Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio ZUORRO | -- | 4239 | 2023-06-01 12:59:55 | | | |
| 2 | Catherine Yang | Meta information modification | 4239 | 2023-06-02 03:22:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms as a Source of Antioxidant Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/45106 (accessed on 07 February 2026).
Rani A, Saini KC, Bast F, Mehariya S, Bhatia SK, Lavecchia R, et al. Microorganisms as a Source of Antioxidant Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/45106. Accessed February 07, 2026.
Rani, Alka, Khem Chand Saini, Felix Bast, Sanjeet Mehariya, Shashi Kant Bhatia, Roberto Lavecchia, Antonio Zuorro. "Microorganisms as a Source of Antioxidant Compounds" Encyclopedia, https://encyclopedia.pub/entry/45106 (accessed February 07, 2026).
Rani, A., Saini, K.C., Bast, F., Mehariya, S., Bhatia, S.K., Lavecchia, R., & Zuorro, A. (2023, June 01). Microorganisms as a Source of Antioxidant Compounds. In Encyclopedia. https://encyclopedia.pub/entry/45106
Rani, Alka, et al. "Microorganisms as a Source of Antioxidant Compounds." Encyclopedia. Web. 01 June, 2023.
Copy Citation
Microorganisms are a diverse group of microscopic organisms including archaea, bacteria, fungi, protozoa, algae, and viruses. Microbial diversity produces a massive pool of unique chemicals, which have become a valuable source for innovative biotechnology. Microorganisms can be used as a source of antioxidants with the advantage of fast growth under controlled conditions.
oxidative stress
natural antioxidant
astaxanthin
mycothiol
1. Actinomycetes
The actinomycetes are gram-positive, aerobic, filamentous, and spore-forming bacteria, with a superior reputation in producing different kinds of metabolites with a broad spectrum of biological activities, including antioxidant, antifungal, antibacterial, and insecticidal activities.
Mycothiol (MSH), a principal “sugar” thiol present in the cell wall of actinomycetes, serves as the glutathione (GSH) analogue. Actinomycetes lack the enzymes for GSH biosynthesis, but as a substitute, they utilize alternative low molecular weight thiols (LMW), such as bacillithiol (BSH) and MSH, ergothioneine (ESH) [1]. MSH maintains the intracellular redox homeostasis, allowing the appropriate working of many biological processes, counting enzyme activation, DNA synthesis, and cell-cycle regulation. MSH is a potent antioxidant, which acts as an electron donor/acceptor and assists as a cofactor in detoxifying free radicals, xenobiotics, and alkylating agents. Unlike GSH, MSH has two sugar components viz. N-glucosamine, inositol, and cysteine components as an alternative to the two amino acids, glycine, and glutamic acid [1].
1.1. Biosynthesis of MSH
MSH biosynthesis involves five enzymes, and the substrates myo-inositol-1-phosphate (Ins-P), UDP-N-acetyl glucosamine (UDP-GlcNAc), and Cysteine (Cys) represented in Figure 1. Firstly Ins-P is conjugated with UDP-GlcNAc to yield N-acetyl glucosamine myo-inositol-1-phosphate (GlcNAc-Ins-P) via enzyme glycosyltransferase MshA. The MSH phosphatase MshA2 dephosphorylated GlcNAc-Ins-P, which is further deacetylated by the metal-dependent deacetylase MshB generating glucosamine inositol [1-O-(2-amino-1-deoxy-a-Dglucopyranosyl)-d-myo-inositol]. The Cys ligase MshC inserted Cys to yield Cys-GlcN-Ins. Finally, the Cys amino group is acetylated by the MSH acetyltransferase MshD to produce MSH [2].
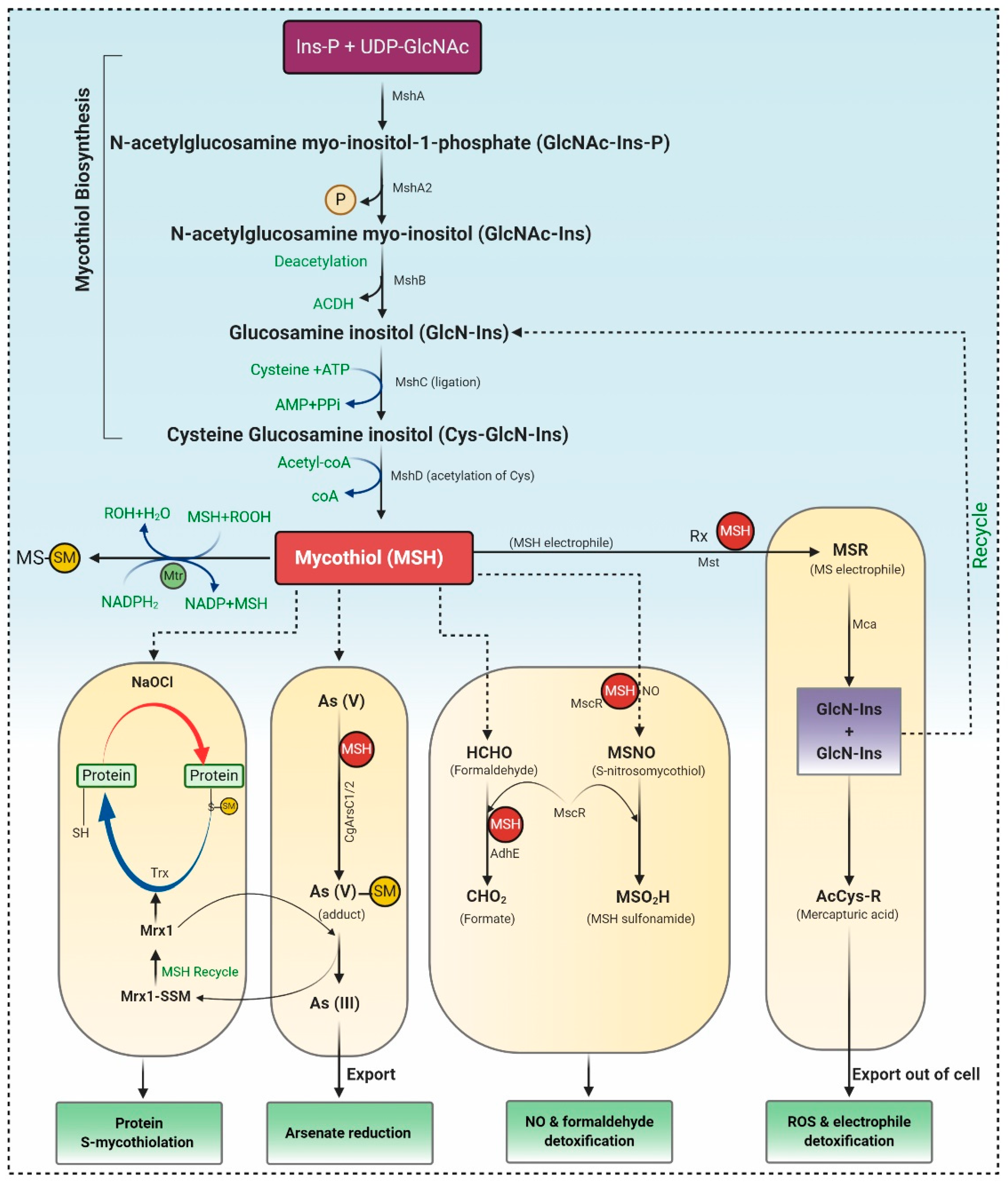
Figure 1. MSH biosynthesis and regulation in actinomycetes. Synthesis of MSH is catalyzed by five enzymes, including MshA, MshA2, MshB, MshC, and MshD. 1) Under Reactive Oxygen Species (ROS), MSH is oxidized to MSSM, which is further reduced by Mtr. 2) S-mycothiolation and protein regeneration occur via Mrx1 (mycoredoxin 1) /MSH/Mtr and thioredoxin/Thioredoxin reductase (Trx /TrxR) pathway. 3) Arsenate reductases CgArsC1/CgArsC2 along with MSH and Mrx1 reduced As (V) to As (III), which is exported through ABC transporter. 4) MSH acts as thiol cofactor for alcohol dehydrogenase MscR and formaldehyde dehydrogenase AdhE and is involved in NO and formaldehyde detoxification. 5) Mycothiol amidase (Mca) and mycothiol-S-transferases are involved in ROS and xenobiotic detoxification.
1.2. The Catalytic Action of the Mycothiol Disulfide Reductase (Mtr)
During the oxidative stress, MSH is oxidized to mycothiol disulfide (MSSM), which is further reduced by the NADPH-dependent Mtr. Mtr is the key enzyme involved in retaining MSH levels, which reduces the oxidized MSH disulfide. Mtr is a homodimeric flavoprotein disulfide isomerase that requires a cofactor, viz. FAD (flavin adenine dinucleotide). The oxidized Mtr (Mtrox) comprises a disulfide bridge between Cys39 and Cys44, which further reduces by accepting electrons from NADPH through FAD cofactor to produce reduced Mtr, as depicted in Figure 1. MSSM is confronted via the exchange of Cys39, resulting in the generation of Cys39-SSM, i.e., reduced MSSM and discharge of the first MSH moiety. Consequently, the disulfide bond in Cys39-SSM is condemned by the thiolate present at Cys44 forming Mtrox [3][4].
1.3. Mycothiol-Dependent Detoxification of Electrophiles
MSH S-transferases (Mst) conjugate with MSH electrophiles (RX), creating MS-electrophiles (MSR), MSH S-conjugate amidase (Mca) cleave an amide bond of MSR to form a glucosaminyl inositols (GlcN-Ins) and mercapturic acid (AcCys-R). AcCys-R or MSH-S-associates of antibiotics or toxins are expelled from cells via ABC transporters, whereas GlcN-Ins is salvaged back to mycothiol (Figure 1) [3][4].
1.4. Detoxification of Nitric Oxide (NO)
Detoxification of NO requires MSH-dependent enzyme nitrosothiol reductase (MscR) that exhibits S-nitrosomycothiol (MSNO) reductase activity, thereby generating MSH sulfonamide (MSO2H). Both MSH-dependent formaldehyde dehydrogenase AdhE and MscR oxidize formaldehyde to formate. In Corynebacterium’s glutamicum, maleylpyruvate is a ring fission output of gentisic acid and is converted to fumarylpyruvate through gentisate pathway by the MSH-dependent maleylpyruvate isomerase (Figure 1) [4][5].
1.5. Detoxification of Arsenate
Arsenate detoxification is catalyzed by the arsenate reductases (CgArsC1/CgArsC2), which associate arsenate As (V) to MSH, generating As (V)-SM adduct, that is later on reduced by mycoredoxin-1 (Mrx1), forming Mrx1-SSM intermediate and As (III). As (III) is disseminated from the cells using two arsenite permeases of the Acr3 family. However, Mrx1-SSM depends on MSH for the regenerating Mrx1 [3][6].
1.6. Protein S-Mycothiolation under NaClO and H2O2 Stress
Proteins are S-mycothiolated and recreated by the Mrx1/MSH/Mtr and Trx/TrxR pathways during NaOCl and H2O2 stress. These proteins control the activity of mycothiol peroxidase (Mpx), thioredoxin peroxidase (Tpx), and methionine sulfoxide reductase A (MsrA) in vitro [3]. Mpx and MsrA result in the formation of intramolecular disulfides and S-mycothiolations and involve both the Trx and Mrx1 pathways for reformation [7]. The Mrx1/Mtr/MSH pathways are likewise involved in reducing the peroxiredoxin AhpE in Mycobacterium tuberculosis (Figure 1). Therefore, it can be concluded that mycothiol is a promising and suitable candidate for antioxidant therapeutics. Only a few studies investigated the antioxidant compounds from actinomycetes. Yang et al. [8] reported the production of 1”-O-methyl-8-hydroxymethyl-daidzein, an isoflavone from Streptomyces sp. YIM 65408, with ROS scavenging activity. Zhou et al. [9] reported antioxidant, 2,6-dimethoxy terephthalic acid (IC50 4.61mg/mL) from endophytic Streptomyces sp. YIM66017.
Several compounds such as antiostatins A1 to A4 and B1 to B4 reported from S. cyaneus, and carbazoquinocins A to F isolated from S. violaceus, were composed of carbazole comprising an o-quinone. Neocarazostatins A to C purified from the mycelium of Streptomyces sp. confirmed antioxidant activity [10]. Therefore, carbazole compounds produced by Streptomyces sp. contributed to be the major class of antioxidants. Tan et al. [11][12] demonstrated antioxidant activity of Streptomyces sp. MUM212 and Streptomyces sp. MUM265, concluded that both strains contain phenolic compounds that inhibit lipid peroxidation and reduce ROS due to their hydrogen-donating and electron transferring capabilities. Praptiwi et al. [13] reported organofluorine, a potent antioxidant from Streptomyces sp. strain TC1. Therefore, it can be considered that microbes are a potential candidate for production of bioactive compounds.
2. Archaea
Archaea specifically grow in harsh environmental conditions such as significantly higher or lower temperature, pH, salinity, etc. Halophilic archaea are a more promising candidate for producing carotenoids, thereby they show red and orange colored colonies. A universal purpose of carotenoids is their antioxidant activity leading to the protection of cells against oxidative stress, thus benefiting human health. Most haloarchaea biosynthesized bacterioruberin (BR), a C50 carotenoid, and its precursor bis-anhydro bacterioruberin (BABR), 2-isopentenyl-3,4-dehydrorhodopin (IDR), and mono-anhydro bacterioruberin (MABR) are represented in Table 1 [14]. BR present in the cell membrane helps halo archaeal cells acclimatize to hypersaline environments, resulting in stabilizing the cell membrane under such stress. Rodrigo et al. [15] investigate and described the haloarcheal BR production and its applications in biomedicine. In contrast, Giani et al. [14] elaborated BR and its derivatives’ production at mid-and large-scale and discussed its recent biotechnology and biomedicine applications.
Table 1. Characterization of bacterioruberin, and its most abundant derivatives.
| Common Name | Molecular Formulae | Scientific Name | Producers | Mode of Action | References |
|---|---|---|---|---|---|
| Bacterioruberin (BR) | C50H76O4 | 2,2′-bis (3- hydroxy-3-methylbutyl)-3,4,3′,4′-tetradehydro-1,2,1′,2′-tetrahydro-γ,γ-carotene-1,1′-diol | Haloarcula japonica, Halobacterium salinarum, Halorubrum sodomense and Haloarcula vallimortis and Halorubrum sp. TBZ126 | Protection against oxidative stress by arachidonic acid and H2O2 | [14][15] |
| Mono-anhydrobacterioruberin (MABR) | C50H74O3 | 30-(2-hydroxypropan-2-yl)-2,6,10,14,19,23,27,33-octamethyl3-(3-methylbut-2-en-1-yl) tetratriaconta 4,6,8,10,12,14,16,18,20,22,24,26,28-tridecaene-2,33-diol | Haloferax volcanii | ROS scavenging activity | [14][15] |
| Bis-anhydrobacterioruberin (BABR) | C50H72O2 | 2,6,10,14,19,23,27,31-octamethyl3,30-bis (3-methylbut-2- en-1-yl)dotriaconta 4, 6, 8, 10, 12,14,16,18,20,22,24,26,28- tridecaene-2,31-diol | Haloferax volcanii | ROS scavenging activity | [15] |
BR acts as a ‘‘rivet” and affects membrane fluidity by imitating as a water barricade and permitting permeability to oxygen and other molecules [16]. In BR, 13 pairs of conjugated double bonds are present compared to the nine pairs of conjugated double bonds present in β-carotene. This transformation makes BR a better ROS scavenger as compared to β -carotene [17]. BR offers resistance to γ irradiation, intense light, and DNA damage caused by UV irradiation, radiography, and H2O2 exposure [18]. Carotenoids from Halorubrum sp. BS2 reported having extraordinary antioxidant capacity compared to ascorbic acid [19]. The antioxidant capabilities of carotenoids produced by Haloterrigena turkmenica, Haloferax volcanii, Halococcus morrhuae, Halogranum rubrum, and Halobacterium salinarum were significantly higher than β-carotene [14]. Hyperthermophilic archaea are furnished with a wide range of antioxidant enzymes that play a crucial role in protecting the living cells from oxidative damage. These enzymes, along with protein disulfide oxidoreductase, thioredoxin, and thioredoxin reductase, act as a core of the antioxidant system and maintain redox homeostasis. As demonstrated by recently published data, the mainstream of aerobic hyperthermophilic archaea utilizes peroxiredoxins (Prxs) and thiol-dependent peroxidases to scavenge peroxides. LMW thiols act as cofactors in the detoxification of xenobiotic compounds. Newton and Javor [20] reported γ-glutamylcysteine (γGC), the first LMW thiol isolated from halophilic archaeon (haloarchaeon) Halobacterium salinarum. Later on, it was reported from other haloarchaea, including Halobacterium saccharovorum, Haloarcula (Halobacterium) marismortui, Haloarcula californiae, Halococcus sp. LS-1), and Haloferax (Halobacterium) volcanii [21][22]. Bacterioferritin comigratory protein 1 (Bcp1) belongs to the Prx family, isolated from hyperthermophilic archaeon Sulfolobus solfataricus, which has been characterized as an antioxidant that inhibits H2O2-induced apoptosis in H9c2 rat cardiomyoblast cells [23]. A 22-kDa protein, structurally similar to a class of DNA-binding protein (Dps) was isolated from hyperthermophilic acidophile Sulfolobus solfataricus, demonstrated to protect nucleic acids by defending DNA from oxidative damage and eliminating constituents that contribute to the formation of hydroxyl radicals [24].
3. Bacteria
Bacteria are closely associated with all life forms on earth and possess the ability to produce ample extracellular metabolites. In bacteria, carotenoids are produced by the extremophiles, including Thermus filiformis, Micrococcus freudenreichii, Flavobacterium sp., Serratia marcescens, Agrobacterium sp., Pseudomonas aeruginosa, Rheinheimera sp., and Chromobacterium sp. Thermophilic bacterium produces all-trans-zeaxanthin, zeaxanthin monoglucoside, thermobiszeaxanthins, and thermozeaxanthins [12]. Carotenoid ingestion might decrease the threat of diseases linked with oxidative stress; therefore, it acts as a proficient scavenger of ROS, RNS, singlet oxygen species (1O2), and nonbiological radicals [25].
Correa et al. [26] analyzed that when Antarctica bacteria belonging to the Pedobacter genus were exposed to cold temperatures and high UV radiation, they had developed an important antioxidant system and produce a variety of pigments that belongs to the carotenoids group and are capable of preventing oxidative damage. The antioxidant capacity of a mix of pigments viz. yelcho2, β-Carotene, and α-Tocopherol, was analyzed using three different methods viz. DPPH, ROS detection, and an oxygen electrode. In December 1988, the Marine Biotechnology Institute Co., Ltd. (MBI, Kamaishi, Japan) was established and began the isolation of novel or rare marine bacteria; many among them have been revealed to yield dicyclic or monocyclic C40 carotenoids, along with several acyclic C30 carotenoids [27]. MBI reported that Paracoccus sp. strain N81106 [28], Brevundimonas sp. strain SD212 [29], and Flavobacterium sp. PC-6 [30] produces astaxanthin glucoside, 2-hydroxyastaxanthin and 4-ketonostoxanthin 3′-sulfate, respectively. These are novel dicyclic C40 carotenoids with β-carotene (β, β-carotene) skeleton. The carotenoid biosynthesis gene cluster responsible for astaxanthin manufacturing was reported from the marine bacterium Agrobacterium aurantiacum [9]. This carotenoid gene cluster consists of five carotenogenic genes viz. crtB, crtW, crtZ, crtY, and crtI with the same orientation. Mishra et al. [31] investigated the capability of a semiquinone glucoside derivative (SQGD) from a Bacillus sp. INM-1 towards SOD, catalase, GSH, GST antioxidant enzymes. There was a significant increase in SOD (35%) activity and GST level (0.46 ± 0.03 μmol/min/mg of protein) in mice’s kidney after SQGD treatment compared to untreated control mice within 12–72 h.
Probiotics contain billions of bacteria that are capable of fermenting foods and beverages and are also used as a supplement due to their numerous beneficial activities including antioxidant properties. They accumulate in the GI tract and produce metabolites, which possess antioxidant activities. Probiotic bacteria have unique antioxidant enzyme systems that stimulate the host antioxidant system. Research on pigs exposed that dietary Lactobacillus fermentum elevated the serum SOD, glutathione peroxidase (GPx) and hepatic CAT, and Cu and Zn-SOD than the control group. Aarti et al. [32] reported the hydrogen peroxide resistant activity of Lactobacillus brevis LAP2, isolated from Hentak, a fermented fish food from Manipur, India. Son et al. [33], isolated Lactobacillus plantarum Ln4 and G72, from kimchi, exhibited (40.97%) DPPH, ROS scavenging, and β-carotene oxidation-inhibitory activities (38.42%). Another strain, SC61 of Lactobacillus plantarum, isolated by Son et al. [34] from jangajji, a Korean traditional fermented food, expresses a high level of IL-1β, IL-6, and TNF-α. These studies did not explore how the probiotic bacteria communicate with the signaling pathways, which is elaborated in Table 2 and Figure 2. In 2018, Ayyanna and colleagues assessed probiotic strains Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 for a significant decrease in rat paw edema, induced by Freund’s adjuvant and carrageenan. This decrease in inflammation is due to the high expression of IL-10, an anti-inflammatory cytokine, inhibiting prostaglandins synthesis, thereby preventing autoimmune and inflammatory diseases [35].
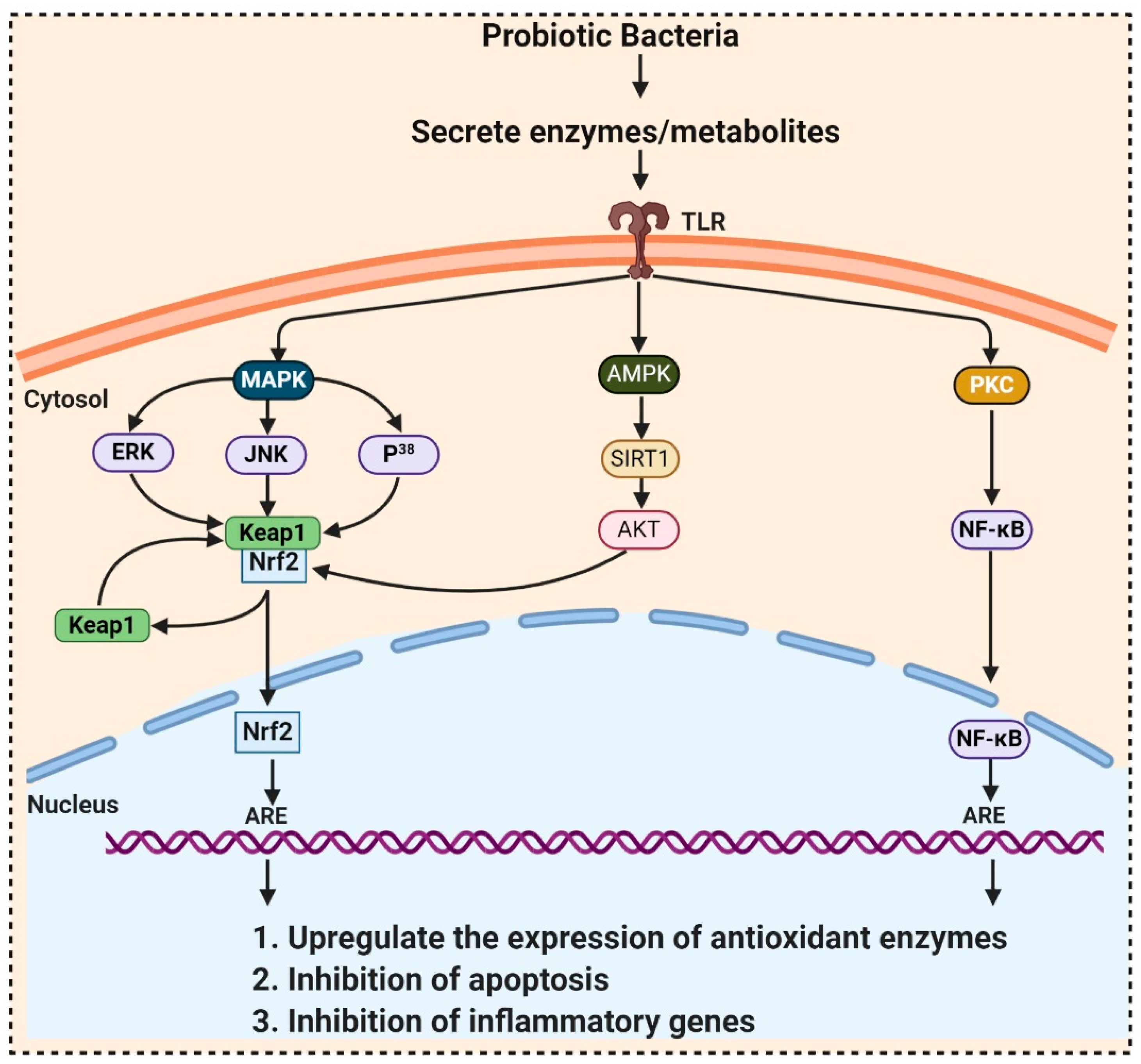
Figure 2. Probiotic activates metabolites and enzymes that enter the cell through toll-like receptors (TLR) and induced Nrf2-Keap1-ARE pathway. During ROS attack, the redox-sensitive cysteine residues of Keap1 react and disrupt the functional conformation of Keap1, thereby activating Nrf2. Nrf2 translocates towards the nucleus and binds to antioxidant response element (ARE) sequences, activating the transcription of ARE-driven genes, encoding antioxidant enzymes, and detoxifying proteins. ROS mediates the expression of redox-sensitive transcription factor NFκB and further expression of inflammatory cytokines. ROS also activates SIRT1 (Silent information regulator T1), mediated Adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway.
Table 2. Some antioxidant probiotic bacteria with the signaling pathways used to activate antioxidant enzymes and molecules.
| Probiotic Bacteria | Pathway | Model Used | Effects | References |
|---|---|---|---|---|
| Probiotic Formulation SLAB51, Bacillus. Longum, Lactobacillus acidophilus | Sirtuin-1 (SIRT1) | Alzheimer’s disease (AD) (3xTg-AD) mice model |
Increases antioxidant enzymes activity | [36] |
| L. plantarum LP6 L. plantarum C88 L. rhamnosus GG |
PKC (protein kinase C) | Caco-2 cells Caco-2 cell Caco-2 cell |
Enhanced activities of SOD and CAT Inhibit malondialdehyde (MDA) formation, raised SOD Ameliorate the oxidative stress-induced disruption of intestinal epithelial tight junction |
[37][38] [39] |
| L. brevis SBC8803 | Integrin–p38 MAPK (mitogen activated protein kinase) | Caco2/BBE cells | Induce cytoprotective heat shock proteins (HSP27) maintaining intestinal homeostasis | [40] |
| L. plantarum, L. rhamnosus, Bacillus subtilis JH642 |
p38 MAPK | RAW 264.7 cells Caco2bbe cells |
Decreases p38, JNK, ERK1/2 phosphorylation Increases p38 phosphorylation |
[41] [42] |
| Bacillus amyloliquefaciens SC06 Clostridium butyricum MIYAIRI 588 L. Plantarum FC255 |
Nrf2-Keap1-ARE | IPEC-1 cell line rat smice |
Increase CAT and glutathione S-transferase (GST) expressions | [43] |
| Activation of ARE-dependent genes i.e., GSTs, and TRX Elevate the activities of SOD and GPX |
[44] | |||
| [45] | ||||
| Lactobacillus caseiBacillus LBP32 L. plantarum CLP-0611 |
NF-κB | mice RAW 264.7 macrophages mice |
Reduce cytokine production Reduce LPS-induced intracellular ROS accumulation Induced expression of M2 macrophage |
[46] [47] [48] |
Kullisaar et al. [49] reported that Lactobacillus fermentum E-3 and E-18 express Mn-SOD that resists oxidative stress. Probiotic bacteria’s ability to locally deliver SOD exposed a novel perspective to bowel diseases caused by ROS production [49]. LeBlanc and colleagues’ demonstrated that the engineered Lactobacillus casei BL23 produce SOD when given to mice with Crohn’s disease and mice showed a quicker recovery from weight loss, improved gut enzyme activities, and a lesser extent of intestinal inflammation than the control mice [50]. CAT also participates in cellular antioxidant defense by decomposing H2O2, but LAB is usually CAT-negative. LeBlanc et al. [51] evidenced that Lactococcus slactis produced CAT and prevented 1,2-dimethylhydrazine (DMH)-induced colon cancer in mice. It was also proved that engineered Lactobacillus casei BL23 produce CAT and decrease or prevent the brutality of intestinal pathologies instigated by ROS. An in vitro study by Wang et al. [43] reported that Bacillus amyloliquefaciens SC06 raised CAT and GST gene expressions and activity in intestinal porcine epithelial cells-1 (IPEC-1). It is still doubtful whether those in vitro and in vivo results are conveyable to humans because maximum probiotics cannot be colonized in the gut and are eliminated immediately after consumption.
4. Fungi
Whenever there is a discussion about fungal metabolites, the story of penicillin has been told many times. Alexander Fleming, in 1929 discovered that mold juice’ from Penicillium notatum had antibacterial action and named this biological activity “Penicillin” [52]. In the past few years, researchers instigated to look for a fungal strain that could be grown quickly and produce bioactive metabolites in submerged culture. Later on, Penicillium chrysogenum was selected for large-scale production of Penicillium [35]. A wide range of primary and secondary metabolites including flavonoids, phenols, steroids, alkaloids, xanthones, etc. produced by the most diverse group of fungi, have antioxidant activities, and are highly exploited by the therapeutical, pharmacological, and medicinal sector. Maximum therapeutical and pharmaceutical investigations focused on the species belonging to Ascomycota and Basidiomycota [53], which include endophytic fungi, exhibiting higher antioxidant potential. A wide range of natural products including antioxidants, anticancerous, antiviral, anti-insecticidal, immune-suppressant, antimycobacterial, antimicrobial, and antimalarial, has been reported from endophytic fungi [34].
The discovery of antioxidant compounds, pestacin and isopestacin from Pestalotiopsis microspore, has opened a new direction towards investigating the antioxidant potential of endophytic fungi [32][54]. Zeng et al. [55] explored 49 endophytic fungi belonging to the family Xylariaceae, for in-vitro antioxidant activities. Among them, endophytic fungi from Scapania verrucosa were reported as a budding and novel source of natural antioxidants. Prihantini et al. [56] isolated seven fungal strains of the genus Pestalotiopsis from the leaves and stems of Elaeocarpus sylvestris. Pestalotiopsis sp. EST 02 and Pseudocercospora sp. ESL 02 possesses terreic acid and 6-methylsalicylic acid, having antioxidant activity. Nuraini et al. [57] reported that Aspergillus minisclerotigens AKF1 and Aspergillus oryzae exhibited antioxidant ability with an IC50 142.96μg/mL and 145.01μg/mL, respectively. GC-MS analysis confirmed that the most active compound isolated from AKF1 and DK7 was dihydropyran and 4H-Pyran-4-one,5-hydroxy-2-(hydroxymethyl-(CAS) Kojic acid, respectively.
Exopolysaccharides and flavonoids produced by Fusarium oxysporum isolated by Caicedo et al. [58] also exhibit such properties. Methanolic extracts from Monascus purpureus, OYRM1, P. 32783, P. salmoneo-stramineus, and T. versicolor inhibit linoleic acid oxidation by at least 53% [59]. Total 42 fungal endophyte strains related to a medicinal plant, Nerium oleander L. (Apocynaceae) were isolated and screened for antioxidant and antimicrobial capacity. Out of these 42 isolated only Chaetomium sp. inhibit xanthine oxidase activity with an IC50 of 109.8 lg/mL, had an antioxidant activity with the highest level of phenolics [60].
5. Microalgae
More than 28,000 potential compounds are reported from microalgae, including hundreds of new compounds discovered every year [61]. Most of them have been described from Chordata (including ascidians) and Porifera (sponges), but they are hard to cultivate, and to attain a sustainable supply of the compounds of interest is problematic. In recent times, high interest is paid in analyzing microalgae’s biotechnological potential as they have short generation time, easier to cultivate, and signify an ecofriendly approach but a less explored resource for drug discovery. Microalgae are photosynthetic eukaryotes comprising one of the key members of freshwater and marine phytoplankton and are exceptional cradles of pigments, carotenoids, ω-3 fatty acids, lipids, sterols, and other biomolecules as depicted in Figure 3 [43]. More than 7000 species are enlisted as “Green microalgae” and they all grow in diverse habitats. Among them, Haematococcus pluvialis is the most important commercial microalgae and is also the richest source of natural astaxanthin which is regarded as “super anti-oxidant” [49]. Apart from β-carotene, astaxanthin has received considerable attention lately. Astaxanthin (3,3’-dihydroxy-β,β’-carotene-4,4’ -dione), a marine xanthophyll carotenoid was first isolated by Kuhn and Soerensen from a lobster [62]. Astaxanthin was initially used as a colorant for aquaculture, besides being approved as a coloring agent for food supplements since 1991. Due to emerging health benefits, demand for astaxanthin has increased rapidly in medicine, food industries, cosmetics, etc. In 2016 market share of astaxanthin was USD 512.8 million, which increased at a CAGR of 6.73% by 2017 and was expected to reach USD 814.1 million and USD 2.57 billion by 2022 and 2025, respectively, worldwide [63][64]. The same is the case with carotenoids, whose market demand was 1.24 billion USD (B$) in 2015 and is expected to rise to 1.6 B$ by 2023 with a CAGR of 3.5%. The demand for lutein in 2015 was 135 M$, which might escalate up to 200 M$ by 2024, with a CAGR of 5.3%. The hike in CAGR is due to the increasing demand for lutein-rich dietary supplements [65].
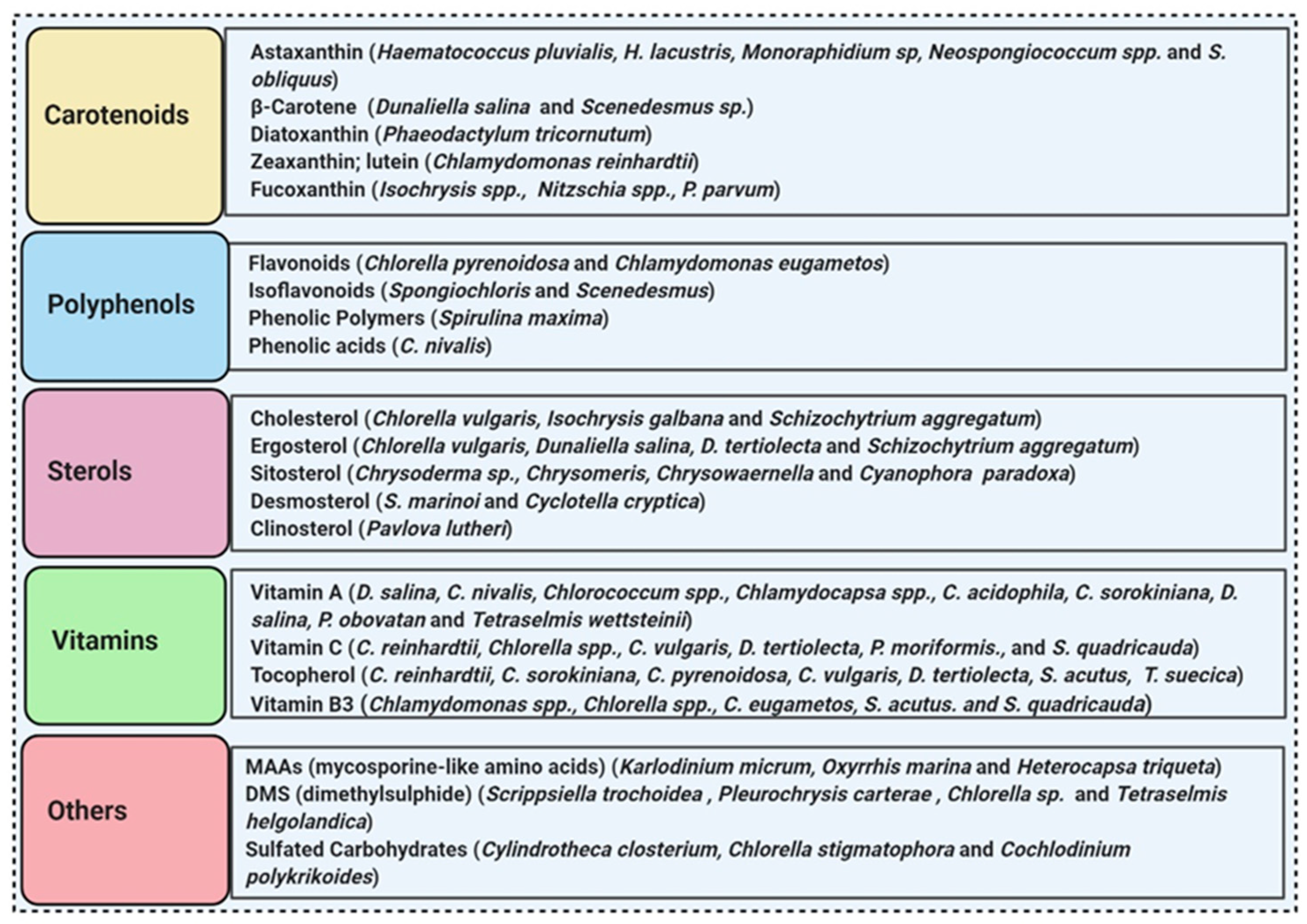
Figure 3. Some antioxidant compounds reported from microalgae.
Polyene chain along with conjugated double bonds permit astaxanthin to pass through the lipid bilayer and allow the quenching of singlet oxygen and ROS removal [49][66].
The presence of hydroxyl and keto moieties on each ionone ring provides a unique structure to astaxanthin, permitting it to pass through the lipid bilayer membrane and allowing it to protect the cell membrane [49]. Unlike many antioxidants, which quench and scavenge ROS and other free radicals including, superoxide anion, hydrogen peroxide, singlet oxygen, etc.) either in the inner (e.g., vitamin E and β-carotene) or the outer side of the bilayer membrane (e.g., vitamin C), astaxanthin can act as both the inner and outer layers of the cellular membrane [67]. Many studies demonstrated that astaxanthin had 500 times higher antioxidant capacity than vitamin E, 65 times more potent than vitamin C, 54 times stronger than β-carotene, and ten times more potent than β-carotene, zeaxanthin, lutein, canthaxanthin. At present, approximately 95% of synthetic astaxanthin is available in the market, whereas only <1% natural astaxanthin derived from H. pluvialis is commercialized [68]. Astaxanthin upregulates the expression of Nrf2, whereas it simultaneously downregulates the Kelch-like ECH-associated protein-1 (Keap1) expression. In the presence of oxidative stress, Nrf2 dissociates from Keap1, translocates into the nucleus, binds to the ARE, and induces the expression of Phase II enzymes, as shown in Figure 4. Astaxanthin prevents lipid peroxidation by activating antioxidant enzymes involved in lipid metabolism, namely thioredoxin reductase (TrxR) and paraoxonase-1 [69][70][71]. Pongkan et al. [72] depicted higher levels of mtROS production, mitochondrial depolarization, and swelling in mitochondria isolated from the ischemic myocardium of mice astaxanthin treatment decreases mtROS production, mitochondria depolarization, and swelling.
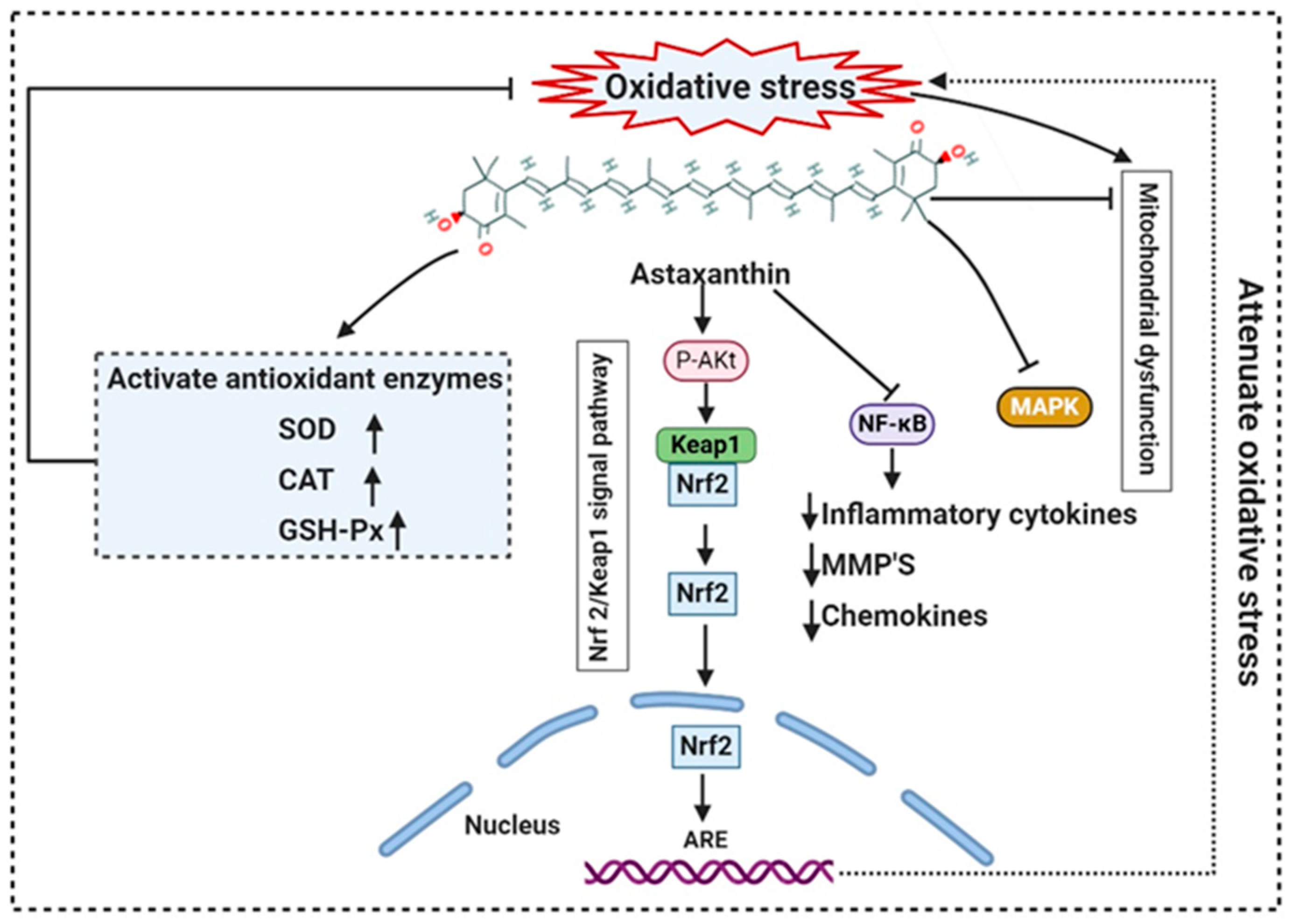
Figure 4. Mode of action of astaxanthin by inhibiting oxidative stress-induced mitochondrial dysfunction and activating transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2). Astaxanthin suppresses nuclear expression of NF-κB and MAPK to reduce downstream production of proinflammatory cytokines. NF-κB: Nuclear factor-κB; MMP: matrix metallopeptidase; MAPK: mitogen-activated protein kinase; ARE: Antioxidant response elements.
Synthetic astaxanthin, synthesized through Wittig reaction between asta-C15 -triarylphosphonium salt and the C10-dialdehyde [73], but it has 20 times lesser antioxidant capacity as compared to its natural counterpart, and until today it has not been permitted for human consumption [74]. However, H. pluvialis has been approved as a color additive and dietary-supplement in the USA, Japan, and some European countries. Astaxanthin from H. pluvialis had been granted “GRAS” status (Generally Recognized As Safe) by US FDA (Food and Drug Administration) [75]. The European Food Safety Authority (EFSA) recommended the use of 0.034 mg/kg of the bodyweight of astaxanthin. Spiller and Dewell [76] reported that 2–4 mg of a daily dose of astaxanthin is safe whereas not toxicity was reported up to 6mg/day consumption. Recently, AIgatechnologies, Mera Pharmaceuticals Inc, Cyanotech Corporation, Fuji Chemical Industry Co. Ltd., etc. are involved in the commercial production of astaxanthin from H. pluvialis. Its global market is predicted to GRASP $2.57 billion by 2025 [63]. L-Ascorbic acid (vitamin C), present both in cytosol and chloroplast, reduce many ROS and act as a scavenger for hydroxyl radicals, superoxide, and lipid hydroperoxides. The maximum concentration of vitamin C was reported from Chlorella sp. [36] and Dunaliella sp. [37], Skeletonema costatum, and Chaetoceros calcitrans. Glutathione (GSH), a tripeptide (Glu-Cys-Gly), acts as a sequence breaker of ROS reactions and ascorbate regeneration. α-tocopherol is the most active antioxidant synthesized in the chloroplasts of Dunaliella tertiolecta and Tetraselmis suecica. The lipophilic carotenoids, synthesized in diatoms and dinoflagellates allow quenching of singlet oxygen [77], leading to the formation of a carotenoid triplet state (Equation (1)).
1O2 * + CAR → 3O2 + 3CAR*
Lutein is a xanthophyll that defends long-chain polyunsaturated fatty acids. Muriellopsis sp. accumulate high lutein levels, whereas, in Scenedesmus, lutein and β-carotene production are accelerated by increasing both the pH and temperature throughout the cultivation [38]. The commercial source of lutein is Muriellopsis sp.
β-carotene, isolated from the Dunaliella salina, is commercially produced for its antioxidant properties. Marennine is isolated from the marine diatom Haslea ostrearia also has antioxidant and antimicrobial activities [78]. Apart from diatoms, additional microalgae, including flagellates, dinoflagellates, and green algae, have also been scrutinized for potential biotechnological applications [79]. Carotenoid, fucoxanthin reported from brown seaweeds, and some diatom species have antioxidant, antidiabetic, antiangiogenic, anti-inflammatory, anticancer, and antimalarial activities. Methanolic extract of Spirulina maxima, and Chlorococcum minutum NIOF17/002 exhibit antioxidant activity with the IC50 of 0.18 mg/mL and 3.92 to 9.83 mg/mL of ascorbic acid, respectively [80][81].
6. Protozoa
Protozoa are regarded as the first and simplest organism of the animal kingdom discovered by Anton van Leeuwenhoek. Most protozoan species are unicellular, free-living, motile, and microscopic, whereas some species are mutualistic and parasitic. More than 10,000 protozoan species are described as parasitic [82]. Entamoeba histolytica, Trypanosoma, Giardia, Leishmania, Trichomonads, and Plasmodium are usually anaerobic but sometimes found to be microaerobic or microaerophilic and produce H202 as a product of metabolism. Some intracellular protozoans are manifest to the toxic oxygen metabolites produced by the host’s effector immune cells. Protozoa either lack or deficient in antioxidant enzyme systems including CAT, GSH, and SOD, necessary for the detoxification of H2O2. However, they have alternative detoxification mechanisms. A higher concentration of NADH-dependent oxidase and a lower concentration of NADH-dependent peroxidase activities were perceived to accomplish this task. Entamoeba histolytica, Trichomonas vaginalis, and Tritrichomonas foetus were reported to encompass NADH oxidase activity. Since protozoa are known to be parasitic, very few reports are available regarding their antioxidant activity. Mastronicola et al. [83] explored the antioxidant defense systems in Giardia intestinalis, which lacks conventional antioxidant enzymes; despite this, it can rely on a well-organized antioxidant system to survive the nitrosative and oxidative stress conditions. Joardar & Babu [84] reviewed the most recent work on parasitic thiol-based enzymatic antioxidant thioredoxin reductase (TrxR), and discuss how to treat filariasis, helminths, and other parasitic infections. Jeelani and Nozaki [85] reported that Entamoeba histolytica, an intestinal parasite, causes amebic dysentery, lacks eukaryotic antioxidative defense systems but owns an efficient thioredoxin system, composed of thioredoxin and thioredoxin reductase, both are crucial for antioxidant activity and sustaining cellular redox balance [85].
References
- Quach, N.T. Functional Analyses of Thiol Switches and Their Impact on the Mycothiol Redox Potential in Actinomycetes. Ph.D. Thesis, Freie University of Berlin, Berlin, Germany, 2020.
- Fan, F.; Vetting, M.W.; Frantom, P.A.; Blanchard, J.S. Structures and mechanisms of the mycothiol biosynthetic enzymes. Curr. Opin. Chem. Biol. 2009, 13, 451–459.
- Loi, V.V.; Rossius, M.; Antelmann, H. Redox regulation by reversible protein S-thiolation in bacteria. Front. Microbiol. 2015, 6, 187.
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145.
- Venkataraman, K.; Raghunathan, C. Coastal and Marine Biodiversity of India. Mar. Faunal Divers. India 2015, 303–348.
- Kumar, V.; Tiwari, S.K. Halocin HA1: An archaeocin produced by the haloarchaeon Haloferax larsenii HA1. Process. Biochem. 2017, 61, 202–208.
- Tossounian, M.-A.; Pedre, B.; Wahni, K.; Erdogan, H.; Vertommen, D.; Van Molle, I.; Messens, J. Corynebacterium diphtheriae Methionine Sulfoxide Reductase A Exploits a Unique Mycothiol Redox Relay Mechanism. J. Biol. Chem. 2015, 290, 11365–11375.
- Yang, X.; Yang, Y.; Peng, T.; Yang, F.; Zhou, H.; Zhao, L.; Xu, L.; Ding, Z. A New Cyclopeptide from Endophytic Streptomyces sp. YIM 64018. Nat. Prod. Commun. 2013, 8, 1753–1754.
- Zhou, H.; Yang, Y.; Peng, T.; Li, W.; Zhao, L.; Xu, L.; Ding, Z. Metabolites of Streptomyces sp., an endophytic actinomycete from Alpinia oxyphylla. Nat. Prod. Res. 2014, 28, 265–267.
- Kobayashi, M.; Kuzuyama, T. Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes. Biomolecules 2020, 10, 1147.
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a Source of Antioxidants with Radical Scavenging and Metal Chelating Properties. Front. Pharm. 2017, 8, 276.
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Yin, W.-F.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol. 2019, 19, 1–16.
- Praptiwi; Fathoni, A.; Putri, A.L.; Wulansari, D.; Agusta, A. (Eds.) Assessment of actinomycetes isolated from soils on Simeuleu Island as antibacterial and antioxidant. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019.
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524.
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.-J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532.
- Madern, D.; Zaccai, G.; Camacho, M.; Rodríguez-Arnedo, A.; Bonete, M.-J. Salt-dependent studies of NADP-dependent isocitrate dehydrogenase from the halophilic archaeon Haloferax volcanii. Extremophiles 2004, 8, 377–384.
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 100.
- Han, H.; Yılmaz, H.; Gülçin, I. Antioxidant Activity of Flaxseed (Linum usitatissimum L.) shell and Analysis of Its Polyphenol Contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402.
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Ghennai, O.; Bonete, M.-J.; León, R.; Kharroub, K. Bioprospecting and characterization of pigmented halophilic archaeal strains from Algerian hypersaline environments with analysis of carotenoids produced by Halorubrum sp. BS2. J. Basic Microbiol. 2020, 60, 624–638.
- Newton, G.L.; Javor, B. gamma-Glutamylcysteine and thiosulfate are the major low-molecular-weight thiols in halobacteria. J. Bacteriol. 1985, 161, 438–441.
- Rawat, M.; Maupin-Furlow, J.A. Redox and Thiols in Archaea. Antioxidants 2020, 9, 381.
- Pedone, E.; Fiorentino, G.; Bartolucci, S.; Limauro, D. Enzymatic Antioxidant Signatures in Hyperthermophilic Archaea. Antioxidants 2020, 9, 703.
- Sarcinelli, C.; Fiorentino, G.; Pizzo, E.; Bartolucci, S.; Limauro, D. Discovering Antioxidant Molecules in the Archaea Domain: Peroxiredoxin Bcp1 from Sulfolobus solfataricus Protects H9c2 Cardiomyoblasts from Oxidative Stress. Archaea 2016, 2016, 1–10.
- Wiedenheft, B.; Mosolf, J.; Willits, D.; Yeager, M.; Dryden, K.A.; Young, M.; Douglas, T. From The Cover: An archaeal antioxidant: Characterization of a Dps-like protein from Sulfolobus solfataricus. Proc. Natl. Acad. Sci. USA 2005, 102, 10551–10556.
- Subramanian, D.; Kim, M.-S.; Kim, D.-H.; Heo, M.-S. Isolation, Characterization, Antioxidant, Antimicrobial and Cytotoxic Effect of Marine Actinomycete, Streptomyces Carpaticus MK-01, against Fish Pathogens. Braz. Arch. Biol. Technol. 2017, 60.
- Correa-Llantén, D.N.; Amenábar, M.J.; Blamey, J.M. Antioxidant capacity of novel pigments from an Antarctic bacterium. J. Microbiol. 2012, 50, 374–379.
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698.
- Yokoyama, A.; Adachi, K.; Shizuri, Y. New Carotenoid Glucosides, Astaxanthin Glucoside and Adonixanthin Glucoside, Isolated from the Astaxanthin-Producing Marine Bacterium, Agrobacterium aurantiacum. J. Nat. Prod. 1995, 58, 1929–1933.
- Yokoyama, A.; Miki, W.; Izumida, H.; Shizuri, Y. New Trihydroxy-keto-carotenoids Isolated from an Astaxanthin-producing Marine Bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 200–203.
- Yokoyama, A.; Izumida, H.; Shizuri, Y. New Carotenoid Sulfates Isolated from a Marine Bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 1877–1878.
- Mishra, S.; Reddy, D.S.K.; Jamwal, V.S.; Bansal, D.D.; Patel, D.D.; Malhotra, P.; Gupta, A.K.; Singh, P.K.; Jawed, S.; Kumar, R. Semiquinone derivative isolated from Bacillus sp. INM-1 protects cellular antioxidant enzymes from γ-radiation-induced renal toxicity. Mol. Cell. Biochem. 2013, 379, 19–27.
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT 2017, 86, 438–446.
- Son, S.-H.; Jeon, H.-L.; Jeon, E.B.; Lee, N.-K.; Park, Y.-S.; Kang, D.-K.; Paik, H.-D. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT 2017, 85, 181–186.
- Son, S.-H.; Yang, S.-J.; Jeon, H.-L.; Yu, H.-S.; Lee, N.-K.; Park, Y.-S.; Paik, H.-D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492.
- Ayyanna, R.; Ankaiah, D.; Arul, V. Anti-inflammatory and Antioxidant Properties of Probiotic Bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar Albino Rats. Front. Microbiol. 2018, 9, 3063.
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000.
- Li, J.-Y.; Jin, M.-M.; Meng, J.; Gao, S.-M.; Lu, R.-R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152.
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275.
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Liver Physiol. 2008, 294, G1060–G1069.
- Segawa, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Kobayashi, N.; Shigyo, T.; Kohgo, Y. Probiotic-Derived Polyphosphate Enhances the Epithelial Barrier Function and Maintains Intestinal Homeostasis through Integrin–p38 MAPK Pathway. PLoS ONE 2011, 6, e23278.
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.-S.; Kim, B.-G.; Chung, D.K.; Kim, H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 2015, 220, 460–466.
- Fujiya, M.; Musch, M.W.; Nakagawa, Y.; Hu, S.; Alverdy, J.; Kohgo, Y.; Schneewind, O.; Jabri, B.; Chang, E.B. The Bacillus subtilis Quorum-Sensing Molecule CSF Contributes to Intestinal Homeostasis via OCTN2, a Host Cell Membrane Transporter. Cell Host Microbe 2007, 1, 299–308.
- Wang, Y.; Wu, Y.; Wang, Y.; Fu, A.; Gong, L.; Li, W.; Li, Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026.
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis. PLoS ONE 2013, 8, e63388.
- Gao, D.; Gao, Z.; Zhu, G. Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct. 2013, 4, 982–989.
- Kim, Y.-G.; Ohta, T.; Takahashi, T.; Kushiro, A.; Nomoto, K.; Yokokura, T.; Okada, N.; Danbara, H. Probiotic Lactobacillus casei activates innate immunity via NF-κB and p38 MAP kinase signaling pathways. Microbes Infect. 2006, 8, 994–1005.
- Diao, Y.; Xin, Y.; Zhou, Y.; Li, N.; Pan, X.; Qi, S.; Qi, Z.; Xu, Y.; Luo, L.; Wan, H. Extracellular polysaccharide from Bacillus sp. strain LBP32 prevents LPS-induced inflammation in RAW 264.7 macrophages by inhibiting NF-κB and MAPKs activation and ROS production. Int. Immunopharmacol. 2014, 18, 12–19.
- Jang, S.-E.; Han, M.J.; Kim, S.-Y.; Kim, D.-H. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. Int. Immunopharmacol. 2014, 21, 186–192.
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224.
- Leblanc, J.G.; Del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; Leblanc, A.D.M.D. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293.
- Leblanc, A.D.M.D.; Leblanc, J.G.; Perdigón, G.; Miyoshi, A.; Langella, P.; Azevedo, V.; Sesma, F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J. Med. Microbiol. 2008, 57, 100–105.
- Photolo, M.M.; Mavumengwana, V.; Sitole, L.; Tlou, M.G. Antimicrobial and Antioxidant Properties of a Bacterial Endophyte, Methylobacterium radiotolerans MAMP 4754, Isolated from Combretum erythrophyllum Seeds. Int. J. Microbiol. 2020, 2020, 1–11.
- Hameed, A.; Hussain, S.A.; Yang, J.; Ijaz, M.U.; Liu, Q.; Suleria, H.A.R.; Song, Y. Antioxidants Potential of the Filamentous Fungi (Mucor circinelloides). Nutrients 2017, 9, 1101.
- Jalgaonwala, R.E.; Mohite, B.V.; Mahajan, R.T. A review: Natural products from plant associated endophytic fungi. J. Microbiol. Biotechnol. Res. 2011, 1, 21–32.
- Zeng, P.; Wu, J.; Liao, L.; Chen, T.-Q.; Wu, J.; Wong, K.H. In vitro antioxidant activities of endophytic fungi isolated from the liverwort Scapania verrucosa. Genet. Mol. Res. 2011, 10, 3169–3179.
- Prihantini, A.I.; Tachibana, S. Antioxidant compounds produced by Pseudocercospora sp. ESL 02, an endophytic fungus isolated from Elaeocarpus sylvestris. Asian Pac. J. Trop. Biomed. 2017, 7, 110–115.
- Nuraini, F.R.; Setyaningsih, R.; Susilowati, A. Antioxidant activity of bioactive compound produced by endophytic fungi isolated from endemic plant of South Kalimantan Mangifera casturi Kosterm. In Proceedings of the International Conference on Biology and Applied Science (ICOBAS), Malang, Indonesia, 13–14 March 2019; AIP Publishing: Melville, NY, USA, 2019; Volume 2120, p. 080013.
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otoba gracilipes. MicrobiologyOpen 2019, 8, e903.
- Smith, H.; Doyle, S.; Murphy, R. Filamentous fungi as a source of natural antioxidants. Food Chem. 2015, 185, 389–397.
- Huang, W.-Y.; Cai, Y.-Z.; Hyde, K.D.; Corke, H.; Sun, M. Endophytic fungi from Nerium oleander L (Apocynaceae): Main constituents and antioxidant activity. World J. Microbiol. Biotechnol. 2007, 23, 1253–1263.
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211.
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7.
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737.
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115.
- Besse, A.; Peduzzi, J.; Rebuffat, S.; Carré-Mlouka, A. Antimicrobial peptides and proteins in the face of extremes: Lessons from archaeocins. Biochimie 2015, 118, 344–355.
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545.
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxidative Med. Cell. Longev. 2019, 2019, 1–14.
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63.
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2020, 47, 37–48.
- Kindlund, P.J. Astaxanthin. Nutrafoods 2011, 10, 27–31.
- Chen, Z.; Xiao, J.; Liu, H.; Yao, K.; Hou, X.; Cao, Y.; Liu, X. Astaxanthin attenuates oxidative stress and immune impairment in d-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food Funct. 2020, 11, 8099–8111.
- Pongkan, W.; Takatori, O.; Ni, Y.; Xu, L.; Nagata, N.; Chattipakorn, S.C.; Usui, S.; Kaneko, S.; Takamura, M.; Sugiura, M.; et al. β-Cryptoxanthin exerts greater cardioprotective effects on cardiac ischemia-reperfusion injury than astaxanthin by attenuating mitochondrial dysfunction in mice. Mol. Nutr. Food Res. 2017, 61.
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17, 103.
- Shah, M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531.
- Mohammadpourfard, I.; Khanjari, A.; Basti, A.A.; Herrero-Latorre, C.; Shariatifar, N.; Hosseini, H. Evaluation of microbiological, chemical, and sensory properties of cooked probiotic sausages containing different concentrations of astaxanthin, thymol, and nitrite. Food Sci. Nutr. 2021, 9, 345–356.
- Spiller, G.A.; Dewell, A. Safety of an Astaxanthin-RichHaematococcus pluvialisAlgal Extract: A Randomized Clinical Trial. J. Med. Food 2003, 6, 51–56.
- Liebler, D.C. Antioxidant Reactions of Carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 20–31.
- Gastineau, R.; Turcotte, F.; Pouvreau, J.-B.; Morançais, M.; Fleurence, J.; Windarto, E.; Prasetiya, F.S.; Arsad, S.; Jaouen, P.; Babin, M.; et al. Marennine, Promising Blue Pigments from a Widespread Haslea Diatom Species Complex. Mar. Drugs 2014, 12, 3161–3189.
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3.
- Miranda, M.; Cintra, R.; Barros, S.; Mancini-Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079.
- Elshobary, M.E.; El-Shenody, R.A.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci. 2020, 35, 100567.
- M, P.; Y, S.; B, M.V. Response of Anaerobic Protozoa to Oxygen Tension in Anaerobic System. Int. Microbiol. 2019, 22, 355–361.
- Mastronicola, D.; Falabella, M.; Forte, E.; Testa, F.; Sarti, P.; Giuffrè, A. Antioxidant defence systems in the protozoan pathogen Giardia intestinalis. Mol. Biochem. Parasitol. 2016, 206, 56–66.
- Joardar, N.; Babu, S.P.S. A review on the druggability of a thiol-based enzymatic antioxidant thioredoxin reductase for treating filariasis and other parasitic infections. Int. J. Biol. Macromol. 2020, 142, 125–141.
- Jeelani, G.; Nozaki, T. Oxidative Stress and Antioxidant Defense Mechanism in the Human Enteric Protozoan Parasite Entamoeba histolytica. In Oxidative Stress in Microbial Diseases; Springer International Publishing: Singapore, 2019; pp. 209–227.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




