Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberta Cassano | -- | 5660 | 2023-06-05 11:12:18 | | | |
| 2 | Camila Xu | Meta information modification | 5660 | 2023-06-06 03:23:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Trombino, S.; Sole, R.; Curcio, F.; Cassano, R. Polymeric Based Hydrogel Membranes for Biomedical Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/45188 (accessed on 12 March 2026).
Trombino S, Sole R, Curcio F, Cassano R. Polymeric Based Hydrogel Membranes for Biomedical Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/45188. Accessed March 12, 2026.
Trombino, Sonia, Roberta Sole, Federica Curcio, Roberta Cassano. "Polymeric Based Hydrogel Membranes for Biomedical Applications" Encyclopedia, https://encyclopedia.pub/entry/45188 (accessed March 12, 2026).
Trombino, S., Sole, R., Curcio, F., & Cassano, R. (2023, June 05). Polymeric Based Hydrogel Membranes for Biomedical Applications. In Encyclopedia. https://encyclopedia.pub/entry/45188
Trombino, Sonia, et al. "Polymeric Based Hydrogel Membranes for Biomedical Applications." Encyclopedia. Web. 05 June, 2023.
Copy Citation
Hydrogel membranes combine the porous architecture and permeability properties of thin membranes with the dynamic mechanical properties and water absorption characteristics of polymeric hydrogels. The characteristics of hydrogel membranes bear a remarkable resemblance to physiological membranes, although the latter are much more complex than their synthetic counterparts.
hydrogel membrane
tissue engineering
drug delivery
wound healing
1. Introduction
Membranes are defined as films that can work as a separation barrier between two adjacent phases, and due to their porosity, they allow the selective passage of substances from one phase to the other one [1][2]. Polymer membranes can have a porous structure, in which separation is based on pore size differences, or a dense structure [3], in which separation is based on the solubility and diffusivity of molecules. Permeability, selectivity and flux are the most important characteristics of polymeric membranes. Their classification takes place according to several criteria: nature of the polymeric material (natural or synthetic); structure (symmetrical or asymmetrical, porous or nonporous); mechanism by which separation is achieved (the size of the permeant species and their solubility in the membrane); and physical chemical properties of the membrane (hydrophobic or hydrophilic).
If the classification of membranes is made according to their nature, they can be divided into natural and synthetic membranes. The latter can be further classified, according to material, into organic, inorganic, or composite membranes [4]. Organic membranes are by far the most widely used. They are made of synthetic polymers such as poly(ethylene glycol) (PEG), poly(acrylic amide), polysulfone, polyurethane, poly(N-vinyl-2-pyrrolidone), or they can be hydrogels based on natural polymers such as hyaluronic acid, cellulose, chitosan, alginate, collagen and others [5][6][7][8]. Membranes can also be classified according to pore size as porous, dense, and asymmetric [9]. In porous membranes polymers usually occupy only a small part of the total volume. Depending on the pore size, these materials can be further subdivided into microporous, if the pore diameter is less than 10 µm, and macroporous if the pore diameter exceeds 10 µm.
Dense membranes have no real pores but still have voids formed by the spaces between molecular chains (the so-called “free volume”) of the order of 5–10 Å.
Asymmetric membranes have a dense and very thin outer film (skin) (0.1–0.5 µm thick), which is responsible for the membrane’s selective behavior, and a thicker porous support (0.1–0.2 mm). The presence of the outer film enables simultaneous selectivity and high fluxes, while the porous support is responsible for mechanical properties and facilitates handling.
The use of membranes has become increasingly established in the biomedical field, and particularly in tissue engineering, with the realization of biological purification systems, protective coatings for wounds that accelerate healing, systems for diagnosis and therapy through the controlled release of active substances [10], and scaffolds. Scaffolds are the main resource of tissue engineering, which is concerned with restoring the functionality of damaged tissues and organs. They are temporary or support artificial structures with nanometric morphological characteristics engineered to emulate the extracellular matrix (ECM) in order to house and support cell cultures and promote their growth until regeneration of the damaged tissue is achieved.
The development of biomedical materials requires that they exhibit particularly high levels of biocompatibility, bioactivity, and biofunctionality [11][12][13][14][15][16][17]. Research has pursued to obtain attractive material properties, including biomimetic methods, inspired by the principle that living organisms within their structures exhibit well-tested strategies [18][19][20][21].
Cells are the basic structures of living organisms. Cell membranes are a nanostructured molecular assembly consisting of lipids, glycolipids, transmembrane proteins and peripheral proteins. The many elements of cell membranes, such as chemical constituents, structure, membrane channels, and receptors can inspire the design of biomaterials, or these elements can often constitute interaction sites for biomedical polymers [22][23][24].
Among the polymers that have recentry successfully employed for biomedical applications, hydrogels have assumed a relevant role. A hydrogel is a material consisting of hydrophilic polymers capable of swelling water and retaining it in amounts that can reach up to 1000 times their dry weight. The three-dimensional structure that such polymers form due to the cross-linking of the individual polymer chains has characteristics of great flexibility very similar to that of natural tissues. Hydrogels can be prepared with two types of cross-linking: hydrogels with covalent cross-linking are called chemical gels, while hydrogels with noncovalent interactions are called physical gels [25]. On the cross-linking density depends the control of the porosity of the hydrogel and thus also the ability to load the drug inside it.
Hydrogel membranes combine the porous architecture and permeability properties of thin membranes with the dynamic mechanical properties and water absorption characteristics of polymeric hydrogels. The characteristics of hydrogel membranes bear a remarkable resemblance to physiological membranes, although the latter are much more complex than their synthetic counterparts. In any case, such characteristics make hydrogel films promising to produce membranes for various applications [26][27], such as antimicrobial coatings, cell culture substrates, wound dressing applications, scaffold production [5], artificial tissues engineering, drug delivery systems.
2. Liver
Chronic liver diseases (CLD) are among the most life-threatening diseases in humans, and have causes that can be traced to obesity, non-alcoholic steatosis, high alcohol consumption, hepatitis B or C infection, autoimmune diseases, cholestatic diseases, and iron or copper overload [28]. Such diseases can give rise to chronic inflammation of the liver and cause a decreased liver function, which can result in the development of cirrhosis.
The obvious inconveniences in approaching the surgical solution of transplantation have suggested researchers develop an artificial liver capable of performing the same functions performed by the liver, namely purification, excretion and biotransformation [29].
Research groups have developed techniques that allow the in vitro production of primary human hepatocytes [30][31].
Numerous publications testify to the use of hydrogels as supporting biomaterials regenerative medicine [32][33][34][35][36][37].
However, there is no well-defined protocol for the use of different hydrogels in the field of liver tissue engineering (LTE). In fact, to date, no hydrogel that mimics liver extracellular matrix (ECM) cells are available. Therefore, the use of LTE is limited to the creation of in vivo models [38][39].
Among natural polymers, cellulose could fulfil, in combination with human liver organoids, many characteristics that could ensure successful liver tissue engineering. Liver organoids derived from adult liver have been found to be very attractive because of their genetic stability and ability to differentiate to hepatocyte-like potential. Until recently, basement membrane hydrogels were used to culture these organoids, among them Matrigel (MG), derived from murine tumour material. It, possessing an indefinite composition and particularly high cost, is not applicable in the clinical setting.
Therefore, CNF hydrogel presents as a viable alternative to MG for liver tissue engineering, with the possibility of being employed for clinical use. Improvements could be achieved by producing smaller CNF microgels, further adjusting the elastic modulus of the hydrogel, introducing cellulase enzymes [40], or even bioactive groups to the nanofibrils.
In recent years, decellularized ECM-based hydrogel materials have attracted attention for their excellent biocompatibility [41][42]. Some studies have reported that decellularized liver matrix coating on 3D cryogels can promote hepatocyte growth and function [43]. As reported by Asadi et al. [44], synthetic hydrogels produced by the combination of decellularized ECM (dECM) and Poly(N-isopropylacrylamide) pNIPAAm (dECM-pN) were found to preserve intact hepatocyte function in cell sheets, and the ECM synthesized from the hydrogels could reconstruct the microenvironment to induce adhesion, proliferation, and differentiation of Adipose Tissue-derived Mesenchymal Stromal Cells AT-MSCs.
Recently, researchers have treated mouse liver after decellularization to form hepatic hydrogels. Experiments have also been conducted to show that hepatic hydrogels can reduce liver fibrosis by replacing necrotic hepatocytes and damaged ECM [45].
In conventional in vitro models, the use of two-dimensional (2D) surfaces for cell seeding can lead to altered cell function [46]. To better mimic the in vivo cell environment [47][48], recently miniaturized bioreactors, in which cells come to be spatially arranged and cultured under dynamic conditions, have been proposed as alternative cell culture models and have become popular as “organs-on-a-chip”. One approach to enhance and prolong cellular functions within organs-on-a-chip is to encapsulate cells within a three-dimensional (3D) matrix of hydrogels that mimics the supporting functions of the extracellular matrix (ECM) [46][49]. Specifically in ref. [46] Christoffersson et al. highlighted the advantages of using a hyaluronan-PEG-based hydrogel modified with RGD peptides to achieve hepatocyte culture within a liver-on-a-chip system.
3. Pancreas Regeneration
Type 1 diabetes mellitus (T1DM) is a chronic metabolic disease characterized by the insufficient or absent production of insulin, due to β-cell destruction [50], which can result in hyperglycaemia. According to the World Health Organization (WHO), diabetes is one of the ten leading cause of death and has a large incidence in risk of heart attacks, strokes, cardiovascular disease, kidney failure, and blindness. The conventional therapeutic approach for treating T1DM is insulin replacement therapy, which requires continuous subcutaneous injections of insulin. Such therapy effectively controls blood glucose levels but is unable to recapitulate physiological pancreatic insulin, and thus cannot completely curb the possibility of developing the diseases listed above. Pancreatic islet transplantation is an alternative and less invasive therapeutic method. Unfortunately, the rejection of the transplant by the immune system and the lack of islet donors do not allow for widespread use of transplant protocol [51]. The limitations of this approach can be made less important by protecting the transplanted secretory cells against the recipient’s immune system, allowing the transfer of insulin, oxygen, and other nutrients [52]. Microencapsulation, intravascular or extravascular, depending on the location of the implant, of β-cells, using biomaterials, has been proposed.
Several studies have considered hydrogels as a material to be exploited for the encapsulation of islets. Haque et al. [53] used injectable Matrigel®, a thermosensitive hydrogel membrane derived from ECM, also used for liver organoid expansion [40], as a carrier to inject subcutaneous β-cells together with liposomal clodronate, a novel agent that could improve islet survival. Results showed that this cell delivery system increased islet survival from 10 to 60 days [54].
The chemical and physical characteristics of the natural polysaccharide alginate, which is biocompatible and inexpensive, make it a biomaterial with suitable prerequisites for the fabrication of hydrogel membranes that can be used for β-cell encapsulation. In a work published in 2018 [55], the preparation of an injectable alginate-based hydrogel was described. This preparation was done by ion cross-linking, and retarding, using Na2HPO4, the gelation time. Lengthening the gelation time of the hydrogel imparted mechanical properties, that were particularly useful in clinical applications, such as manageability and flexibility, but also physiological properties that improved biocompatibility and β-cell growth.
Wang et al. [56] produced interpenetrating thermosensitive networks (IPNs) based on alginate and ECM derived from human adipose tissue, to encapsulate β islets. The procedure was conducted by introducing the islets into an alginate solution and then performing cross-linking by ionic gelation. This structure was added to the hydrogel of the ECM. In vitro cell studies have found the system to be biocompatible and not susceptible to immune attack. Within the time span of one week, there was a significant (seven-fold) increase in cell population compared with when the cells are not encapsulatedSynthetic PEG-based hydrogels, due to their low protein adsorption, minimal inflammatory aggressiveness, immunoprotective properties, and high biocompatibility, are promising vectors for cell delivery [57]. In a study Knobeloch et al. [58] developed an injectable PEG hydrogel that supports islet survival in vitro and in vivo The hydrogel was produced by subjecting a multiarm of PEG-vinyl sulfone (VS) and PEG dithiol as the cross linker to reaction (Michael’s addition). The islets were encapsulated in the hydrogel and the solution was injected about 90 s before gelation. Determination of blood glucose levels showed a significant reduction from 600 to 200 mg/mL as early as about 2 days after implantation.
4. Artificial Oxygenators
Recently, due to the COVID-19 pandemic, the need for the development of artificial oxygenators has grown. Oxygenators are defined as medical devices designed to provide breathing support. Among those, most widely used in the treatment of critically ill patients with cardiopulmonary compromise caused by infection [59][60][61], is extracorporeal membrane oxygenation (ECMO). The operation of oxygenators is based on this principle: when blood passes through the oxygenator, the oxygen level increases and the CO2 level decreases, to oxygenate the non-oxygenated blood [62][63][64]. Extracorporeal membrane oxygenators consist of a pump that has the role of pumping the blood and an oxygenating membrane that has the role of oxygenating the blood. The most common causes of complications in patients using an oxygenator are clotting and bleeding [59]. The material from which the oxygenator membrane is obtained must have high permeability, high mechanical strength, no defects, high biocompatibility and hemocompatibility [65]. Adhesion of proteins, bacteria and platelets on biomedical devices in contact with blood is the main cause of thrombosis and infection [66]. To prevent clot formation in the oxygenation system, anticoagulant drugs, such as heparin, are administered. Unfortunately, this method is not without serious risks, such as those of haemorrhage [67][68].
Functional coatings [69] play an important role in the emerging field of medical devices, helping to achieve appropriate molecular interactions that can curb problems inherent in the activity for whose purpose the membrane was designed. Numerous studies have shown that zwitterionic polymers are ideal candidates for preventing thrombosis and infection formation due to their superhydrophilic properties through equal amounts of positively and negatively charged groups on the same side chain [70][71]. In general, the formation of zwitterionic polymer brushes on biomedical devices in contact with blood is a promising strategy. Hydrogel coatings show even more promise due to characteristics such as thickness, lubricity, and hydrophilicity, which can be adjusted [72]. However, limitations to the application of zwitterionic hydrogel coatings in anti-thrombosis and anti-infection medical devices arise from the superhydrophilic characteristics [73], which weaken their mechanical properties, while it is important that the coatings have robust mechanical properties and strong device adhesion [74]. To improve mechanical properties, non-zwitterionic components are usually introduced. Yao et al. [75] created a zwitterionic hydrogel coating reinforced with poly(carboxybetaine) (pCBM) microgel. The microgel was first prepared by reverse polymerization in miniemulsion, and then combined with poly(-sulfobetaine) (pSB). In this system, the pSB, as a continuous phase, passes through the pCBM (which acted as a crosslinker), to increase the mechanical strength. The pCBM/pSB hydrogel was used as a coating of polyvinyl chloride (PVC) pipes. Detailed studies of the mechanical and stability properties of the pCBM/pSB hydrogel coating were carried out. In addition, the antithrombogenic properties were investigated by making an extracorporeal circuit (ECC) of SD rats and New Zealand white rabbits.
An anticoagulant coating consisting of a methacrylate alginate hydrogel (MA-SA) was synthesized using a UV cross-linking reaction and then applied to PVC pipes [76]. Natural polysaccharides exhibit similar anticoagulation mechanisms to heparin, and thus can replace it as a viable alternative. Sodium alginate, through its sulfated polysaccharide site, can bind to antithrombin III (AT-III), catalyzing its action and antagonizing coagulation factors IIa, Xa, IXa, XIa and XIIa. Thus, the intrinsic coagulation pathway, which involves the conversion of prothrombin to (IIa), and the activity of thrombin come to be inhibited; in addition, the conversion of fibrinogen to fibrin monomer is hindered.
The surface of MA-SA-hydrogel coatings was irradiated with UV light to polymerize the hydrogel layer. The obtained MA-SA hydrogel coating was found to be uniform and transparent. The morphology of the ECMO surface coated with MA-SA-hydrogel has a porosity of about 5 mm, however, the diameter of RBCs observed under the light microscope is less than 8 mm [77]. The contact angle is 112°, which proves the hydrophobicity of MA-SA-hydrogel coating, that is a low inclination to thrombosis.
5. Wound Healing
Wound healing is a process that involves several interdependent steps, in which the activity of cellular components that promote tissue regeneration and growth takes place [78][79]. The overall process is divided into homeostasis, inflammation, proliferation, and re-epithelialization [80][81]. Infections at the hands of microorganisms, that can take over when wounds are left untreated, adversely affect the healing process by lengthening its time.
In order to find a versatile wound dressing, different types of materials such as membranes, hydrogels, films, foams, microparticles, and nanoparticles have been used [82][83].
Among the most used materials for acute and chronic wound management are hydrogels. Hydrogel membranes act as a moist dressing [84]. The release of moisture increases collagenase production and provides a suitable environment for tissue regeneration (Figure 1).
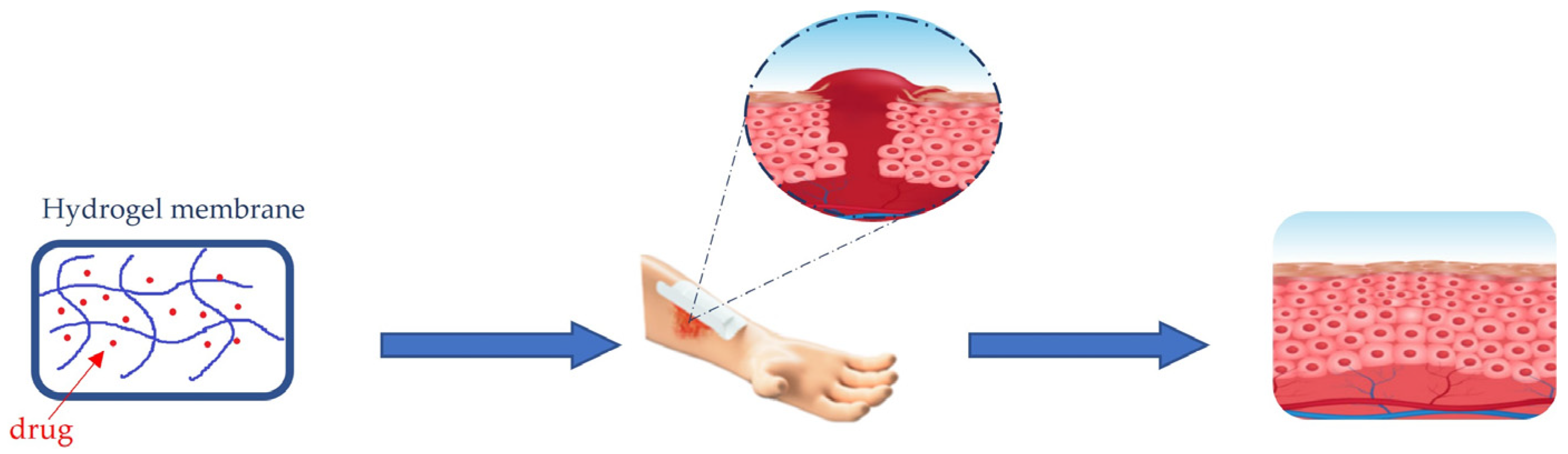
Figure 1. Healing process of wounds trough hydrogel membrane dressing.
The cross-linked structure of polymer chains in hydrogels can expand as a gel mass, absorbs and retains exudates, isolates bacteria, odorous molecules and debris from the exudate. The high aqueous content helps diffuse oxygen and vapor into the wound, thus providing a soothing effect.
Abbasi et al. [85] synthesized heat-sensitive hydrogel membranes, using sodium alginate biopolymer, synthetic polymer F127 and PVA as the cross-linker. The thermosensitive hydrogel showed good mechanical properties, elasticity, flexibility and tensile properties related to the degree of polymer crosslinking. Porosity on the polymer surface allowed ‘oxygenation of the wound, contributing to a stimulating environment for its re-epithelialization. The porosity allowed sustained release of the antimicrobial drug, which promoted accelerated healing. The drug loaded was amikacin, which has strong activity against gram-positive (S. aureus) and gram-negative (P. aeruginosa) organisms. Histological examination performed on an animal model attested the complete wound healing in 21 days.
Batool et al. [86] made a PVA/Starch-based membrane hydrogel in which silver nanoparticles (NPs) were embedded. These NPs were extracted from the Diospyros lotus plant through two different methods, one green and one nongreen. The former used water and the latter used methanol as a solvent.
Nanoparticles, in general, have several advantages, such as greater stability, longer shelf life and pharmacological effect of the drug, which in turn increases bioavailability and reduces dosing frequency [87]. Nanomaterials can promote wound healing through direct regulation of the extracellular matrix, promoting stem cell growth and skin regeneration by modulating growth factors at the wound site. Due to the unique properties of high surface-to-volume ratio, nanoscale size and porosity, they are used in wound dressings care and management [87][88]. The silver NPs obtained by the two different methods were studied and showed very similar properties between them. Next, the mechanical and also antibacterial properties of the membranes with and without the NPs were compared. The mechanical properties of membranes without NPs were found to be better, while NP membranes showed superior swelling and moisture retention capabilities. Membranes that had incorporated NP prepared with organic extract also exhibited antibacterial activity, which was totally absent in membranes without NP. Membranes containing “green” Ag NP thus have great aptitude for use in wound dressing applications.
Among the natural polymers most widely used in the manufacture of hydrogel membranes, there are hyaluronic acid and chitosan. Hyaluronic acid is a non-sulfur anionic glycosaminoglycan [89], which often occurs in the form of a sodium salt. It exhibits unique characteristics, including biocompatibility, biodegradability, nonimmunogenicity, and hydrophilicity [90].
Chitosan is a linear cationic polyamide, it is obtained by deacetylation of chitin, the second most abundant biopolymer in nature, after cellulose. It has bactericidal and bacteriostatic action. It is biocompatible and has low toxicity in wound dressings. It provides a moist environment to heal wounds, prevents the accumulation of exudates and reduces the chances of bacterial infection [91].
Shafique et al. [92] used hyaluronic acid and chitosan to produce membranes consisting not only of these two natural polymers, but also of pullulane and polyvinyl alcohol (PVA). Pullulane is a polysaccharide polymer composed of maltotriose units connected by α 1–6 bonds [93]. It possesses great advantages such as low toxicity and mutagenicity, high biodegradability, and water solubility. Above all, pullulane can form thin films with structural flexibility, adhesive properties and great mechanical strength [94][95]. PVA is a hydrophilic polymer, which can retain water, providing a moist environment and thus conducive to wound healing. It is biocompatible and biodegradable with good mechanical properties. The antimicrobial properties of the hydrogel membrane, due to the presence of chitosan, have been enhanced through loading with nanoparticles of cefepime, an antibiotic belonging to the fourth generation cephalosporins, which is usually parenterally administered. It is effective against Gram-positive pathogens such as MRSA, PRSP, Streptococcus pyogenes, and Gram-negative pathogens such as Escherichia coli, Klebsiella pneumonia and Serratia, Citrobacter [96][97]. The membrane hydrogel, tested on an excisional rat model that showed rapid recovery, demonstrated inhibitory action especially against the proliferation of Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. The important antibacterial activity seems likely to warrant promising use by skin application of the produced membrane as a potential accelerator in the wound healing process.
During wound healing, if a disproportionate inflammatory response occurs, the resulting increase in wound size complicates the process of tissue regeneration. (S)-ibuprofen (IBP), a nonsteroidal anti-inflammatory agent used for healing muscle injuries and treating venous leg ulcers, has also been studied as an active ingredient for skin wound healing. Agujar-Ricardo et al. [98] designed IBP-β-cyclodextrin carriers to modulate the release of IBP from poly (vinyl alcohol)/chitosan (PVA/CS) dressings with the aim of achieving faster skin regeneration. In vitro studies showed that β-cyclodextrins allowed controlled release of IBP from hydrogels, while in vivo assays revealed that the presence of PVA/CS membranes prevented crusting and excessive inflammation, accelerating the healing.
6. Bone Tissue Engineering
Bone tissue is made up of various types of cells, and its ECM contains both organic elements, such as type I collagen, and inorganic elements, such as hydroxyapatite [99]. Inorganic substances can give bone a special hardness. Research in recent years has developed many membranes to accelerate bone regeneration after traumatic events resulting in injury. Hydrogels have been used in various therapeutic treatments (Figure 2), such as, for example, for filling bone gaps [100][101].

Figure 2. Bone tissue regeneration through the use of hydrogel membranes supplemented with inorganic nanoparticles The system can promote the differentiation of bone Marrow Stromal Cells (Msc) and can be integrated in the damaged bone.
A multilayer hydrogel membrane consisting of chemically converted graphene (CCG) has been used as a barrier membrane for bone regeneration [102]. In a rat model both osteoinductivity and osteoconductivity were increased, which resulted in improved mineralization of mature lamellar bone. This was interpreted as an effect of the osteogenic activity of CCG and multilayer membrane nanostructure.
Fracture healing is a process that takes place in several stages [103]. There are factors that can hinder this process: soft tissue damage, location of the injury, age of the patient, osteoporosis, and use of particular drugs. In orthopaedic and joint/prosthetic surgery, infections are a not uncommon complication [104][105].
Johnson et al. [106] designed injectable hydrogels to treat infections caused by Staphylococcus aureus in orthopaedic implants used for fracture repair. A mouse model of femoral fracture infection was used to evaluate the therapeutic potential of lysostaphin therapy incorporated into a formulation consisting essentially of a PEG hydrogel. By adhering to exposed fracture surfaces, the formulation allowed lysostaphin to be effectively administered locally. Lysostaphin encapsulated in this synthetic hydrogel maintained its stability. The released lysostaphin showed greater antibiofilm activity than the unencapsulated lysostaphin. Thus, the authors demonstrated that PEG-based hydrogels can restore the fracture healing process, which has been altered by infection sustained by Staphylococcus aureus. In addition, hydrogels can deliver growth factors added directly to the gel to promote fracture healing.
The main constituents of osteochondral tissue are subchondral bone and articular cartilage. To correct defects in this tissue, regeneration of both articular cartilage and subchondral bone is necessary [107][108].
Due to their characteristics of biocompatibility, biodegradability, and control of cell-ECM interactions, hydrogels have emerged as a material of choice for the fabrication of membranes suitable for cartilage tissue repair [109].
In recent years, great strides have been made in the field of cartilage tissue engineering, such as using 3D printing and doping of hydrogels with porous and/or biodegradable microspheres to induce cartilage structure formation. The porosity plays an important role. This is demonstrated by the fact that in scaffolds with closed pores, cells are poorly distributed, thus generating an inhomogeneous ECM, characterized by poor mechanical properties. Hydrogels are being used as a basic biomaterial for cartilage recovery through two modes: the first involving a carrier action of cells that go on to promote tissue regeneration, and the second as a constituent of permanent implants for the replacement of damaged cartilage tissue [109]. The polymers most commonly used as base material of hydrogels are Polyethylene glycol diacrylate (PEGDA), hyaluronic acid thiolates, chitosan, graphene, and alginate.
Zhu et al. [110] combined 3D-printed acellular chondrocytes, extracellular matrix (ECM), polyethylene glycol diacrylate (PEGDA), and Honokiol, a natural compound that revealed good anti-inflammatory properties for the treatment of various diseases, inclu-ding osteoarthritis. The combination tested showed promising results for the recovery of osteochondrial defects.
Yuan et al. [111] prepared composite material of HAPNW HydroxyAPatite NanoWires embedded in a double network of bovine serum albumin/sodium alginate. HAPNWs were added to the hydrogel membranes not only to improve their microstructure, but also to increase their mechanical properties. In fact, the resulting material possesses higher porosity, swelling and compressive modulus properties. In addition, in vivo studies have confirmed that the obtained material can increase the proliferation and differentiation of bone marrow stromal cells (BMSCs) and promote the integration of the regenerated tissue with the surrounding normal tissue.
7. Neural Tissue Engineering
Neurological diseases can be severe and difficult to treat. Neural tissue engineering offers valuable help through the selection of basic materials to produce suitable membranes to promote neural cell differentiation and growth [112]. Hydrogels are among these materials. They have been exploited for the delivery of neural growth-promoting agents and neurotrophic factors that oppose neural growth inhibitors (chondroitin sulphate proteoglycans (CSPG) [113], Nogo [114], and myelin-associated glycoprotein [115]. Recently encapsulating hydrogels have been used to protect neural cells from immune activity.
The term “neural tissue” seems to refer mainly to neurons. Actually, neural tissue engineering is aimed at developing functional neural tissue not only of neurons but also of non-neuronal glial cells [116].
An important element, which influences attachment, the creation of neuronal synapses and the regulation of their diameter, is the maintenance of mechanical tension along the neurite. It also influences the arborized arrangement of neurons [117]. In synthetic and natural hydrogels such as those of polyacrylamide and fibrin, it has been observed that cell survival and neuritic extension of cortical neurons cultured on them are higher when the elastic modulus of the hydrogel is closer to that of the extracellular matrix [118]. Softer gels increase neuronal sprouting to a greater extent than harder gels. In contrast, astrocytes develop much better on stiffer substrates.
Several research papers in the last two decades have reported [119] that electrical stimulation of damaged neural tissue can give important contributions to its repair and regeneration. The mechanism underlying electrical stimulation has not been fully elucidated, but several hypotheses have been made, including those regarding the role of voltage-dependent calcium channels [120] and changes in the local electric field of extracellular matrix molecules [121].
Lee et al. [122] developed a material based on a PEG hydrogel substrate micro patinated with a silver nanowire (AgNW). The introduction of silver NWs increased the conductivity of the substrate, which was sensitive to electrical stimuli applied to differentiate neural stem cells (NSCs) and to drive the growth of neurites. To further guide the growth of neurites, parallel micro arrays were created from the hybrid hydrogel material. The combination of electrical stimuli and physical micropatterns containing AgNWs in one device resulted in synergistic effects, with a neurite outgrowth rate higher than that obtained using electrical stimuli or micropatterns alone.
Liu et al. [123] produced a perfluoro polyether thin film functionalized with dimethacrylate and subjected to crosslinking by UV. It can perform localized neuromodulation by interfacing with peripheral nerves. The Young’s modulus of this material was adjusted to match that of nerve tissue. The authors then described the lithographic process developed to pattern the soft and intrinsically stretchable material in a multi-electrode array. The results of the study validated the biocompatibility and the stability of the system in an aqueous environment. It was able to perform good electrical stimulation with ultra-low voltages.
For the future, hydrogel membranes are presented with ideal properties for neuronal tissue growth and regeneration, and may supply a platform that can provide, separately or synergistically, cues for the replacement and the repair of neural components, with restoration of function (Figure 3) [124].
Figure 3. Properties of the ideal nerve guidance conduit [124] (License CC BY).
8. Drug Delivery
Ideal drug delivery must ensure efficiency and avoid side effects. From this it follows that the drug concentration at the plasma level must be effective and below the level of toxicity as far as possible [125]. The traditional method of drug administration generally results in the plasma concentration rising to a peak and then decreasing. This leads to an inevitable risk of toxicity and drug wastage. One solution could be smart membranes, which can ensure stable and targeted drug release, plus incorporating features responsive to environmental stimuli into drug delivery systems [126][127]. For controlled drug release, smart membranes can be made in the form of capsules, which are easy to design and apply. Such stimuli-reactive capsule membranes, with a shell structure, can provide a large internal volume for encapsulation of various drugs and in a versatile manner for controlled release [128].
Hydrogels, due to their three-dimensional polymer structure and their ability to absorb a large fraction of water, can be exploited for controlled drug release (Figure 4). Control can be accomplished through special stimuli such as changes in pH, temperature, and electrical potentials. What is particularly interesting to researchers is the possibility of making drug release very precise and timed [129][130]. That is why scientists have increasingly turned toward the study of smart systems that can pick up signals produced by the disease and release the specific amount of drug responsive to the physiological condition, minimizing the risk of side effects. To achieve efficient controlled release and low side effects, an on/off model seems to offer the greatest assurance. It is ideal for the treatment of chronic diseases that require frequent administration. For example, for blood glucose level control, subcutaneous insulin injections are given in diabetes, an inconvenient and painful therapy with low patient compliance. These drawbacks can be overcome by producing a smart capsule with a blood glucose concentration-sensitive envelope that can transition from off to on level for insulin release governed by glucose concentrations [127].
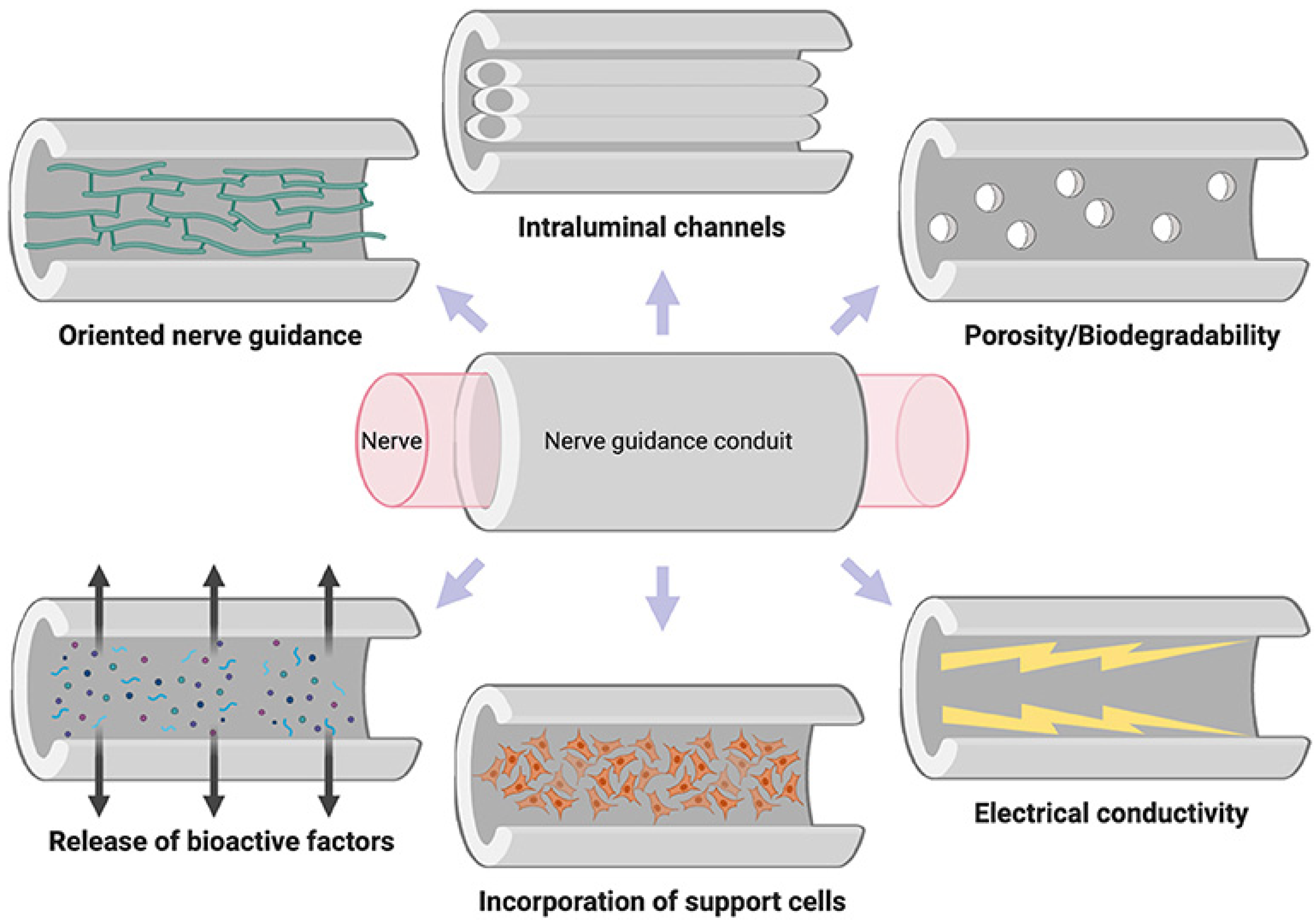
Figure 4. Loading and releasing of a drug by a hydrogel membrane.
A classic method to produce smart capsules is to use stimuli-responsive polymeric materials embedded on the pores or surfaces of porous membranes as smart on/off switches [131]. Polymers could be introduced onto porous membranes using “grafting to” and “grafting from” methods.
For the “grafting from” method, there is pre-activation of polymerization sites on the membranes through chemical agents, UV light, plasma or heat; on these activated sites the functional monomers “grow” and form smart gates. For the grafting-to method, polymerization of functional monomers occurs before grafting onto the porous membrane.
The drug release scheme predicts that in the absence of the predetermined specific stimulus, the gate remains closed and thus there is no release. Under the action of the stimulus, the gate opens, and drug release occurs [125].
Smart capsules can be developed using hydrogel as the membrane of the entire capsule. Among the membrane modeling methods, the microfluidic technique comes up with special features of precision in manipulating the shape, structure and composition of monodisperse emulsion droplets [132].
Zhang et al. [133] obtained, through a plate-emulsion microfluidic methodology, a system encapsulated in a glucose-reactive hydrogel. 3-Acrylamidophenylboronic acid (AAPBA) was used as the glucose sensor, and thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) was used as the activator. In the range of physiological blood glucose concentration at 37 °C, the capsule showed a reversible and repeated swelling/shrinking response. The system developed by Zhang and coworkers thus provides a promising model to produce smart drug delivery systems.
Implantable systems that can release drugs in a programmable mode according to therapeutic needs also have a viable use in cancer treatment. Wang et al. [134] fabricated a composite membrane by incorporating both pH and temperature-sensitive hydrogel microparticles with magnetic silk fibroin nanoparticles inside. The application of an alternating magnetic field with the subsequent generation of heat by the magnetic nanoparticles led to the contraction and swelling of the microgels. This induced a reversible change in membrane permeability that allowed immediate release of the model drug Rhodamine B (Rh.B). By adjusting the thickness of the membrane and the ratio of the amount of microgel to the number of magnetic nanoparticles, control of the release rate could be achieved.
The release rate of Rh.B is increased under acidic conditions compared with its value at physiological pH. It is well known that oncological diseases lead to a lowering of pH, and thus this experimental observation has prospected a relevant potential for the use of the membrane in selective cancer therapy.
Still for the development of an oncology drug delivery system, more particularly for breast and liver cancer, a membrane of polyvinyl/cellulose nanocrystals (PVA/CNCs) loaded with curcumin [135] has been made. The strategy to maximize the encapsulation capacity of the hydrogel was directed toward finding an optimal preparation method. This was found in the solution fusion method using citric acid as a crosslinker. FT-IR spectroscopy revealed that curcumin and membrane components are bound through an intermolecular hydrogen bond in the amorphous phase of the PVA/CNC system. Curcumin was released in bursts (41%) during the first hour, after which a sustained release of 70% and 94% was shown in 24 h and 48 h, respectively.
Kamoun et al. [136] developed hydrogel membranes based on hyaluronic acid (HA) and poly(N-isopropylacrylamide) sensitive to pH and temperature. Such membranes were produced by redox polymerization, using N,N-methylenbisacrylamide (BIS) and epichlorohydrin (EPI) as crosslinkers. The membranes were loaded with ampicillin antimicrobial drug, and it was observed that, as the ratios of HA varied, the swelling capacity and release rate varied too. In addition, the reactivity to heat and pH allowed a rapid release. Thus, this intelligent system could be used for rapid drug release to different districts.
In 2016, Nagarjuna et al. [137] developed a hydrogel membrane consisting of a mixture of sodium alginate (SA) and Karaya rubber (KG). It was used for testing the sustained release, in physiological conditions obtained by phosphate buffer (pH 7.4 T 37 °C), of flutamide (FLT), a potent nonsteroidal antiandrogenic prostate anticancer drug, which was membrane-loaded. The results showed a decreased swelling with increasing KG amount. FT-IR spectroscopical analysis confirmed the absence of chemical interactions between drug and polymer. Results of controlled release tests showed that the amount of the released flutamide increased with the amount of SA in the membrane.
References
- Siddique, T.; Dutta, N.K.; Choudhury, N.R. Mixed-Matrix Membrane Fabrication for Water Treatment. Membranes 2021, 11, 557.
- Toniato, T.V.; Stocco, T.D.; Martins, D.D.S.; Santanna, L.B.; Tim, C.R.; Marciano, F.R.; Silva-Filho, E.C.; Campana-Filho, S.P.; Lobo, A. Hybrid chitosan/amniotic membrane-based hydrogels for articular cartilage tissue engineering application. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 961–970.
- Arefi-Oskoui, S.; Khataee, A.; Safarpour, M.; Orooji, Y.; Vatanpour, V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrason. Sonochemistry 2019, 58, 104633.
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2018, 76, 4879–4901.
- Mbituyimana, B.; Mao, L.; Hu, S.; Ullah, M.W.; Chen, K.; Fu, L.; Zhao, W.; Shi, Z.; Yang, G. Bacterial cellulose/glycolic acid/glycerol composite membrane as a system to deliver glycolic acid for anti-aging treatment. J. Bioresour. Bioprod. 2021, 6, 129–141.
- Wsoo, M.A.; Razak, S.I.A.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98.
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274.
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419.
- Donato, L. A Spasso con le Membrane; CNR Edizionii: Roma, Italy, 2021; pp. 21–22.
- Woźniak-Budych, M.J. Polymeric membranes for biomedical applications. Phys. Sci. Rev. 2021, 15, 619.
- Chen, X.; Li, J. Bioinspired by cell membranes: Functional polymeric materials for biomedical applications. Mater. Chem. Front. 2020, 4, 750–774.
- Wu, J.; Qu, Y.; Yu, Q.; Chen, H. Gold nanoparticle layer: A versatile nanostructured platform for biomedical applications. Mater. Chem. Front. 2018, 2, 2175–2190.
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162.
- Zhang, X.; Liu, M.; Zhang, X.; Deng, F.; Zhou, C.; Hui, J.; Liu, W.; Wei, Y. Interaction of tannic acid with carbon nanotubes: Enhancement of dispersibility and biocompatibility. Toxicol. Res. 2015, 4, 160–168.
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228.
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544.
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14.
- Parodi, A.; Molinaro, R.; Sushnitha, M.; Evangelopoulos, M.; Martinez, J.O.; Arrighetti, N.; Corbo, C.; Tasciotti, E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 2017, 147, 155–168.
- Kim, Y.; Chortos, A.; Xu, W.; Liu, Y.; Oh, J.Y.; Son, D.; Kang, J.; Foudeh, A.M.; Zhu, C.; Lee, Y.; et al. A bioinspired flexible organic artificial afferent nerve. Science 2018, 360, 998–1003.
- Zhang, Z.; Wen, L.; Jiang, L. Bioinspired smart asymmetric nanochannel membranes. Chem. Soc. Rev. 2018, 47, 322–356.
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Khademhosseini, A.; Peppas, N.A. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 2019, 106, 100589.
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411.
- Garni, M.; Thamboo, S.; Schoenenberger, C.-A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 619–638.
- Lu, Y.; Huskens, J.; Pang, W.; Duan, X. Hypersonic poration of supported lipid bilayers. Mater. Chem. Front. 2019, 3, 782–790.
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242.
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. 2020, 114, 111023.
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811.
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376.
- Khakpour, S.; Ahmed, H.M.M.; De Bartolo, L. Membrane-Based Bioartificial Liver Devices. In Biomedical Membranes and (Bio)Artificial Organs; World Scientific Series in Membrane Science and Technology: Biological and Biomimetic Applications, Energy and the Environment; World Scientific: Singapore, 2017; Volume 2, pp. 149–178.
- Zhang, K.; Zhang, L.; Liu, W.; Ma, X.; Cen, J.; Sun, Z.; Wang, C.; Feng, S.; Zhang, Z.; Yue, L.; et al. In Vitro Expansion of Primary Human Hepatocytes with Efficient Liver Repopulation Capacity. Cell Stem Cell 2018, 23, 806–819.e4.
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19.
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846.
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61.
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663.
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 159–182.
- Khorshidi, S.; Karkhaneh, A. A review on gradient hydrogel/fiber scaffolds for osteochondral regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1974–e1990.
- Gradinaru, V.; Treweek, J.; Overton, K.; Deisseroth, K. Hydrogel-Tissue Chemistry: Principles and Applications. Annu. Rev. Biophys. 2018, 47, 355–376.
- Ye, S.; Boeter, J.W.B.; Penning, L.C.; Spee, B.; Schneeberger, K. Hydrogels for Liver Tissue Engineering. Bioengineering 2019, 6, 59.
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells 2020, 9, 304.
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.W.; Kock, L.M.; Spee, B. Cellulose Nanofibril Hydrogel Promotes Hepatic Differentiation of Human Liver Organoids. Adv. Healthc. Mater. 2020, 9, 1901658.
- McCrary, M.W.; Bousalis, D.; Mobini, S.; Song, Y.H.; Schmidt, C.E. Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues. Acta Biomater. 2020, 111, 1–19.
- Lu, S.; Cuzzucoli, F.; Jiang, J.; Liang, L.-G.; Wang, Y.; Kong, M.; Zhao, X.; Cui, W.; Li, J.; Wang, S. Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 2018, 18, 3379–3392.
- Damania, A.; Kumar, A.; Teotia, A.K.; Kimura, H.; Kamihira, M.; Ijima, H.; Sarin, S.K.; Kumar, A. Decellularized Liver Matrix-Modified Cryogel Scaffolds as Potential Hepatocyte Carriers in Bioartificial Liver Support Systems and Implantable Liver Constructs. ACS Appl. Mater. Interfaces 2017, 10, 114–126.
- Asadi, M.; Lotfi, H.; Salehi, R.; Mehdipour, A.; Zarghami, N.; Akbarzadeh, A.; Alizadeh, E. Hepatic cell-sheet fabrication of differentiated mesenchymal stem cells using decellularized extracellular matrix and thermoresponsive polymer. Biomed. Pharmacother. 2021, 134, 111096.
- Hussein, K.H.; Park, K.-M.; Yu, L.; Kwak, H.-H.; Woo, H.-M. Decellularized hepatic extracellular matrix hydrogel attenuates hepatic stellate cell activation and liver fibrosis. Mater. Sci. Eng. 2020, 116, 111160.
- Christoffersson, J.; Aronsson, C.; Jury, M.; Selegård, R.; Aili, D.; Mandenius, C.-F. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device. Biofabrication 2018, 11, 015013.
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260.
- Christoffersson, J.; van Noort, D.; Mandenius, C.-F. Developing organ-on-a-chip concepts using bio-mechatronic design methodology. Biofabrication 2017, 9, 025023.
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414.
- Lin, Y.-J.; Mi, F.-L.; Lin, P.-Y.; Miao, Y.-B.; Huang, T.; Chen, K.-H.; Chen, C.-T.; Chang, Y.; Sung, H.-W. Strategies for improving diabetic therapy via alternative administration routes that involve stimuli-responsive insulin-delivering systems. Adv. Drug Deliv. Rev. 2019, 139, 71–82.
- Dinnyes, A.; Schnur, A.; Muenthaisong, S.; Bartenstein, P.; Burcez, C.; Burton, N.; Cyran, C.; Gianello, P.; Kemter, E.; Nemeth, G.; et al. Integration of nano- and biotechnology for beta-cell and islet transplantation in type-1 diabetes treatment. Cell Prolif. 2020, 53, e12785.
- Hwang, P.T.J.; Shah, D.K.; Garcia, J.A.; Bae, C.Y.; Lim, D.-J.; Huiszoon, R.C.; Alexander, G.C.; Jun, H.-W. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016, 3, 28.
- Haque, M.R.; Lee, D.Y.; Ahn, C.-H.; Jeong, J.-H.; Byun, Y. Local Co-Delivery of Pancreatic Islets and Liposomal Clodronate Using Injectable Hydrogel to Prevent Acute Immune Reactions in a Type 1 Diabetes. Pharm. Res. 2014, 31, 2453–2462.
- Ghasemi, A.; Akbari, E.; Imani, R. An Overview of Engineered Hydrogel-Based Biomaterials for Improved β-Cell Survival and Insulin Secretion. Front. Bioeng. Biotechnol. 2021, 9, 662084.
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Orive, G.; Hernández, R.M.; Saenz del Burgo, L.; Pedraz, J. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics 2019, 11, 597.
- Wang, J.K.; Cheam, N.M.J.; Irvine, S.A.; Tan, N.S.; Venkatraman, S.; Tay, C.Y. Interpenetrating Network of Alginate–Human Adipose Extracellular Matrix Hydrogel for Islet Cells Encapsulation. Macromol. Rapid Commun. 2020, 41, 2000275.
- Bai, X.; Pei, Q.; Pu, C.; Chen, Y.; He, S.; Wang, B. Multifunctional Islet Transplantation Hydrogel Encapsulating A20 High-Expressing Islets. Drug Des. Dev. Ther. 2020, 14, 4021–4027.
- Knobeloch, T.; Abadi, S.E.M.; Bruns, J.; Petrova Zustiak, S.; Kwon, G. Injectable polyethylene glycol hydrogel for islet encapsulation: An in vitro and in vivo characterization. Biomed. Phys. Eng. Express 2017, 3, 035022.
- Abada, E.N.; Feinberg, B.J.; Roy, S. Evaluation of silicon membranes for extracorporeal membrane oxygenation (ECMO). Biomed. Microdevices 2018, 20, 86.
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975.
- He, T.; He, J.; Wang, Z.; Cui, Z. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO). Adv. Compos. Hybrid Mater. 2021, 4, 847–864.
- He, T.; Yu, S.; He, J.; Chen, D.; Li, J.; Hu, H.; Zhong, X.; Wang, Y.; Wang, Z.; Cui, Z. Membranes for extracorporeal membrane oxygenator (ECMO): History, preparation, modification and mass transfer. Chin. J. Chem. Eng. 2022, 49, 46–75.
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Bréchot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.-L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013, 39, 838–846.
- Schlanstein, P.C.; Limper, A.; Hesselmann, F.; Schmitz-Rode, T.; Steinseifer, U.; Arens, J. Experimental method to determine anisotropic permeability of hollow fiber membrane bundles. J. Membr. Sci. 2018, 546, 70–81.
- Valencia, E.; Nasr, V.G. Updates in Pediatric Extracorporeal Membrane Oxygenation. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1309–1323.
- Lavery, K.S.; Rhodes, C.; Mcgraw, A.; Eppihimer, M.J. Anti-thrombotic technologies for medical devices. Adv. Drug Deliv. Rev. 2017, 112, 2–11.
- Mi, M.Y.; Matthay, M.A.; Morris, A.H. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 379, 884–887.
- Winnersbach, P.; Hosseinnejad, A.; Breuer, T.; Fechter, T.; Jakob, F.; Schwaneberg, U.; Rossaint, R.; Bleilevens, C.; Singh, S. Endogenous Nitric Oxide-Releasing Microgel Coating Prevents Clot Formation on Oxygenator Fibers Exposed to In Vitro Blood Flow. Membranes 2022, 12, 73.
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2020, 8, nwaa254.
- Naito, N.; Ukita, R.; Wilbs, J.; Wu, K.; Lin, X.; Carleton, N.M.; Roberts, K.; Jiang, S.; Heinis, C.; Cook, K.E. Combination of polycarboxybetaine coating and factor XII inhibitor reduces clot formation while preserving normal tissue coagulation during extracorporeal life support. Biomaterials 2021, 272, 120778.
- Wang, W.; Lu, Y.; Zhu, H.; Cao, Z. Superdurable Coating Fabricated from a Double-Sided Tape with Long Term “Zero” Bacterial Adhesion. Adv. Mater. 2017, 29, 1606506.
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, 1903062.
- Guo, Q.; Sun, H.; Wu, X.; Yan, Z.; Tang, C.; Qin, Z.; Yao, M.; Che, P.; Yao, F.; Li, J. In Situ Clickable Purely Zwitterionic Hydrogel for Peritoneal Adhesion Prevention. Chem. Mater. 2020, 32, 6347–6357.
- Xie, X.; Doloff, J.C.; Yesilyurt, V.; Sadraei, A.; McGarrigle, J.J.; Omami, M.; Veiseh, O.; Farah, S.; Isa, D.; Ghani, S.; et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2018, 2, 894–906.
- Yao, M.; Wei, Z.; Li, J.; Guo, Z.; Yan, Z.; Sun, X.; Yu, Q.; Wu, X.; Yu, C.; Yao, F.; et al. Microgel reinforced zwitterionic hydrogel coating for blood-contacting biomedical devices. Nat. Commun. 2022, 13, 5339.
- Gao, W.; Wang, H.; Liu, Y.; Tang, Q.; Wu, P.; Lin, T.; Li, T.; Sun, D. Sodium alginate-hydrogel coatings on extracorporeal membrane oxygenation for anticoagulation. Front. Cardiovasc. Med. 2022, 9, 966649.
- Hyono, A.; Yonezawa, T.; Kawai, K.; Abe, S.; Fujihara, M.; Azuma, H.; Wakamoto, S. SEM observation of the live morphology of human red blood cells under high vacuum conditions using a novel RTIL. Surf. Interface Anal. 2014, 46, 425–428.
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695.
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166.
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923.
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229.
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13.
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In vitro and in vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373.
- Murphy, P.S.; Evans, G.R.D. Advances in Wound Healing: A Review of Current Wound Healing Products. Plast. Surg. Int. 2012, 2012, 190436.
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765.
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414.
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26.
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574.
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The Role of Hyaluronic Acid in Wound Healing. Am. J. Clin. Dermatol. 2005, 6, 393–402.
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993.
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers, Composites Renewable Resources; Gandini, A., Belgacem, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 5; pp. 517–542.
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C.; et al. Bio-functional hydrogel membranes loaded with chitosan nanoparticles for accelerated wound healing. Int. J. Biol. Macromol. 2021, 170, 207–221.
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial sources, production and applications. Carbohydr. Polym. 2008, 73, 515–531.
- Tamer, T.M.; Hassan, M.A.; Omer, A.M.; Valachová, K.; Eldin, M.S.M.; Collins, M.N.; Šoltés, L. Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr. Polym. 2017, 169, 441–450.
- Priya, V.S.; Iyappan, K.; Gayathri, V.S.; William, S.; Suguna, L. Influence of pullulan hydrogel on sutureless wound healing in rats. Wound Med. 2016, 14, 1–5.
- Pawar, V.; Dhanka, M.; Srivastava, R. Cefuroxime conjugated chitosan hydrogel for treatment of wound infections. Colloids Surf. B 2019, 173, 776–787.
- Yahav, D.; Paul, M.; Fraser, A.; Sarid, N.; Leibovici, L. Efficacy and safety of cefepime: A systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 338–348.
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145.
- Mabrouk, M.; Rajendran, R.; Soliman, I.E.; Ashour, M.M.; Beherei, H.H.; Tohamy, K.M.; Thomas, S.; Kalarikkal, N.; Arthanareeswaran, G.; Das, D.B. Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics 2019, 11, 294.
- Liu, F.; Wang, M.; Wang, X.; Wang, P.; Shen, W.; Ding, S.; Wang, Y. Fabrication and application of nanoporous polymer ion-track membranes. Nanotechnology 2018, 30, 052001.
- Baptista, D.; Teixeira, L.M.; Birgani, Z.T.; van Riet, S.; Pasman, T.; Poot, A.; Stamatialis, D.; Rottier, R.J.; Hiemstra, P.S.; Habibović, P.; et al. 3D alveolar in vitro model based on epithelialized biomimetically curved culture membranes. Biomaterials 2021, 266, 120436.
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30.
- AI-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. J. Dent. Res. 2008, 87, 107–118.
- Drago, L.; Clerici, P.; Morelli, I.; Ashok, J.; Benzakour, T.; Bozhkova, S.; Alizadeh, C.; Del Sel, H.; Sharma, H.K.; Peel, T.; et al. The World Association against Infection in Orthopaedics and Trauma (WAIOT) Procedures for Microbiological Sampling and Processing for Periprosthetic Joint Infections (PJIs) and Other Implant-Related Infections. J. Clin. Med. 2019, 8, 933.
- George, D.A.; Drago, L.; Scarponi, S.; Gallazzi, E.; Haddad, F.S.; Romano, C.L. Predicting lower limb periprosthetic joint infections: A review of risk factors and their classification. World J. Orthop. 2017, 8, 400.
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969.
- Dang, W.; Wang, X.; Li, J.; Deng, C.; Liu, Y.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. 3D printing of Mo-containing scaffolds with activated anabolic responses and bi-lineage bioactivities. Theranostics 2018, 8, 4372–4392.
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48.
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the Repair of Articular Cartilage Defects. Tissue Eng. 2011, 17, 281–299.
- Zhu, S.; Chen, P.; Chen, Y.; Li, M.; Chen, C.; Lu, H. 3D-Printed Extracellular Matrix/Polyethylene Glycol Diacrylate Hydrogel Incorporating the Anti-inflammatory Phytomolecule Honokiol for Regeneration of Osteochondral Defects. Am. J. Sport. Med. 2020, 48, 2808–2818.
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B 2020, 192, 111041.
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505.
- Silver, D.J.; Silver, J. Contributions of chondroitin sulfate proteoglycans to neurodevelopment, injury, and cancer. Curr. Opin. Neurobiol. 2014, 27, 171–178.
- Buchli, A.D.; Schwab, M.E. Inhibition of Nogo: A key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann. Med. 2005, 37, 556–567.
- McKerracher, L.; Rosen, K.M. MAG, myelin and overcoming growth inhibition in the CNS. Front. Mol. Neurosci. 2015, 8, 51.
- Madhusudanan, P.; Reade, S.; Shankarappa, S.A. Neuroglia as targets for drug delivery systems: A review. Nanomedicine 2017, 13, 667–679.
- Franze, K.; Gerdelmann, J.; Weick, M.; Betz, T.; Pawlizak, S.; Lakadamyali, M.; Bayer, J.; Rillich, K.; Gögler, M.; Lu, Y.-B.; et al. Neurite Branch Retraction Is Caused by a Threshold-Dependent Mechanical Impact. Biophys. J. 2009, 97, 1883–1890.
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with Compliance Comparable to that of Brain Tissue Select Neuronal over Glial Growth in Mixed Cortical Cultures. Biophys. J. 2006, 90, 3012–3018.
- Goganau, I.; Sandner, B.; Weidner, N.; Fouad, K.; Blesch, A. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp. Neurol. 2018, 300, 247–258.
- André, S.; Boukhaddaoui, H.; Campo, B.; Al-Jumaily, M.; Mayeux, V.; Greuet, D.; Valmier, J.; Scamps, F. Axotomy-Induced Expression of Calcium-Activated Chloride Current in Subpopulations of Mouse Dorsal Root Ganglion Neurons. J. Neurophysiol. 2003, 90, 3764–3773.
- Nguyen, H.T.; Wei, C.; Chow, J.K.; Nguy, L.; Nguyen, H.K.; Schmidt, C.E. Electric field stimulation through a substrate influences Schwann cell and extracellular matrix structure. J. Neural Eng. 2013, 10, 046011.
- Lee, J.M.; Moon, J.Y.; Kim, T.H.; Lee, S.W.; Ahrberg, C.D.; Chung, B.G. Conductive hydrogel/nanowire micropattern-based sensor for neural stem cell differentiation. Sens. Actuators B 2018, 258, 1042–1050.
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.H.; et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68.
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for Neural Tissue Engineering. Front. Nanotechnol. 2021, 3, 643507.
- Zou, L.-B.; Gong, J.-Y.; Ju, X.-J.; Liu, Z.; Wang, W.; Xie, R.; Chu, L.-Y. Smart membranes for biomedical applications. Chin. J. Chem. Eng. 2022, 49, 34–45.
- Wang, C.; Wang, S.; Pan, H.; Min, L.; Zheng, H.; Zhu, H.; Liu, G.; Yang, W.; Chen, X.; Hou, X. Bioinspired liquid gating membrane-based catheter with anticoagulation and positionally drug release properties. Sci. Adv. 2020, 6, eabb4700.
- Liu, Z.; Ju, X.-J.; Wang, W.; Xie, R.; Jiang, L.; Chen, Q.; Zhang, Y.-Q.; Wu, J.-F.; Chu, L.-Y. Stimuli-Responsive Capsule Membranes for Controlled Release in Pharmaceutical Applications. Curr. Pharm. Des. 2017, 23, 295–301.
- Chu, L.; Xie, R.; Ju, X. Stimuli-responsive Membranes: Smart Tools for Controllable Mass-transfer and Separation Processes. Chin. J. Chem. Eng. 2011, 19, 891–903.
- Wise, D.L. Handbook of Pharmaceutical Controlled Release Technology; CRC Press: Boca Raton, FL, USA, 2000.
- Misra, A.; Jogani, V.; Jinturkar, K.; Vyas, T. Recent Patents Review on Intranasal Administration for CNS Drug Delivery. Recent PAt. Drug Deliv. Formul. 2008, 2, 25–40.
- Liu, Z.; Wang, W.; Xie, R.; Ju, X.-J.; Chu, L.-Y. Stimuli-responsive smart gating membranes. Chem. Soc. Rev. 2016, 45, 460–475.
- Zhang, M.-J.; Zhang, P.; Qiu, L.-D.; Chen, T.; Wang, W.; Chu, L.-Y. Controllable microfluidic fabrication of microstructured functional materials. Biomicrofluidics 2020, 14, 061501.
- Zhang, M.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, L.; Gu, Y.-Y.; Chu, L.-Y. Microfluidic fabrication of monodisperse microcapsules for glucose-response at physiological temperature. Soft Matter 2013, 9, 4150.
- Wang, Y.; Boero, G.; Zhang, X.; Brugger, J. Thermal and pH Sensitive Composite Membrane for On-Demand Drug Delivery by Applying an Alternating Magnetic Field. Adv. Mater. Interfaces 2020, 7, 2000733.
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced anti-cancer activity by localized delivery of curcumin form PVA/CNCs hydrogel membranes: Preparation and in vitro bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122.
- Kamoun, E.A.; Fahmy, A.; Taha, T.H.; El-Fakharany, E.M.; Makram, M.; Soliman, H.M.A.; Shehata, H. Thermo-and pH-sensitive hydrogel membranes composed of poly(N-isopropylacrylamide)-hyaluronan for biomedical applications: Influence of hyaluronan incorporation on the membrane properties. Int. J. Biol. Macromol. 2018, 106, 158–167.
- Nagarjuna, G.; Babu, P.; Maruthi, Y.; Parandhama, A.; Madhavi, C.; Subha, M.; Chowdojirao, K. Interpenetrating Polymer Network Hydrogel Membranes of Karayagum and Sodium Alginate for Control Release of Flutamide Drug. J. Appl. Pharm. Sci. 2016, 6, 011–019.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
726
Revisions:
2 times
(View History)
Update Date:
06 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





