
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | engila khan | -- | 1323 | 2023-06-05 09:43:06 | | | |
| 2 | Catherine Yang | Meta information modification | 1323 | 2023-06-05 10:15:55 | | |
Video Upload Options
Parkinson's disease is an advancing condition characterized by different types of physical and mental impairments. The characteristic features of Parkinson's disease include the buildup of improperly folded protein known as α-synuclein as Lewy bodies, as well as the deterioration of dopamine-producing neurons in the substantia nigra pars compacta (SNc) region, which impacts the patient's motor functions. Significant studies have been conducted to investigate the use of animal models for Parkinson's disease.
1. Introduction
Over two hundred years ago, James Parkinson, an English physician, published an article titled 'An Essay on the Shaking Palsy,' describing a clinical syndrome characterized by involuntary tremors and weakened muscle power. This syndrome eventually became known as Parkinson's disease (PD) and was named after James Parkinson [1][2]. PD is a chronic neurodegenerative disorder typically marked by a significant loss of dopaminergic neurons in the SNc region and the presence of Lewy bodies, which are abnormal protein aggregations containing α-synuclein and ubiquitin. These Lewy bodies are a major pathological feature of the disease [3]. The primary manifestation of this neurodegeneration is the development of abnormal motor symptoms. These symptoms, collectively referred to as parkinsonism or parkinsonian syndrome, include bradykinesia (slowness of movement), postural instability, muscle rigidity, resting tremors, and gait abnormalities [3]. Alongside these motor symptoms, non-motor symptoms may also occur, such as sleep disturbances, dementia, sensory and autonomic dysfunction, and various abnormalities like constipation, pain, depression, and loss of sense of smell.
Numerous research findings indicate that factors such as aging, genetics, and environmental influences interact and contribute to the prognosis of Parkinson's disease (PD), making it a multifactorial condition. Several underlying disease processes are involved, including disturbances in cellular proteostasis, mitochondrial dysfunction, neuronal inflammation, oxidative stress, and failures in lysosomal and autophagy mechanisms. PD can be categorized into sporadic or familial subtypes. Familial PD, which involves dopaminergic loss, is associated with mutations in specific genes, including SNCA (α-synuclein), Parkin/PARK2, ubiquitin carboxy terminal hydrolase-1 (UCHL1), PINK1, DJ-1/PARK7, and LRRK2 [4][5]. Normally, α-synuclein exists abundantly in different forms (oligomeric, monomeric, aggregated) within presynaptic terminals and functions to regulate synaptic vesicle transport and neurotransmitter release [6][7][8][9][10][11][12]. However, in PD, these proteins misfold and aggregate, leading to the formation of Lewy bodies/Lewy neurites, which gradually spread throughout the brain similar to prions. Various preclinical studies suggest that α-synuclein-induced inclusion toxicity plays a significant role in the death of dopaminergic neurons. Current treatment approaches involve the use of levodopa or other dopaminergic agonists to alleviate motor symptoms by restoring neurotransmission. However, these interventions often come with undesirable side effects and complications. Unfortunately, there are currently no treatments available that have demonstrated the ability to halt the neurodegenerative process in PD patients [3].
2. Parkinson’s Disease Model Systems
The use of experimental model systems in emulating the Parkinson's disease (PD) phenotype serves the purpose of exploring potential therapies, discovering new treatments, and gaining a deeper understanding of disease progression. These model systems provide a platform for identifying novel therapeutic targets for intervening in the disease. In recent years, researchers have made significant progress in elucidating the genetics, pathology, and heterogeneity of PD by employing various experimental models.
However, it should be noted that current PD models only partially capture the pathology of the disease. This may be attributed to the fact that ideal "model systems" should be capable of developing the disease pathology within a relatively shorter timeframe compared to the natural progression of PD in humans [13]. Given the variations and heterogeneity in the causes and origins of PD, efforts have been made to model the disease pathology by replicating α-synucleinopathy, genetic forms of PD, and dysfunction in midbrain dopaminergic neuronal signaling using toxin-induced or pharmacological interventions. These model systems often represent specific PD characteristics such as behavioral changes, alterations in electrical activity, and molecular or cellular changes [14].
PD experimental models can be classified into animal-based and cell-culture-based systems (Figure 1).
These models employ environmental or synthetic neurotoxins or genetics-based approaches to investigate the disease pathology. Each model group has its own advantages and disadvantages, and understanding the existing variations and their applications in studying PD helps researchers make informed decisions when selecting an appropriate model system for their specific experiments. Traditional toxin-based animal models of PD, which involve the destruction of dopaminergic neurons, have contributed to the development of treatments for PD symptoms and the exploration of potential adverse effects associated with dopamine replacement therapies. However, these models have not been able to modify, reduce, or reverse the course of the disease. Other model systems focusing on α-synuclein-induced dysfunction and neuronal death (rather than direct neuronal death) are closer to mimicking the chronic degenerative progression of the disease [1].
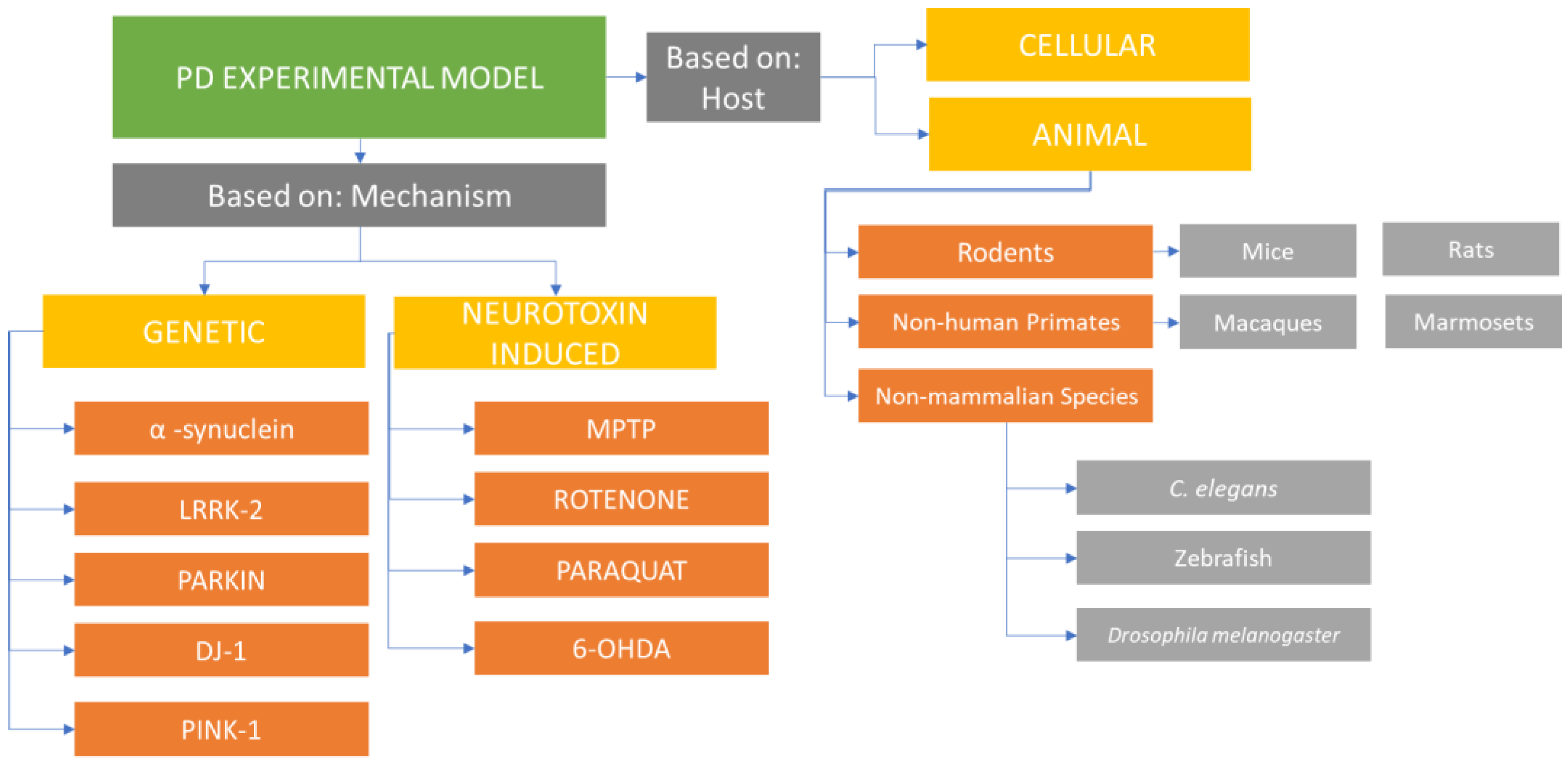
Cellular models of Parkinson's disease have been observed to successfully replicate key features such as degeneration of dopaminergic neurons and the presence of α-synuclein protein aggregates, which are characteristic of the disease. Compared to in vivo animal models, cellular models offer advantages in terms of cost-effectiveness, time efficiency, and ease of use. However, the selection of an appropriate model depends on the specific aspect of Parkinson's disease being studied [13].
In order to make informed decisions and obtain relevant outcomes, it is crucial to explore different experimental models and methods for inducing Parkinson's disease. Researchers need to consider which model best mimics the pathology of interest and aligns closely with the objectives of their investigation [15]. By carefully selecting the most appropriate model, researchers can enhance the relevance and applicability of their studies in understanding and developing treatments for Parkinson's disease.
3. Recent Development in PD Model System
Reproducibility is a crucial aspect in clinical research, and addressing this challenge is of utmost importance and efficiency. One approach to improve the quality of experiments in Parkinson's disease (PD) research is by implementing guidelines that govern the conduct of pre-clinical studies. Adhering to these guidelines can enhance reproducibility. Another approach involves hypothesis-driven research, which requires a comprehensive understanding of the basic biology and physiology of the human dopaminergic system at both behavioral and cellular levels [16].
When developing new model systems, it is important to achieve a decreasing level of α-synucleinopathy and aggregated α-synuclein, as these are crucial in identifying new therapies for PD. Measuring α-synuclein levels in blood can reflect its concentration in the brain and emphasize the need for quantifiable targets to monitor disease progression and clinical outcomes [16].
Recent technological advancements have addressed issues related to the availability of human neuronal cells and ethical concerns. The development of stem cell-derived midbrain dopaminergic progenitors has allowed PD investigations using cultured human neurons, providing a valuable tool for studying the disease [17][18]. Research goals in PD have shifted towards a focus on neuroprotection, aiming to develop better PD models. Dopaminergic neurons differentiated from induced pluripotent stem cells (iPSCs) derived from both sporadic and inherited PD patients successfully replicate the PD pathological environment, including increased stress, mitochondrial and synaptic abnormalities, and pathological protein accumulation [17][18].
Another significant development in PD modeling is the use of midbrain organoids. These organoids offer a more advanced modeling system by capturing the interactions between glial and neuronal cells [19][20][21]. Although midbrain organoids are a relatively recent development, they hold promise as a PD model. One drawback is the lack of established, robust protocols. While human organoids can be transplanted into the adult mouse brain, they have yet to provide the opportunity to study experimental neurorestorative treatments for the impaired motor phenotype. However, midbrain organoids offer an ideal environment for the growth and development of neuronal cells, making them a promising PD model [22]. This strategy has been successfully employed to develop "humanized brains chimeric" models [23].
In summary, addressing the challenge of reproducibility in PD research involves following guidelines for conducting pre-clinical studies, understanding the basic biology of the dopaminergic system, developing model systems that replicate PD pathology, and utilizing advancements such as stem cell-derived neurons and midbrain organoids. These approaches contribute to improving the quality and relevance of PD research.
References
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464.
- Parkinson, J. An Essay on the Shaking Palsy.1817. J. Neuropsychiatry Clin.Neurosci. 2002, 14, 223–236.
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet Lond. Engl. 2015, 386, 896–912.
- Gasser, T.; Hardy, J.; Mizuno, Y. Milestones in PD Genetics. Mov.Disord. 2011, 26, 1042–1048.
- Nalls, M.A.; Pankratz, N.; Lill, C.M.; Do, C.B.; Hernandez, D.G.; Saad, M.; DeStefano, A.L.; Kara, E.; Bras, J.; Sharma, M.; et al. Large-Scale Meta-Analysis of Genome-Wide Association Data Identifies Six New Risk Loci for Parkinson’s Disease. Nat. Genet. 2014, 46, 989–993.
- Larsen, K.E.; Schmitz, Y.; Troyer, M.D.; Mosharov, E.; Dietrich, P.; Quazi, A.Z.; Savalle, M.; Nemani, V.; Chaudhry, F.A.; Edwards, R.H.; et al. Alpha-Synuclein Overexpression in PC12 and Chromaffin Cells Impairs Catecholamine Release by Interfering with a Late Step in Exocytosis. J. Neurosci. 2006, 26, 11915–11922.
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased Expression of Alpha-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis. Neuron 2010, 65, 66–79.
- Scott, D.A.; Tabarean, I.; Tang, Y.; Cartier, A.; Masliah, E.; Roy, S. A Pathologic Cascade Leading to Synaptic Dysfunction in α-Synuclein-Induced Neurodegeneration. J. Neurosci. 2010, 30, 8083–8095.
- Scott, D.; Roy, S. α-Synuclein Inhibits Intersynaptic Vesicle Mobility and Maintains Recycling-Pool Homeostasis. J. Neurosci. 2012, 32, 10129–10135.
- Vargas, K.J.; Makani, S.; Davis, T.; Westphal, C.H.; Castillo, P.E.; Chandra, S.S. Synucleins Regulate the Kinetics of Synaptic Vesicle Endocytosis. J. Neurosci. 2014, 34, 9364–9376.
- Wang, L.; Das, U.; Scott, D.A.; Tang, Y.; McLean, P.J.; Roy, S. α-SynucleinMultimers Cluster Synaptic-Vesicles and Attenuate Recycling. Curr. Biol. CB 2014, 24, 2319–2326.
- Sun, J.; Wang, L.; Bao, H.; Premi, S.; Das, U.; Chapman, E.R.; Roy, S. Functional Cooperation of α-Synuclein and VAMP2 in Synaptic Vesicle Recycling. Proc. Natl. Acad. Sci. USA 2019, 116, 11113–11115.
- Falkenburger, B.H.; Saridaki, T.; Dinter, E. Cellular Models for Parkinson’s Disease. J. Neurochem. 2016, 139, 121–130.
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci.2016, 9, 503.
- Koprich, J.B.; Kalia, L.V.; Brotchie, J.M. Animal Models of α-Synucleinopathy for Parkinson Disease Drug Development. Nat. Rev. Neurosci. 2017, 18, 515–529.
- Airavaara, M.; Parkkinen, I.; Konovalova, J.; Albert, K.; Chmielarz, P.; Domanskyi, A. Back and to the Future: From NeurotoxinInduced to Human Parkinson’s Disease Models. Curr.Protoc.Neurosci.2020, 91, e88.
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Björklund, A.; et al. Human ESC-Derived Dopamine Neurons Show Similar Preclinical Efficacy and Potency to Fetal Neurons When Grafted in a Rat Model of Parkinson’s Disease. Cell Stem Cell 2014, 15, 653–665.
- Quick, J.; Grubaugh, N.D.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR Method for MinION and Illumina Sequencing of Zika and Other Virus Genomes Directly from Clinical Samples. Nat. Protoc. 2017, 12, 1261–1276.
- Galet, B.; Cheval, H.; Ravassard, P. Patient-Derived Midbrain Organoids to Explore the Molecular Basis of Parkinson’s Disease. Front. Neurol. 2020, 11, 1005.
- Smits, L.M.; Schwamborn, J.C. Midbrain Organoids: A New Tool to Investigate Parkinson’s Disease. Front. Cell Dev. Biol. 2020, 8, 359.
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s Disease in Midbrain-like Organoids. NPJ Park.Dis. 2019, 5, 5.
- Beal, M.F. Experimental Models of Parkinson’s Disease. Nat. Rev. Neurosci. 2001, 2, 325–334.
- Windrem, M.S.; Schanz, S.J.; Morrow, C.; Munir, J.; Chandler-Militello, D.; Wang, S.; Goldman, S.A. A Competitive Advantage by Neonatally Engrafted Human Glial Progenitors Yields Mice Whose Brains Are Chimeric for Human Glia. J. Neurosci. 2014, 34, 16153–16161.




