiago, M.B.; Souza, A.B.; Furtado, R.A.; Tavares, D.C.; Veneziani, R.C.S.; Ambrósio, S.R.; Bastos, J.K. In vitro evaluation of Copaifera oblongifolia oleoresin against bacteria causing oral infections and assessment of its cytotoxic potential. Curr. Pharm. Biotechnol. 2016, 17, 894–904.
- Borges, C.H.; Cruz, M.G.; Carneiro, L.J.; da Silva, J.J.; Bastos, J.K.; Tavares, D.C.; de Oliveira, P.F.; Rodrigues, V.; Veneziani, R.C.; Parreira, R.L. Copaifera duckei oleoresin and its main nonvolatile terpenes: In vitro schistosomicidal properties. Chem. Biodivers. 2016, 13, 1348–1356.
- Alves, J.M.; Senedese, J.M.; Leandro, L.F.; Castro, P.T.; Pereira, D.E.; Carneiro, L.J.; Ambrósio, S.R.; Bastos, J.K.; Tavares, D.C. Copaifera multijuga oleoresin and its constituent diterpene (−)-copalic acid: Genotoxicity and chemoprevention study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017, 819, 26–30.
- Abrão, F.; Alves, J.A.; Andrade, G.; De Oliveira, P.F.; Ambrósio, S.R.; Veneziani, R.; Tavares, D.C.; Bastos, J.K.; Martins, C.H. Antibacterial effect of Copaifera duckei Dwyer oleoresin and its main diterpenes against oral pathogens and their cytotoxic effect. Front. Microbiol. 2018, 9, 201.
- Furtado, R.A.; de Oliveira, P.F.; Senedese, J.M.; Ozelin, S.D.; de Souza, L.D.R.; Leandro, L.F.; de Oliveira, W.L.; da Silva, J.J.M.; Oliveira, L.C.; Rogez, H. Assessment of genotoxic activity of oleoresins and leaves extracts of six Copaifera species for prediction of potential human risks. J. Ethnopharmacol. 2018, 221, 119–125.
- De Souza, M.G.M.; Leandro, L.F.; da Silva Moraes, T.; Abrão, F.; Veneziani, R.C.S.; Ambrosio, S.R.; Martins, C.H.G. ent-Copalic acid antibacterial and anti-biofilm properties against Actinomyces naeslundii and Peptostreptococcus anaerobius. Anaerobe 2018, 52, 43–49.
- Abrão, F.; Silva, T.S.; Moura, C.L.; Ambrósio, S.R.; Veneziani, R.C.S.; de Paiva, R.E.F.; Bastos, J.K.; Martins, C.H.G. Oleoresins and naturally occurring compounds of Copaifera genus as antibacterial and antivirulence agents against periodontal pathogens. Sci. Rep. 2021, 11, 4953.




