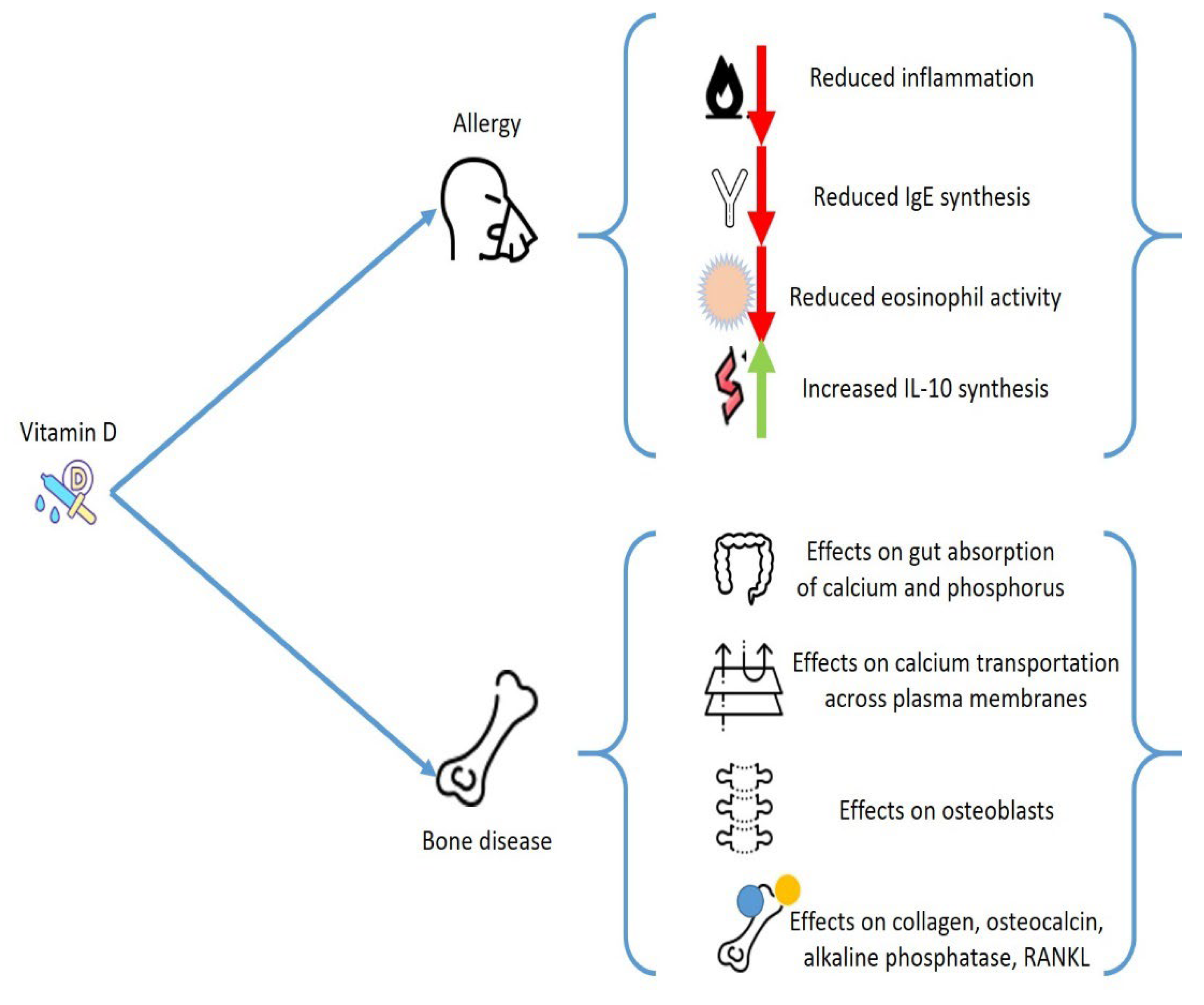
2. General Considerations on Vitamin D
Vitamin D3 (cholecalciferol) is mainly produced in the epidermis when pre-vitamin D3, the compound originating from 7-dehydrocholesterol after ultraviolet-B (UVB) irradiation of the skin, undertakes thermal isomerization. The transformation of vitamin D3 to its active form 1α,25(OH)2D3, is due to a sequence of hydroxylation procedures, initially caused by liver cytochrome P450 proteins, which can produce the transitional metabolite, 25OHD3 and, subsequently, 25-hydroxyvitamin 1αhydroxylase in the renal tubule to generate 1α,25(OH)2D3. This compound operates by joining to the vitamin D receptor (VDR), which provokes the enrolment of the retinoid X receptor, to generate a heterodimeric structure that affects vitamin D (VD) response factors in the promoter areas of genes. According to the concurrent joining of nuclear co-stimulators or coinhibitory factors, the complex can operate as a ligand-dependent stimulator or an inhibitor of gene transcription
[12][13][14].
VD deficit has been recognized as a main health problem, which is constantly more prevalent
[15]. Even though there is no agreement on the ideal concentrations of 25(OH)D, a VD deficit is identified when concentrations of VD less than 50 nmol/L occurs
[16].
All organs and cells feature a VDR, including immune and bone cells, skin, brain, gonads, and heart cells. A reduction on VD levels may change the activity of these tissues. This factor explains the extensive effects of VD and justifies why a decrease in VD levels has been related with several different chronic pathologies. Regarding the subject of analysis, VD has a main action in calcium/phosphate equilibrium and provokes profound consequences on the bone metabolism, beyond having a pivotal effect as an anti-inflammatory mediator. VD deficit increases the possible occurrence of osteoporosis and several other pathologies that present alterations of bone metabolisms, such as hematological malignancies, bone marrow transplantation, inflammatory bowel diseases, and endocrinological diseases
[17].
Furthermore, VD modifies relevant activities of the immune system and may change the development of immune-mediated diseases, including allergies and autoimmune diseases. VD operates by steering T lymphocytes to Th2 polarization and blocking Th1 and Th17 function and growth. The stimulation of T regulatory cells (Treg) might be its principal immunological function
[18]. Numerous reports indicate a VD favorable action on diseases correlated to hyperstimulation of Th1 cells, such as psoriasis, multiple sclerosis, rheumatoid arthritis, and type 1 diabetes
[19]. In allergic pathologies, where Th2 cells have a central effect, VD has a more complex effect. However, several experimentations displayed a positive action on the progression of allergic conditions, although the causal mechanisms have not always been fully explained
[20].
3. Vitamin D and Allergies
Epidemiological data have also indicated the existence of a correlation of VD reduction with several conditions including allergies. The relationship of VD deficit and asthma is described, and several studies stated a connection with disease course worsening and a worse outcome. It is possible that VD, by improving the inflammation state, can decrease the rate of respiratory sepsis and exacerbations
[21][22]. Although the correlation between VD concentrations and allergies has been questioned in a cross-sectional analysis including only allergic subjects
[23], an effect of VD in the eosinophil activities is demonstrated. VD decreases the immunoglobulin E (IgE) synthesis and increases expression of interleukin-10
[24].
Other studies confirmed the correlation of VD with allergic diseases
[25][26]. Some experimentations were conducted to evaluate the effect of VD in chronic urticaria (CU), allergic contact dermatitis (ACD), and atopic dermatitis (AD)
[27][28], and a relationship between VD deficit with ACD and AD severity was reported
[29][30], while a work proved a relevant difference between CSU subjects and controls in blood concentrations of VD
[31].
An interesting analysis has reported that the period of birth and UBV exposure is correlated to the incidence of food allergy (FA). The concentrations of VD generated from skin after UBV might justify this correlation, while maternal or VD deficiency occurring in the first years of life also remarkably predisposed the FA onset in an experimental animal model
[32][33].
Still in the framework of FA, employing results obtained from a nutritional investigation, it was stated that allergic sensitization to several different allergens, including foods, was more frequent in young subjects with a 25(OH)D deficit, while no relevant correlation was found between VD concentrations and FA in adults
[34]. Although, a different study performed by Beak et al. reported that reduced concentrations of VD might be correlated to polysensitization of nutrition allergens
[35]. Therefore, the correlation between VD and FA is still debatable.
However, other findings support the existence of a tight relationship between VD and allergies, and the beneficial action of VD supplementation in CSU subjects has been proved in several small clinical experimentations
[36][37]. In a prospective research, patients suffering from CSU were treated with low or high vitamin D3 supplement for 12 weeks. All patients demonstrated a significantly decreased urticaria severity score, but the reduction was greater in patients treated with a higher VD dosage at week six. The medication score was also remarkably decreased, without distinguishing the two groups
[38].
Some researches report in the table some of the works that confirm the existence of a relationship between VD levels and allergic diseases
[27][34][37][38][39][40][41][42][43][44][45][46], (
Table 1).
Table 1. Effects of Vitamin D on allergies.
4. Vitamin D and Immune Response
Several other relevant connections have been reported between blood cells, both lymphoid and myeloid cells, and VD concentrations. For instance, B cells can produce VD
[47], while naïve T cells grown with VD-primed B cells demonstrated decreased proliferation, provoked by the presence of CD86 on B cells
[48].
As reported above, VD has a main effect in IgE provoked responses, and it was displayed that IgE serum concentrations are enhanced in VDR-knockout animals
[49]. Similarly, VD reduced IgE generation by B lymphocytes, and the IgE reaction in a type 1 allergy animal model can be altered by employing a VDR agonist
[50].
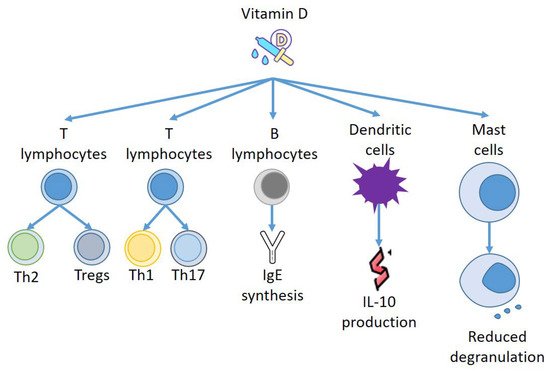
Furthermore, VD can act on other effectors of the immune system, and Szeles et al. have stated that an increase of 25(OH)D provokes dendritic cells (DCs) to switch on VD related genes, and stimulation of VDRs by VD resets the DCs to turn out to be tolerogenic
[51]. Remarkably, when VD was added to monocyte, DCs were less mature, presenting different amounts of MHC class II molecules and transforming CD4+ T cells to IL-10-producing Tregs
[52]. Thus, VD not only produces a repressive consequence on DC development but also instructs the DCs to stimulate Tregs to generate IL-10
[53]. Almerighi et al. have confirmed that VD reduces inflammation caused by CD40L and increases IL-10 generation by CD4+ T cells
[54], stimulating the expansion of Tregs presenting forkhead box P3 and cytotoxic T-lymphocyte-associated protein 4
[55] (
Figure 2).
Figure 2. Effects of Vitamin D on immune effectors.
5. Vitamin D and Mast Cells: Effects on Allergies
VD also seems to have a role in allergic diseases and bone pathologies mediated by Th2 cells, via the IL-31/IL-33 axis
[56], which is another new area to investigate the intricate overlapping mechanisms that connect allergies and osteoporosis. IL-31 is a cytokine that is able to provoke inflammation, which was proposed as a marker of tissue remodeling and different allergic and immunologic pathologies
[56]. It is released by Th2 cells and, in smaller amounts, also by DCs and MCs. IL-31 stimulates eosinophils and fibroblasts, and its receptor is present in skin and endothelium. It is correlated with the onset of itch and chronic skin inflammation. An increased amount of IL-31 was reported in the skin and serum of patients suffering from AD, CSU, contact dermatitis, prurigo nodularis, cutaneous lymphoma, and mastocytosis, correlating with disease severity
[57][58]. It is therefore interesting that increased concentrations of serum IL-31 are present in postmenopausal women with a reduction of bone mineral density (BMD), although there is no correlation with the degree of osteoporosis
[59].
Mast cells are myeloid cells that transfer into practically all tissues, where they perform tissue–specialized evolution. They are a constituent of connective tissue and are numerous in areas such as the gut, mucous membranes, and skin
[60][61], near to vessels and nerves
[62]. This tactical settlement and the mediators generated from MCs explain their ability to quickly modify their milieu, and to control other forms of inflammatory cells
[63][64].
MCs have a relevant effect in immediate hypersensitivity reactions, but also in late phase responses and innate immune response, by generating a large number of different mediators either from storage places in their granules or by delivering substances which are able to regulate different signaling pathways after adequate stimulation
[65].
In allergic reactions, IgE joins to the IgE receptor to produce complexes on the cellular membrane of MCs to induce MC sensitization. After new contact with particular antigens, they combine with the IgE/FceRI complex to stimulate MCs
[66]. Moreover, MCs also can be stimulated by temperature modifications and microbial factors
[67][68].
However, beyond allergic diseases, MCs are involved in the genesis of a massive variety of pathological conditions including chronic inflammation, autoimmunity, and cancer
[69].
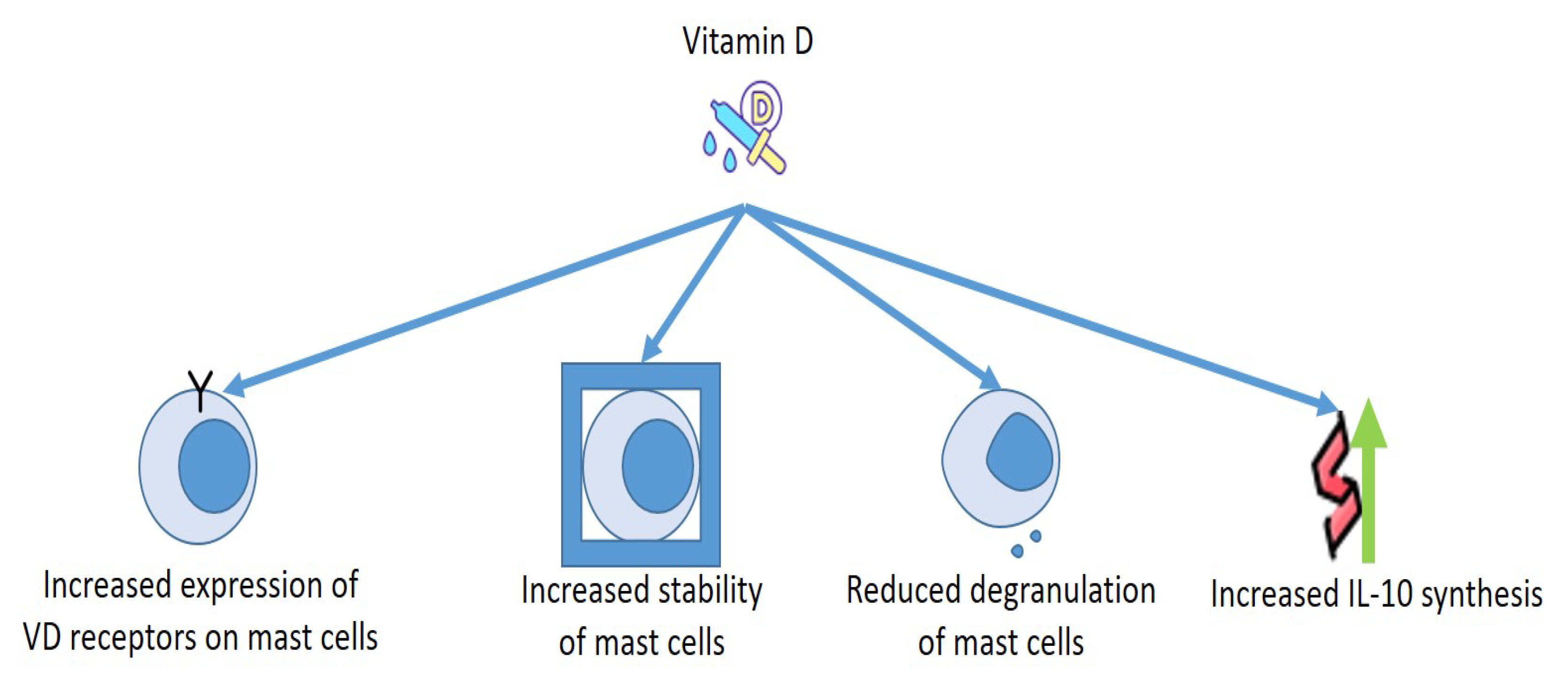
As for the relationship between MCs and VD, the cells discharged several mediators in a VD-lacking milieu without the need of any activators. Presence of calcitriol in the culture medium increased the number of VDRs in the MCs, and VDRs provoked complexes with Lyn in MCs to block the connection of Lyn to MyD88 and to the β chain of FcεRI, which diminished the amounts of NF-kB and MAPK and reduced the phosphorylation of Syk. Moreover, VDRs connected to the promoter of TNF-α reduce the acetylation of RNA polymerase II and histone H3/H4, reducing the production of TNF-α in MCs. These findings make evident that VD is necessary to preserve the steadiness of MCs, whereas the deficit of VD provokes the stimulation of MCs
[70][71].
This has been demonstrated under numerous experimental conditions. It is notorious that the release of granules and the discharge of histamine from MCs are involved in the genesis of urticaria
[72][73]. VD has been suggested for this therapy and for the one concerning other allergic pathology
[74][75], as MCs own the VDR capable of blocking degranulation of compounds provoked by IgE
[76].
Finally, it may be important to consider how the strong correlations between MCs and VD can aid to clarify some contradictory actions provoked by MCs. Although MCs were once believed to operate essentially as cells which were able to induce inflammation that can provoke allergic responses and inflammation induced by exogenic factors such as UV irradiation
[63][77], novel findings suggest that, in specific conditions, MCs can reduce cellular damage and decrease inflammation provoked by UVB irradiation
[78]. Even though most UVB rays do not enter the dermis, the UVB–supported stimulation of dermal MCs is believed to be obtained through a nerve growth factor, that originated from the epidermis
[79], or by nervous sensory C fibers that have been stimulated by cis-urocanic acid
[80]. This secondary mechanism of MC stimulation seems to participate in the UVB-provoked systemic immunosuppression in animals exposed to an acute single dose of UVB irradiation
[79]. The intimate mechanism of this condition could be constituted by the production of specific cytokines, including IL-10 (
Figure 3).
Figure 3. Effects of Vitamin D on mast cells.