Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Gureeva | -- | 6391 | 2023-06-01 12:57:50 | | | |
| 2 | Camila Xu | Meta information modification | 6391 | 2023-06-02 04:28:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gureeva, M.V.; Gureev, A.P. Azospirillum in Plant Adaptation to Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/45105 (accessed on 07 February 2026).
Gureeva MV, Gureev AP. Azospirillum in Plant Adaptation to Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/45105. Accessed February 07, 2026.
Gureeva, Maria V., Artem P. Gureev. "Azospirillum in Plant Adaptation to Stress" Encyclopedia, https://encyclopedia.pub/entry/45105 (accessed February 07, 2026).
Gureeva, M.V., & Gureev, A.P. (2023, June 01). Azospirillum in Plant Adaptation to Stress. In Encyclopedia. https://encyclopedia.pub/entry/45105
Gureeva, Maria V. and Artem P. Gureev. "Azospirillum in Plant Adaptation to Stress." Encyclopedia. Web. 01 June, 2023.
Copy Citation
Azospirillum is one of the most studied genera of plant growth-promoting rhizobacteria (PGPR), and species of this genus are recognized as biofertilizers due to their ability to stimulate plant growth and productivity. Representatives of this genus have different sensitivities or resistances to osmotic stress, pesticides, heavy metals, hydrocarbons, and perchlorate and also have the ability to mitigate the consequences of such stresses for plants.
Azospirillum
stress
hydrocarbons
heavy metals
1. Introduction
Farming is a risky industry, the result of which largely depends on environmental factors. Agricultural plants are regularly exposed to a variety of stress factors. This may be drought, infection by pathogenic micro-organisms, growth on saline soils or on soils contaminated with hydrocarbons, heavy metals, pesticides, radioactive elements, or perchlorate. One of the ways to reduce the stress impact on plants and increase their productivity is the use of plant growth-promoting rhizobacteria (PGPR) [1]. PGPR are the rhizosphere bacteria that can enhance plant growth via a wide variety of mechanisms, such as phosphate solubilization, siderophore production, biological nitrogen fixation, rhizosphere engineering, the production of 1-Aminocyclopropane-1-carboxylate deaminase (ACC), the quorum sensing signal interference, the inhibition of biofilm formation, phytohormone production, exhibiting antifungal activity, the production of volatile organic compounds, the induction of systemic resistance, promoting beneficial plant–microbe symbioses, interference with pathogen toxin production, etc. [2][3].
Depending on the degree of association of bacteria with plant root cells, PGPR can be divided into extracellular plant growth-promoting rhizobacteria (ePGPR) and intracellular plant growth-promoting rhizobacteria (iPGPR) [4]. ePGPR includes such genera as Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcus, Pseudomonas, and Serratia [5]. iPGPR includes endophytes (Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, and Rhizobium) and Frankia species [6].
Microbial inoculants consisting of PGPR are the most widely used in Latin America, Southeast Asia, and Africa, where inoculated seeds are sown on a large scale, with millions of hectares of Fabaceae (e.g., soybean or bean) and Poaceae (e.g., maize, sorghum, or wheat) inoculated through PGPR, belonging mainly to the genera Bacillus, Paenibacillus, Pseudomonas, or Azospirillum [7]. Each of the most commonly used genera is most effective in some way for plant growth promotion: phytohormone production (e.g., Azospirillum spp., Pseudomonas spp.), phosphate dissolution (e.g., Bacillus spp.), or biological control (e.g., Pseudomonas spp., Bacillus spp.) [8].
A number of reviews on the use of bacteria from the genera Bacillus [9][10][11][12][13][14][15][16], Paenibacillus [17][18], and Pseudomonas [19][20][21] for plant growth promotion have been published in the last decade. In particular, the role of these bacteria in the protection against biotic and abiotic stresses [9][12][19] and molecular mechanisms that determine their interactions with plants [14][15][21] were considered.
As for bacteria from the genus Azospirillum, the last review on the molecular basis of the interaction of the representatives of this genus with plants was published in 2012 [22]. In 2019, a review was published on the use of Azospirillum as inoculants in crop plants [8], but it focused more on the effectiveness of using commercial inoculants in different countries rather than the molecular mechanisms of their effect on plants. In 2018, a review was published on the role of azospirilla in the protection against biotic stress as well as two types of abiotic stress, namely osmotic and oxidative [23].
2. Bacteria from the Genus Azospirillum
The genus Azospirillum currently includes 28 species, 24 of which have validly published names (https://lpsn.dsmz.de/genus/azospirillum accessed on 25 April 2023). Most azospirilla species were isolated from the soil or plant rhizosphere, although individual species were isolated from water bodies, oil-producing mixtures, discarded tar, fermented cattle products, fermenter, microbial fuel cells, and karst caves.
Azospirillum is one of the most studied genera of PGPR, and species of this genus are recognized as biofertilizers due to their ability to stimulate plant growth and productivity [24]. Bacteria of this genus are resistant to many types of biotic and abiotic stress and are also capable of activating plant defense mechanisms upon inoculation into the rhizosphere, increasing crop yields in stress conditions (Table 1).
Table 1. Influence of inoculation with Azospirillum strains on the yield of agricultural plants under stress.
| The Crop | Stress Type | Azospirillum Species | The Percentage of Improved Growth or Yield | Reference |
|---|---|---|---|---|
| wheat | arsenic | A. brasilense | plant height 2.36–3.21% spike length 11.42–22.19% number of spikelets per spike 4.46–6.60% number of grains per spike 4.67–5.69% 1000 grain weight 5.17–9.63% grain yield per plant 3.42–17.6% |
[25] |
| arabidopsis | cadmium | A. brasilense | shoot fresh weight about 100% | [26] |
| pak choi | cadmium | A. brasilense | biomass 26–255% | [27] |
| barley | cadmium | A. lipoferum | root biomass 22.22% root elongation 12.5% |
[28] |
| pak choi | cadmium | A. brasilense | shoot biomass 16.2% root biomass 12.2% |
[29] |
| cucumber | copper | A. brasilense | root weight 55.32% root length 73.65% root tips 35.85% |
[30] |
| tomato | Pseudomonas syringae pv. tomato, the causal agent of bacterial speck on tomato | A. brasilense | dry weight about 100% | [31] |
| tomato | Pseudomonas syringae pv. tomato, the causal agent of bacterial speck on tomato | A. brasilense | dry weight 7.81–28.79% | [32] |
| green gram | nematode disease | A. lipoferum | shoot length 10.26% fresh weight 18.28% dry weight 18.45% |
[33] |
| cherry tomato | Clavibacter michiganensis subsp. michiganensis (bacterial canker), Xanthomonas campestris pv. vesicatoria (bacterial spot) | A. brasilense and Azospirillum sp. BNM-65 | leaves 32–43% shoot height 12–143% shoot dry weight 81–107% root dry weight 37–80% |
[34] |
| teosinte | fungal diseases caused by Alternaria, Bipolaris and Fusarium | A. brasilense | total dry mass from −8.6 to 73.0% | [35] |
| komatsuna | radioactive 137Cs | Azospirillum sp. strain TS13 | dry weight 40–51% | [36] |
| wheat | drought | A. lipoferum | wheat yield up to 109% | [37] |
| arabidopsis | drought | A. brasilense | rosettes diameter 7.7% rosettes DW 86.21% seed yield 328.66% |
[38] |
| maize | drought | A. brasilense | total biomass 26% | [39] |
| maize | drought | A. lipoferum | height 35.33–43.89% | [40] |
| coriander | salinization | A. brasilense and Azotobacter chroococcum | grain yield 11.6% stem fresh weight 11.3% stem dry weight 17.2% total plant fresh weight 6.1% total plant dry weight 10.2% |
[41] |
| flax | salinization | A. brasilense | shoot length 16.5% root length 36.6% fresh weight of shoot 17.07% dry weight of shoot 13.43% fresh weight of root 57.7% dry weight of root 78.6% number of leaves 10.5% |
[42] |
| white clover | salinization | A. brasilense | shoot height 57.8–70% root length 58.82–70.85% |
[43] |
| tomato | salinization | A. brasilense | root biomass 118% | [44] |
Azospirillum Response to Stress
The stress response in many bacteria is activated via the extracytoplasmic function σ factors (ECF). Due to their diversity and relative simplicity of the mechanism of action, they stand out as a versatile and powerful bacterial tool for the effective activation of stress responses [45]. They are subunits of the RNA polymerase holoenzyme required for transcription initiation. ECFs belong to group IV σ factors and consist of two domains, σ2 at the N-terminus and σ4 at the C-terminus. Upon transcription initiation, σ2 binds to the −10-box in the promoter, while σ4 binds to the −35-box, and two-stranded DNA begins to melt in the −10-box. ECF activity can be regulated through anti-σ factors, through serine/threonine protein kinases, and through C- and N-terminal extensions. In addition, some ECFs may be regulated only by controlling their production at the transcriptional level [45].
The regulation of the stress response in the representatives of the genus Azospirillum was studied using the strains of the species A. brasilense. It was shown that adaptation to many types of stress is mediated through ECF, which can be regulated by anti-sigma factors (Figure 1). The role of ECF, known as RpoE or σ E, in the adaptation to salt, ethanol, and methylene blue stress was shown for A. brasilense Sp7 [46].
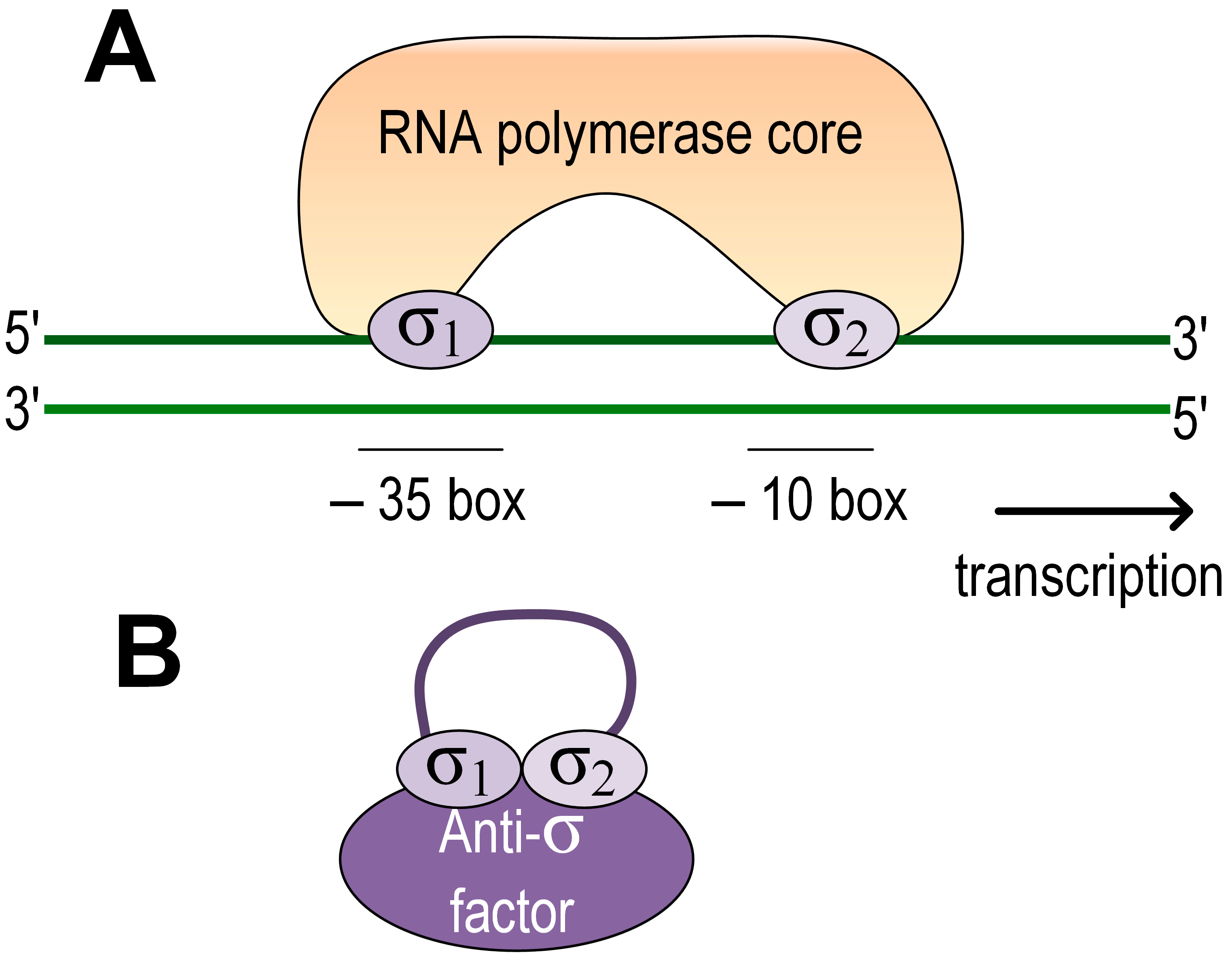
Figure 1. Scheme of ECF regulation in Azospirillum. (A) Inactive state. ECF is associated with the anti-sigma factor. (B) Active state. The σ 2 domain binds to the promoter at the −10 box and the σ4 domain at the −35 box. DNA begins to melt from the −10 to the start codon.
The synthesis of carotenoids in response to stress in A. brasilense is regulated by ECF rpoE, which, in turn, is regulated by the anti-sigma factor chrR [47][48]. Kumar et al., 2012, showed that ECF RpoH2 controls the response to photooxidative stress in A. brasilense [49]. Gupta et al., 2014, showed that A. brasilense contains two redox-sensitive zinc-binding anti-sigma factors (ZAS) (ChrR1 and ChrR2), which negatively regulate the activity of their related ECFs (RpoE1 and RpoE2), blocking their binding to bovine enzyme. At the same time, two A. brasilense ZAS anti-σ factors also interact with their unrelated ECFs and affect gene expression [50].
ECF RpoH2 in A. brasilense regulates the use of ethanol as an additional carbon source when growing on fructose or glycerol [51]. Pandey et al., 2022 described a new ECF RpoE7-RpoH3 regulatory cascade that negatively regulates ampicillin resistance in A. baldaniorum Sp245 by controlling the expression of β-lactamase and lytic transglycosylase [52].
Authors also paid attention to ECF-encoding genes in works on the sequencing and analysis of the genomes of azospirilla. The genome of A. brasilense Sp7 encodes one home and twenty-two alternative ECFs, consisting of ten RpoE, five RpoH, one RpoN, and six FecI sigma factors [53]. Fourteen rpoE genes and five rpoH genes were found in the genome of A. brasilense Az19 [54].
3. Azospirillum Participation in Plants’ Defense against Stress Factors
3.1. Hydrocarbon Pollution
Hydrocarbons are the largest group of organic pollutants. The increasing dependence of humanity on fossil fuels, especially petroleum hydrocarbons, has led to the pollution of agricultural lands through the spillage of crude oil during extraction and processing operations in many oil producing countries [55]. These hydrocarbons are highly resistant, can accumulate in plants, as well as in humans and animals, and exhibit carcinogenic and neurotoxic properties [56]. One of the ways to effectively remove hydrocarbons from the soil is microbial biodegradation.
Bacteria from the genus Azospirillum are found in microbial communities that break down hydrocarbons [57]. There are few data on the ability of individual strains to remove oil. Some Azospirillum strains have been shown to biodegrade crude oil [58], phenol, and benzoate [59], as well as polycyclic organic compounds [57][60][61][62][63][64]. Additionally, representatives of the genus Azospirillum were found in biofilms that decompose hydrocarbons [65][66] and, as part of the microbiome in the maize rhizosphere, bioremediate soil contaminated with crude oil [67]. It has been suggested that this bacterium appears to enrich biofilms with nitrogenous compounds known to enhance the microbiological degradation of hydrocarbons [66].
In addition, two Azospirillum species isolated from oil-bearing samples, A. rugosum [68] and A. oleiclasticum [24], were described. For the latter species, the ability to biodegrade crude oil was shown [24].
Thus, the metabolic potential of the genus Azospirillum allows its representatives to participate in the biodegradation of hydrocarbons, thereby contributing to the bioremediation of polluted soil and, consequently, reducing the damaging effect of this pollutant on plants.
3.2. Heavy Metal Pollution
Heavy metals are an essential part of the environment, but in places of active anthropogenic activity, their concentration significantly exceeds the permissible limits, which adversely affects agriculture [69]. In plants, heavy metal stress has both direct and indirect effects, including oxidative stress through various indirect mechanisms (e.g., the depletion of glutathione or its binding to protein sulfhydryl groups) or through the inhibition of antioxidant enzymes, thereby inducing ROS (reactive oxygen species)-producing enzymes (for example, NADPH oxidases) [70].
PGPR biostimulants are incredibly effective at reducing heavy metal toxicity in plants. They inhibit the transfer of heavy metals to various areas of the plant, changing their mobilization through complexation, precipitation, redox processes, chelation, and adsorption [71][72][73]. In addition, rhizospheric bacteria produce extracellular polymeric substances (EPS) [74][75], such as polysaccharides, glycoproteins, lipopolysaccharides, and soluble peptides, which contain many anion binding sites, and thus contribute to the displacement or recovery of heavy metals from the rhizosphere through biosorption [76].
Bacteria from the genus Azospirillum are able to tolerate high concentrations of heavy metals: arsenic [77][78][79], cadmium [26][27][80], copper [81], and lead [80]. Moreover, bacteria can reduce the negative effects of heavy metals on plants growing in contaminated soil (Figure 2). Vezza et al., 2019, showed that arsenic-resistant genes can mediate the redox transformation of As and its displacement outside the cell [77].
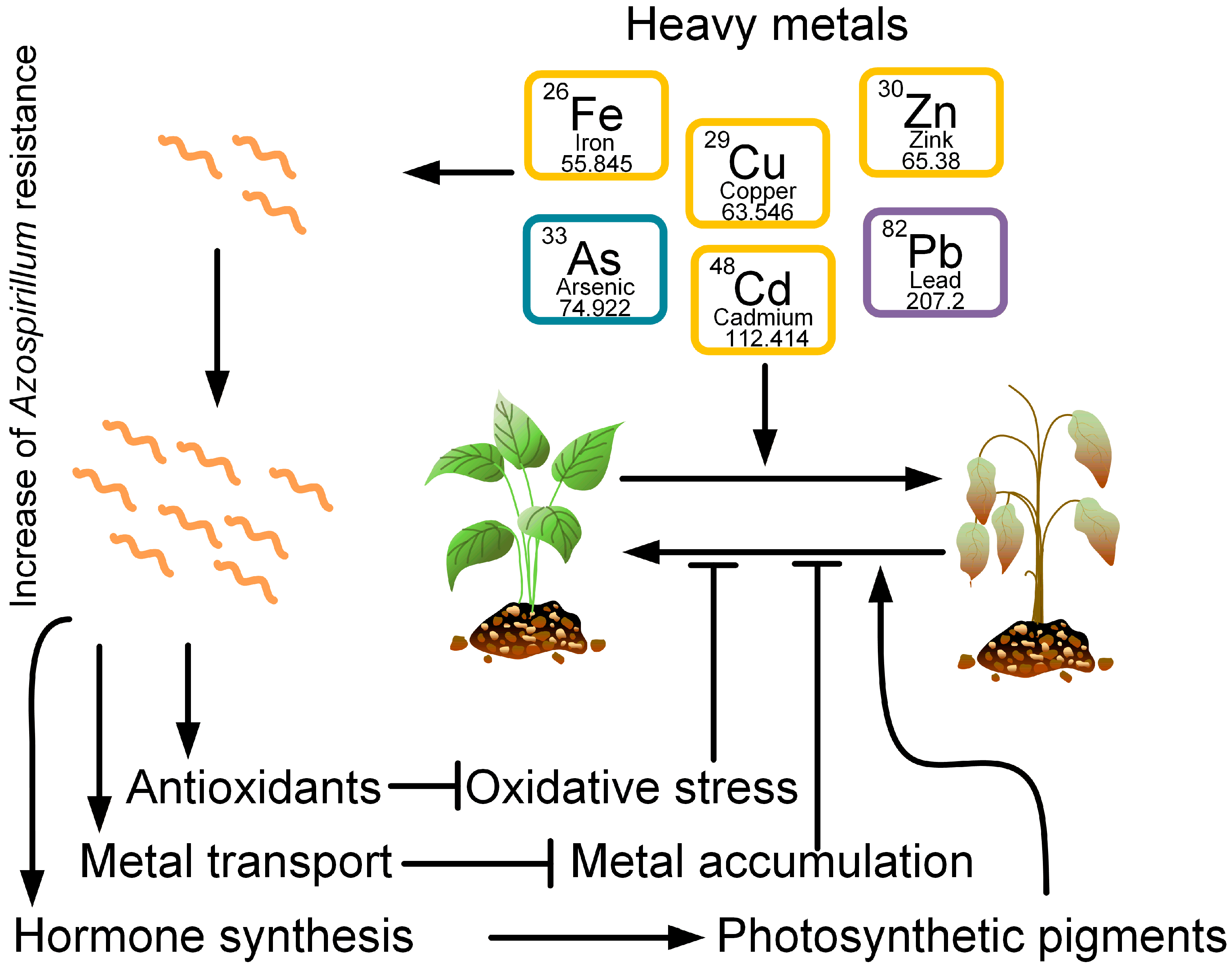
Figure 2. Heavy metals, such as iron, arsenic, copper, cadmium, zinc, and lead, have negative effects on plant growth and vitality. However, at the same time, certain Azospirillum strains can be resistant to these metals. Azospirillum produces antioxidants that neutralize oxidative stress induced by heavy metals. Azospirillum also promotes the transport of heavy metals from plant cells, preventing its intracellular accumulation. In addition, Azospirillum produces plant hormones that promote the formation of photosynthetic pigments. Together, the reduction of oxidative stress, the removal of metals from plants, and the synthesis of photosynthetic pigments contribute to an increase in plant resistance to heavy metal stress.
Peralta et al., 2021, showed different effects of different strains of A. brasilense on the content of photosynthetic pigments in maize in the presence of arsenic: strain CD caused their significant decrease, while strain Az39 did not affect their amount [82]. The use of A. brasilense as biological additives reversed the effects of arsenic toxicity by increasing wheat plant growth rate, leaf area, and photosynthesis, and yield [25]. Additionally, the co-inoculation of soybean seeds with the bacteria Bradyrhizobium japonicum E109 and A. brasilense Cd had a positive effect on nodule formation, photosynthetic pigment content, and antioxidant system activity, as well as a significant reduction in the accumulation of arsenic in plant tissues exposed to AsV and AsIII [77].
It has been shown that bacteria from the genus Azospirillum, alone or in combination with another rhizosphere bacterium, Bacillus subtilis, are able to reduce cadmium toxicity for arabidopsis, pakchoi, and barley [26][27][28][29]. A decrease in the concentration of cadmium in plants and an increase in the biomass of shoots occurred due to an increase in the concentration of abscisic acid (ABA) [26][27][29]. The action of ABA was mediated through IRT1 (IRON-REGULATED TRANSPORTER 1) [26]. A decrease in the level of cadmium toxicity for plants could also be due to a decrease in oxidative stress and an increase in the activity of antioxidant enzymes [29].
Bacteria from the species A. brasilense are able to reduce copper stress in wheat [81], cucumber [30], and an algae Chlorella sorokiniana [83] by activating antioxidant defense enzymes. Moreover, it has been shown for wheat that the copper content in plants increases upon inoculation with azospirilla but its toxicity decreases [81]. For Chlorella sorokiniana, it has also been shown that inoculation with azospirilla increases the content of chlorophyll due to the secretion of IAA (indoleacetic acid) [83]. The ability of bacteria from the genus Azospirillum to produce auxin affects the accumulation of zinc and iron in corn in different ways: a low ability of azospirilla to produce auxin leads to an increase in the zinc content in plants and a high ability leads to an increase in the iron content [84].
Thus, several main mechanisms of reducing the toxicity of heavy metals to plants by bacteria from the genus Azospirillum can be identified: through a decrease in oxidative stress, through an increase in the activity of antioxidant enzymes and the amount of photosynthetic pigments, and through the regulation of the amount of phytohormones.
3.3. Infection of Plants with Phytopathogens
Plant pathogens have a negative impact on the marketable yield (i.e., quality and quantity) of agricultural products, with an adverse impact on the economy. Approximately 14% of crops worldwide are killed by disease, and worldwide crop losses can be as high as 20–40% in sensitive strains [85]. In this regard, the issue of protecting agricultural plants from pathogens is of great importance.
Traditional methods of controlling plant pathogens include implementing good agricultural practices that prevent further infestation; the physical destruction of infected plant tissues; the use of chemicals, such as pesticides and antibiotics, to fight bacterial infections; the development of genetically modified plants resistant to pests and pathogens; and the use of bacteriophages [85].
In addition to the above methods, the PGPR inoculation of agricultural plants has been actively used in recent years to reduce the negative effect of phytopathogens. In particular, the bacteria of the genus Azospirillum have been shown to be capable of the biological control of phytopathogens [31][32][33][34][86]. This may be due to the synthesis of siderophores that limit the availability of iron (Fe) to phytopathogens [86] or the induction of changes in the host plant metabolism, which increases plant resistance to pathogen infection—the induced systemic resistance (ISR) [23].
Siderophores are compounds with low molecular weight (<1500 Da) and high iron affinity that allow soil micro-organisms to bind and dissolve ferric iron in iron-poor environments. The conversion of iron into an available form and the subsequent increase in the uptake of the available form of iron by plants can lead to the prevention of the growth of soil pathogens due to iron deficiency. Siderophores vary greatly in chemical structure; however, they can be divided into two main groups, namely catechols and hydroxamates, according to the chemical group involved in iron(III) chelation [86].
Among the catechols, salicylic acid (SA) has received particular attention, because it can be active in pathogen biocontrol in two ways. On the one hand, it can act as a siderophore, reducing the availability of iron in an environment with a low iron content [87], and on the other hand, it can act as a signal molecule that triggers a systemic response of plant resistance to pathogens [88]. It is the synthesis of catechol siderophores, including SA, that allows A. brasilense to exhibit antifungal activity against Colletotrichum acutatum, the causative agent of anthracnose, and reduce its negative effect on strawberry plants [86]. Additionally, the synthesis of siderophores via the bacterial strains of A. brasilense is able to determine the resistance of the teosinte plant (Zea mays L. ssp. mexicana) to the phytopathogenic fungi Alternaria (causative agent of Alternaria), Bipolaris (causative agent of helminthosporiasis), and Fusarium (causative agent of Fusarium) [35].
Another form of Azospirillum limitation regarding the development of phytopathogens is the induction of systemic resistance in plants. Plant systemic resistance can be divided into ISR and systemic acquired resistance (SAR) induced by non-pathogenic microbes and pathogenic microbes, respectively [89][90]. Colonization with beneficial microbes induces a physiological state of the host plant called “priming”. When “priming” is activated, plants exhibit stronger and faster defense responses against the subsequent pathogen invasion [91].
The classic difference between ISR and SAR, adopted in 1996, is the type of activated signaling pathway. For ISR, these are the jasmonic acid (JA) and ethylene (ET) pathways, and for SAR, these are the SA pathway and the activation of PR (pathogenesis-related) proteins [92]. However, there have been numerous reports of the activation of both the SA and JA/ET signaling pathways in ISR triggered by beneficial microbes [91]. As for PR proteins, the activation of PR1, PR2, and PR5 depends on SA signaling, while PDF1.2, as well as the PR3 and PR4 genes, are activated via an SA-independent and JA-dependent pathway [93].
In the SA pathway, the activation/repression of PR genes is mediated by NPR1 (“nonexpressor of PR-gene1”, related to the plant’s defense system). When SA levels are low, NPR4 (paralog of NPR1) interacts with NPR1, resulting in its degradation. Thus, when SA levels are high, binding between NPR1 and NPR3 (paralog of NPR1) is increased, which also leads to the removal of NPR1 [94]. When SA is intermediate, the interaction between NPR1 and NPR3 is suppressed, resulting in the accumulation of NPR1 and the activation of SA-dependent protective genes [95].
The major players In the JA pathway are the CORONATINE INSENSITIVE 1 (COI1) protein, JASMONATE ZIM DOMAIN PROTEIN (JAZ), and MYC. In the absence of stress, the endogenous level of the active form of JA, isoleucine jasmonate (JA-Ile), is very low in plants. JAZ repressors bind to MYC2 to inhibit its transcriptional activation on downstream genes. Under stress conditions, the endogenous level of JA-Ile is activated to a large extent, which is perceived by the JA-receptor COI1. SKP1/CULLIN/F-box (SCF)COI1 then binds to JAZ for ubiquitination and degradation via the 26S proteasome pathway, resulting in the release of downstream transcription factors, such as MYC, and the activation of JA responses [96].
The classical ET pathway is a linear sequence of the following components: the ET receptor family; the protein kinase CTR1; the transmembrane protein with unknown biochemical activity, EIN2; the transcription factors EIN3, EIL and ERF; and the ET response. In the absence of ET, the receptors activate CTR1, which negatively regulates downstream signaling [97].
There have been several attempts to identify the signaling pathways leading to the emergence of systemic plant resistance upon inoculation with bacteria from the genus Azospirillum. In a study of strawberries (Fragaria ananassa) inoculated with A. brasilense REC3, Elias et al. (2018) reported increased ET synthesis and the upregulation of genes associated with ET signaling (Faetr1, Faers1, Faein4, Factr1, Faein2, and Faaco1) [98]. Kusajima et al., 2018, also showed that A. brasilense induces ISR in rice through the ET pathway [99]. Yasuda et al. (2009) showed that rice plants inoculated with Azospirillum sp. B510 increased resistance to the pathogenic fungus Magnoporthe oryzae (the causative agent of blast) and to the bacteria Xanthomonas oryzae (the causative agent of bacterial blight of rice) through the mechanisms independent of SA signaling, without the accumulation of SA or PR proteins [100].
However, other studies showed that PR proteins play a role in the formation of systemic resistance in plants in response to inoculation with azospirilla. A transcriptome study showed that Azospirillum sp. strain B510 (isolated from cv. Nipponbare) inoculated into rice induced one and repressed five PR genes, while strain A. lipoferum 4B (isolated from cv. Cigalon) induced more protection-related genes in rice cv. Nipponbare than in rice cv. Cigalon [101]. In another study with Arabidopsis thaliana, PR genes were induced when the plant was inoculated with A. brasilense Sp245 [102]. A study was also conducted using A. brasilense Ab-V5 and Ab-V6 cells and metabolites, which led to the induction of PR-1 SAR-associated genes and PRP-4 ISR-associated genes [103].
Thus, at present, it is not possible to draw an unambiguous conclusion about the systemic resistance pathway induced by azospirilla in inoculated plants. Most likely, this is a combination of different pathways, and their relationship and regulation needs to be studied in more detail.
3.4. Pesticide Pollution
The third agricultural revolution, or green revolution, which took place in the second half of the 20th century, made it possible to significantly increase the productivity of many agricultural crops. Much of this was made possible through the widespread use of pesticides [104]. However, only 1% of the pesticide reaches the pest, while the rest accumulates in soil, water, and air and affects non-target organisms, including agricultural plants [105]. Pesticides accumulate in the plant body and can target the electron transport chains in photosystems in chloroplasts [106], inhibit respiratory complexes in mitochondria, uncouple phosphorylated respiration, damage DNA [107], cause oxidative stress [108], disrupt the metabolism of polyphenols, reduce the bioavailability of trace elements [109], and negatively influence rhizospheric bacteria [110].
Data on pesticide toxicity for azospirilla are inconsistent and not abundant. Several works on this subject were carried out at the end of the 20th century and beginning of the 21st century. In vitro studies showed that methidathion is able to reduce nitrogen fixation, intracellular ATP levels, and cell growth, while profenophos also inhibits the production of a number of hormones in A. brasilense [111]. At the same time, terbufos has little effect on the growth of A. lipoferum on a solid medium, while carbofuran, chlormephos, and benfuracarb do not affect it at all [112]. Bromopropylate and diazinon are also completely harmless to A. brasilense [111].
Under field conditions, the population of Azospirillum sp. decreased in vigna treated with thiram but not in plants treated with carbendazim, Bordeaux mixture, carbofuran, and phorate. A mixture of thiram and carbofuran and phorate reduced the population of azospirilla, but after treatment, a gradual accumulation of bacteria was observed in the rhizosphere [113]. Additionally, the soil isolates of Azospirillum sp. were able to degrade the pesticide Ethion [114].
In recent years, there has been renewed interest in research on the interaction of azospirilla and pesticides regarding the joint treatment of cereal seeds before sowing. The treatment of plant seeds with pesticides Standak™ Top (BASF) (a mixture of insecticide fipronil and fungicide pyraclostrobin and thiophanate-methyl) and Helicur 250 EW (tebuconazole) is known to reduce the survival of Azospirillum bacteria [115][116]. It has been shown in terms of insecticides (imidocloprid and thiodicarb) and fungicides (triadimenol) that azospirilla can survive only if the interval between the inoculation of pesticide-treated seeds and sowing in the soil does not exceed 4 h [117].
Thus, the joint treatment of seeds with azospirilla and pesticides is possible; however, for each pesticide, it is necessary to choose compatible strains and it is necessary to follow a certain treatment technology that preserves the viability of the strains used.
3.5. Pollution with Radioactive Elements
There was an attempt to inoculate plants with Azospirillum strains in contaminated soil in Fukushima for the purpose of bioremediation by translocating radioactive caesium to the aerial parts of the plants. Despite the positive effects of inoculation, the concentrations of (137)Cs during their transfer to the tested plants were not very high, and the removal of (137)Cs from the soil would therefore be very slow [36].
3.6. Perchlorate Pollution
Perchlorate is a persistent pollutant produced by natural and human processes [118]. Perchlorates were shown to easily accumulate in plants [119]. Xie et al. (2014) showed that the rice plant Oryza sativa L. is easily contaminated with perchlorate and suggested that perchlorate can inhibit plant growth [120]. Perchlorates also affect the chlorophyll content and root systems of Acorus calamus, Canna indica, Thalia dealbata, and Eichhornia crassipes [121]. A study by Acevedo-Barrios et al. (2018) showed that perchlorate significantly reduced the survival of freshwater algae Pseudokirchneriella subcapitata (LC50 = 72 mM) [122]. However, the exact way in which perchlorate damages the photosystem is unclear [120].
One of the methods for removing perchlorate from ecosystems is microbial degradation. It is cost effective, easy to implement, and environmentally friendly, making it a viable method for reducing perchlorate pollution. Perchlorate-reducing bacteria (PRB) reduce ClO4− or chlorate (ClO3−) to chlorite (ClO2−) with perchlorate reductase (pcrABCD) and then disproportionate ClO2− to Cl− and O2 with chlorite dismutase (cld) [123] (Figure 3). Electron donors for the reduction of perchlorates are often organic compounds such as methanol and acetate [124][125]. Inorganic donors, such as H2 and S, are also capable of causing the reduction of perchlorates [126][127]. Moreover, researchers have recently reported that PRBs are able to reduce perchlorates using methane as an electron donor [128][129][130].
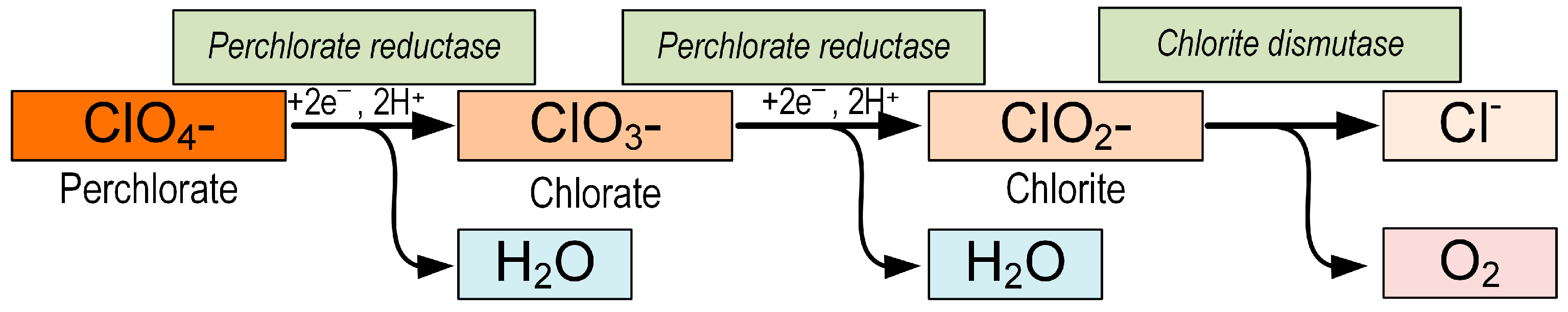
Figure 3. Reactions of perchlorate reduction by perchlorate-reducing bacteria. Perchlorate reductase catalyzes perchlorate reduction to chlorate and chlorate reduction to chlorite. Chlorite dismutase catalyzes chlorite dismutation to chloride and oxygen.
The reduction of perchlorate is usually inhibited by the presence of nitrates [125][131], as some reducing micro-organisms prefer other electron acceptors to perchlorates [132]. To prevent this, donors are added in excess to remove non-perchlorate electron acceptors before reduction is performed; this is carried out because non-perchlorate electron acceptors can activate bacteria that do not degrade perchlorate, resulting in inefficient processing. Oxygen is another inhibitor of microbial perchlorate reduction, as its presence can cause bacteria to use donors to consume oxygen [132][133]. Research showed that perchlorate recovery should ideally be performed under facultative anaerobic conditions [134][135].
Azospirillum strains capable of degrading perchlorate have been repeatedly isolated from samples contaminated with perchlorate. At the same time, they could use acetate [136][137] or hydrogen [138] as electron donors. It was recently shown that in a batch membrane biofilm reactor, representatives of the genus Azospirillum, along with the genus Denitratisoma, were the main genera involved in the reduction of perchlorates and nitrates, and both were able to use NO3− and ClO4− as electron acceptors [129].
The ability of bacteria from the genus Azospirillum to biodegrade perchlorate makes it possible to use them for the remediation of contaminated soils, and therefore, the negative effect of perchlorate on plants can be reduced.
3.7. Osmotic Stress
Osmotic stress in a plant cell occurs when the concentration of the solvent (water) in the environment is lower than in the cell. This is possible in two cases: with salinity and with drought. The physical way to reduce osmotic stress is the synthesis of osmolytes—low molecular weight organic substances that are soluble in the intracellular environment and change the properties of biological fluids. The main osmolytes are prolines, soluble sugars, and glycine–betaine [139].
Proline has very strong moisturizing properties. Its hydrophobic part is able to bind to proteins, while its hydrophilic part is able to bind to water molecules, allowing proteins to access more water to increase their solubility and prevent protein denaturation through dehydration under osmotic stress conditions [140]. Trehalose is a reducing disaccharide. Under the conditions of drought stress, the intercellular content of trehalose rapidly increases, which blocks the transition of the phospholipid bilayer membrane from the liquid crystal state to the solid state and stabilizes the structure of proteins, nucleic acids, and other biological macromolecules [141]. Betaine is a metabolic intermediate belonging to the water-soluble alkaloid compounds of quaternary ammonium. It helps to stabilize the structures and activity of photosynthesis, including protective enzymes, and also helps to maintain membrane integrity from widespread damage under drought stress conditions [142][143][144][145].
Bacteria from the genus Azospirillum are not only capable of mitigating the consequences of osmotic stress for plants, but they themselves have a number of mechanisms of resistance to osmotic stress.
The mechanism of osmoadaptation was investigated in relatively more detail in A. brasilense, where glycine–betaine was shown to enhance growth and nitrogen fixation under salt stress conditions [146]. In addition to betaine, proline was shown to be the predominant osmolyte at higher salt concentrations [147]. In response to salt stress, a periplasmically located glycine–betaine-binding protein, a component of the ProU system, is induced, which is expressed as one of the “early genes” in the process of osmoadaptation. This protein binds glycine–betaine with a high degree of activity and contributes to its high intracellular accumulation [148][149]. However, Chowdhury et al., 2007, showed that the production of exopolysaccharides and cell aggregates is a more consistent physiological response of A. brasilense to salt stress than osmoprotection through glycine–betaine [150]. Nagarajan et al., 2007, also showed that most of the genes induced by salt stress in A. brasilense seem to be involved in functions associated with the cell membrane [151].
3.7.1. Drought
Drought stress is one of the major constraints on global agricultural production. Approximately one third of the Earth’s land area is in arid and semi-arid regions, while most of the other land areas are often subject to periodic and unexpected climatic droughts. Water deficit can be fatal to plants and lead to huge social problems and economic losses [152].
Drought stress results in reduced nutrient diffusion, induces the formation of free radicals that affect antioxidant protection, leads to a decrease in chlorophyll content, and affects nitrate reductase activity due to the lower uptake of nitrates from the soil [153]. Drought also enhances ET biosynthesis, which inhibits plant growth [154].
PGPR were shown to reduce the negative effects of drought on plants. It may be due to several factors: the production of phytohormones, such as ABA, gibberellic acid, cytokinins, and IAA; the ability of PGP bacteria with ACC deaminase enzyme to degrade plant ET precursor ACC, thereby reducing ET levels in stressed plants; the induction of systemic tolerance by bacterial compounds though microbe-induced physical and chemical changes in plants that lead to increased resistance to abiotic stresses; and the synthetization through bacteria of exopolysaccharides capable of binding Na+ ions [155].
Strains of Azospirillum brasilense are most often used as inoculants among the representatives of the genus Azospirillum in studies on the negative drought effects on plants (Figure 4). Sometimes, they are used in combination with other PGPRs, mycorrhizal fungi, or zinc or silicon oxide nanoparticles. Studies have also been conducted on A. baldaniorum Sp245 [156] (previously A. brasilense) and A. lipoferum [157].
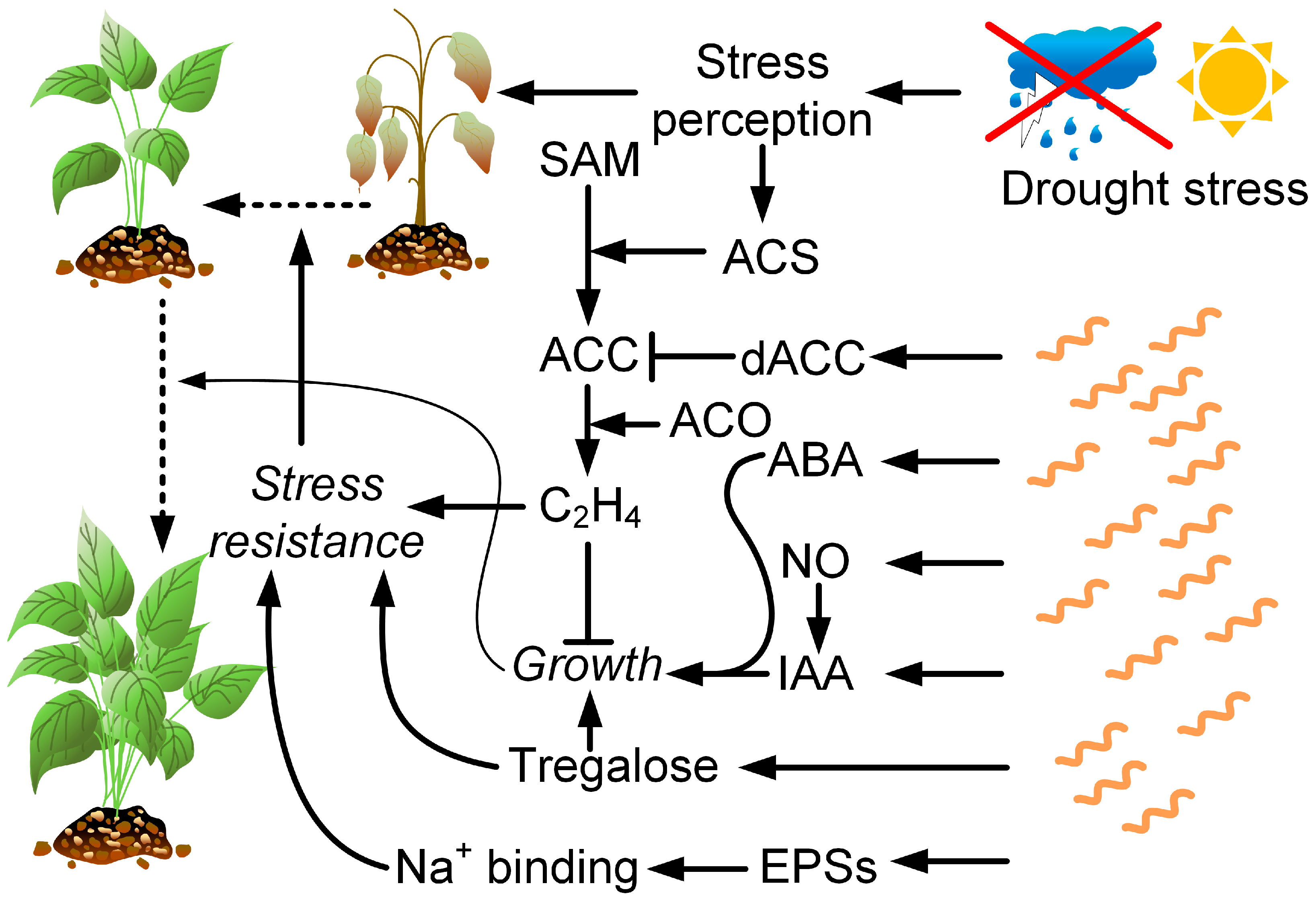
Figure 4. Drought has a complex negative effect on plants. However, some defense mechanisms are activated. For example, drought activates ACS (ACC synthase), which catalyzes the formation of ACC (1-aminocyclopropane-1-carboxylic acid) from SAM (S-adenosyl-L-methionine). Further, ethylene (C2H4) is formed from ACC by ACO—ACC oxidase. Ethylene, through a variety of mechanisms, increases plant resistance to drought, but at the same time, limits their growth, which can adversely affect crop productivity. The bacteria of the genus Azospirillum produce ACC deaminase (dACC), thereby limiting ethylene synthesis in plants. In addition, they produce ABA and IAA, as well as nitric oxide, which contributes to the synthesis of IAA. Together, these factors cause the induction of plant growth even in drought conditions. Azospirillum produces trehalose, which simultaneously promotes plant growth and increases its resistance to drought. Azospirillum also synthesizes exopolysaccharides capable of binding Na+ ions.
Azospirilla are able to increase plant resistance to drought stress through the production of auxins [37][158] or through the synthesis of nitric oxide, which acts as a signaling molecule in the IAA-inducing pathway [159][160]. Auxins, in small concentrations, enhance root growth and stimulate the formation of lateral roots. Thus, their effect on the plant leads to an increase in the area of the root system, and therefore, has a positive effect on water absorption and prevents the occurrence of drought stress [158].
ABA is considered to be one of the most important growth regulators involved in osmotic stress signaling and tolerance [161]. ABA accumulates to high levels during drought stress [162]. Data on the effect of azospirilla on the level of ABA in plants are contradictory. On the one hand, A. lipoferum has been shown to reduce drought stress through the production of ABA and gibberellins [163]. The level of ABA also increased in Arabidopsis plants inoculated with A. brasilense Sp245 [164]. The production of this hormone by A. lipoferum increased the concentration of ABA in inoculated maize seedlings (Z. mays), which led to stomatal closure [38]. However, stomatal closure inhibits photosynthesis, which leads to the inhibition of plant growth [165].
On the other hand, the inoculation of maize with the A. brasilense strain SP-7 in combination with the Herbaspirillum seropedicae strain Z-152 under drought conditions led to a decrease in the expression of the ZmVP14 gene, which is involved in the biosynthesis of ABA, and a decrease in the level of ABA in the plant. Additionally, in this work, inoculation caused a decrease in ET levels in corn [39].
One of the ways to stimulate drought resistance in plants through bacteria is to change the elasticity of root cell membranes [158]. It has been shown that A. brasilense reduces the membrane potentials of wheat seedlings and the content of phospholipids in cowpea cell membranes due to altered proton efflux activity [166]. Inoculation with azospirilla can prevent an increase in the level of phosphatidylcholine and a decrease in the level of phosphatidylethanolamine in water-deficient conditions in wheat seedlings [167].
Trehalose [168] and the polyamine cadaverine [169] can be mentioned as signaling molecules secreted by azospirilla that stimulate drought resistance in plants. Maize inoculation with A. brasilense, which overexpresses the trehalose biosynthesis gene, conferred drought tolerance on maize and significantly increased plant biomass. A very small amount of trehalose is thought to move into maize roots and signal pathways for plant stress tolerance [168].
Another indicator of a decrease in osmotic stress during drought, namely a decrease in the amount of proline, was observed when plants were inoculated with bacteria from the genus Azospirillum [38][39][170]. The inoculation of maize plants with A. lipoferum improved plant growth by accumulating free amino acids and soluble sugars compared to untreated plants under drought stress conditions [40].
It was also shown that under drought conditions, inoculation with bacteria from the genus Azospirillum leads to the activation in plants of enzymatic [157][171][172][173] and non-enzymatic [172][174] antioxidant pathways. Bacterial inoculation also led to lower levels of hydrogen peroxide and lipid peroxidation in plants [170].
The role of polysaccharides in plant adaptation to drought was also shown for members of the genus Azospirillum. A. brasilense Sp245 capsule material contains high molecular weight carbohydrate complexes (the lipopolysaccharide–protein complex and the polysaccharide–lipid complex) responsible for protection under extreme conditions, such as desiccation. The addition of these complexes to a suspension of decapsulated A. brasilense Sp245 cells significantly increased survival under drought stress conditions [175].
So, bacteria from the genus Azospirillum are actively used to mitigate the effects of drought in plants. The mechanism of stress factor mitigation is associated with the modulation of the level of phytohormones: auxins, ABA, ET, changes in the elasticity of root cell membranes, changes in the content of osmolytes, the activation of the antioxidant defense system, and the synthesis of polysaccharides.
3.7.2. Salinization
Salinity affects more than 6% of the world’s total land area (approximately 800 million hectares of land worldwide) [176]. Soil salinity has increased due to inefficient irrigation, improper fertilizer application, and industrial pollution [177]. Salinity causes Na+ toxicity and ionic imbalance and disrupts vital metabolic processes in plant cells, such as protein synthesis, enzymatic reactions, and ribosome functions [178].
PGPR can mitigate salinity-induced stress in plants through many synergistic mechanisms, including osmotic regulation, the stimulation of osmolyte accumulation and phytohormone signaling, the increase in nutrient uptake, the achievement of ion homeostasis, the reduction of oxidative stress via enhancing antioxidant activity [179], the increased synthesis of volatile organic compounds [180], and improved photosynthesis [76].
Representatives of the genus Azospirillum have repeatedly shown their effectiveness in reducing salt stress in plants. The possibility of their use as inoculants under salinity is due to the halotolerance of some strains [181][182].
They are used for the inoculation of plants under saline conditions, both alone and in combination with fungi [183], other PGPBs [41][42][184], and even with phosphogypsum [185].
The softening effect of inoculation can be manifested in the modulation of the concentration of osmolytes in plants. For example, one of the responses of corn to salinity is the accumulation of a powerful osmolite, i.e., raffinose, in the leaves. The inoculation of plants with A. brasilense (HM053) resulted in a decrease in the content of raffinose in the leaves and an increase in the content of sucrose [186]. Inoculation with azospirilla also improves the content of soluble sugars and proline in plants [42][44] and increases the content of glycine–betaine [187] under salt stress.
In addition, azospirilla can increase the K+/Na+ ratio in plants [41][42][43][183][184][188]; increase the content of nitrogen, phosphorus, calcium [183][188], and magnesium [183] in the crop; increase the content of nitrates; and reduce the content of chlorides [184], as well as increase the activity of nitrogenase and phosphatase [183] under salinity.
Additionally, inoculation with azospirilla leads to an improvement in the morphological characteristics of plants [41][42][43][44] and an increase in yield [41][185] and protein content [42][183] under saline conditions.
Azospirilla also affects the level of oxidative stress in plants under saline conditions. This results in a decrease in the content of malonic aldehyde [42][43] and hydrogen peroxide [42]. Data on the effect of azospirilla on antioxidant defense enzymes under salt stress are contradictory. They can cause both an increase [41][44][185][187] and a decrease [41][42] in the activity of antioxidant enzymes.
In most studies, an increase in the content of chlorophylls and carotenoids was shown when plants were inoculated with azospirilla under salt stress conditions [41][42][185]. However, Del Amor and Cuadra-Crespo, 2012, showed that the co-inoculation of A. brasilense and Pantoea diversa on sweet peppers did not affect the photochemical efficiency of photosystem II and the relative content of chlorophyll but contributed to maintaining a higher stomatal conductivity; therefore, they concluded that the influence of inoculants on the response to salinity was due mainly to the stomatal regulation of photosynthesis and not to the influence on the biochemical limitations of photosynthesis [184].
It was also shown that the most important compounds of secondary metabolism (phenylpropanoids, alkaloids, and other N-containing metabolites, as well as membrane lipids) and phytohormones (brassinosteroids, cytokinins, and methyl salicylate) showed the most pronounced modulation in response to treatment with azospirilla under salt stress [44]. Thus, the effect that azospirilla inoculation has on plants can be varied, but in most cases, it leads to the mitigation of salt stress.
References
- Jalmi, S.K.; Sinha, A.K. Ambiguities of PGPR-Induced Plant Signaling and Stress Management. Front. Microbiol. 2022, 13, 899563.
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350.
- Waqas, M.; Hawkesford, M.J.; Geilfus, C. Feeding the world sustainably: Efficient nitrogen use. Trends Plant Sci. 2023, 28, 505–508.
- Martinez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319.
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signalling processes. Soil Biol. Biochem. 2005, 37, 395–412.
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Lavakush, S.; Singh, V. Impact of plant growth promoting rhizobacteria on crop production. Int. J. Agric. Res. 2010, 5, 954–983.
- Renoud, S.; Abrouk, D.; Prigent-Combaret, C.; Wisniewski-Dyé, F.; Legendre, L.; Moënne-Loccoz, Y.; Muller, D. Effect of Inoculation Level on the Impact of the PGPR Azospirillum lipoferum CRT1 on Selected Microbial Functional Groups in the Rhizosphere of Field Maize. Microorganisms 2022, 10, 325.
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 21, 205.
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297.
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711.
- Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 5, 1763.
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562.
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594.
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 16, 2491.
- Chowdhury, S.P.; Hartmann, A.; Gao, X.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—A review. Front. Microbiol. 2015, 28, 780.
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39.
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 1, 203.
- Jeong, H.; Choi, S.K.; Ryu, C.M.; Park, S.H. Chronicle of a Soil Bacterium: Paenibacillus polymyxa E681 as a Tiny Guardian of Plant and Human Health. Front. Microbiol. 2019, 15, 467.
- Costa-Gutierrez, S.B.; Adler, C.; Espinosa-Urgel, M.; de Cristóbal, R.E. Pseudomonas putida and its close relatives: Mixing and mastering the perfect tune for plants. Appl. Microbiol. Biotechnol. 2022, 106, 3351–3367.
- Balthazar, C.; Joly, D.L.; Filion, M. Exploiting Beneficial Pseudomonas spp. for Cannabis Production. Front. Microbiol. 2022, 14, 833172.
- Zboralski, A.; Filion, M. Genetic factors involved in rhizosphere colonization by phytobeneficial Pseudomonas spp. Comput. Struct. Biotechnol. J. 2020, 19, 3539–3554.
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108.
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73.
- Wu, D.; Zhang, X.J.; Liu, H.C.; Zhou, Y.G.; Wu, X.L.; Nie, Y.; Kang, Y.Q.; Cai, M. Azospirillum oleiclasticum sp. nov, a nitrogen-fixing and heavy oil degrading bacterium isolated from an oil production mixture of Yumen Oilfield. Syst. Appl. Microbiol. 2021, 44, 126171.
- Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Okon, O.G.; Nadeem, M.A.; Nawaz, M.; Bodlah, M.A.; Waqas, M.M.; Iqbal, J.; et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 33909–33919.
- Xu, Q.; Pan, W.; Zhang, R.; Lu, Q.; Xue, W.; Wu, C.; Song, B.; Du, S. Inoculation with Bacillus subtilis and Azospirillum brasilense produces abscisic acid that reduces Irt1-mediated cadmium uptake of roots. J. Agric. Food Chem. 2018, 66, 5229–5236.
- Pan, W.; Lu, Q.; Xu, Q.R.; Zhang, R.R.; Li, H.Y.; Yang, Y.H.; Liu, H.J.; Du, S.T. Abscisic acid-generating bacteria can reduce Cd concentration in pakchoi grown in Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 177, 100–107.
- Belimov, A.A.; Dietz, K.J. Effect of associative bacteria on element composition of barley seedlings grown in solution culture at toxic cadmium concentrations. Microbiol. Res. 2000, 155, 113–121.
- Cui, Q.; Liu, D.; Chen, H.; Qiu, T.; Zhao, S.; Duan, C.; Cui, Y.; Zhu, X.; Chao, H.; Wang, Y.; et al. Synergistic interplay between Azospirillum brasilense and exogenous signaling molecule H2S promotes Cd stress resistance and growth in pak choi (Brassica chinensis L.). J. Hazard. Mater. 2023, 444, 130425.
- Marastoni, L.; Pii, Y.; Maver, M.; Valentinuzzi, F.; Cesco, S.; Mimmo, T. Role of Azospirillum brasilense in triggering different Fe chelate reductase enzymes in cucumber plants subjected to both nutrient deficiency and toxicity. Plant Physiol. Biochem. 2019, 136, 118–126.
- Bashan, Y.; De-Bashan, L.E. Protection of tomato seedlings against infection by Pseudomonas syringae pv. tomato by using the plant growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2002, 68, 2637–2643.
- Bashan, Y.; de-Bashan, L.E. Reduction of bacterial speck (Pseudomonas syringae pv. tomato) of tomato by combined treatments of plant growth-promoting bacterium, Azospirillum brasilense, streptomycin sulfate, and chemo-thermal seed treatment. Eur. J. Plant Pathol. 2002, 108, 821–829.
- Khan, M.R.; Kounsar, K.; Hamid, A. Effect of certain rhizobacteria and antagonistic fungi on root-nodulation and root-knot nematode disease of green gram . Nematol. Mediterr. 2002, 30, 85–89.
- Romero, A.M.; Correa, O.S.; Moccia, S.; Rivas, J.G. Effect of Azospirillum-mediated plant growth promotion on the development of bacterial diseases on fresh-market and cherry tomato. J. Appl. Microbiol. 2003, 95, 832–838.
- López-Reyes, L.; Carcaño-Montiel, M.G.; Lilia, T.L.; Medina-de la Rosa, G.; Armando, T.H.R. Antifungal and growth-promoting activity of Azospirillum brasilense in Zea mays L. ssp. mexicana. Arch. Phytopathol. Plant Prot. 2017, 50, 727–743.
- Djedidi, S.; Terasaki, A.; Aung, H.P.; Kojima, K.; Yamaya, H.; Ohkama-Ohtsu, N.; Bellingrath-Kimura, S.D.; Meunchang, P.; Yokoyama, T. Evaluation of the possibility to use the plant–microbe interaction to stimulate radioactive 137 Cs accumulation by plants in a contaminated farm field in Fukushima, Japan. J. Plant Res. 2015, 128, 147–159.
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205.
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90.
- Curá, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria Increases the Tolerance of Maize to Drought Stress. Microorganisms 2017, 5, 41.
- Bano, Q.; Ilyas, N.; Bano, A.; Zafar, N.; Akram, A.; Hassan, F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013, 45, 13–20.
- Rabiei, Z.; Hosseini, S.J.; Pirdashti, H.; Hazrati, S. Physiological and biochemical traits in coriander affected by plant growth-promoting rhizobacteria under salt stress. Heliyon 2020, 6, e05321.
- Omer, A.M.; Osman, M.S.; Badawy, A.A. Inoculation with Azospirillum brasilense and/or Pseudomonas geniculata reinforces flax (Linum usitatissimum) growth by improving physiological activities under saline soil conditions. Bot. Stud. 2022, 63, 15.
- Khalid, M.; Bilal, M.; Hassani, D.; Iqbal, H.; Wang, H.; Huang, D. Mitigation of salt stress in white clover (Trifolium repens) by Azospirillum brasilense and its inoculation effect. Bot. Stud. 2017, 58, 1–7.
- Alzate Zuluaga, M.Y.; Miras-Moreno, B.; Monterisi, S.; Rouphael, Y.; Colla, G.; Lucini, L.; Cesco, S.; Pii, Y. Integrated Metabolomics and Morpho-Biochemical Analyses Reveal a Better Performance of Azospirillum brasilense over Plant-Derived Biostimulants in Counteracting Salt Stress in Tomato. Int. J. Mol. Sci. 2022, 23, 14216.
- De Dios, R.; Santero, E.; Reyes-Ramírez, F. Extracytoplasmic function σ factors as tools for coordinating stress responses. Int. J. Mol. Sci. 2021, 22, 3900.
- Mishra, M.N.; Kumar, S.; Gupta, N.; Kaur, S.; Gupta, A.; Tripathi, A.K. An extracytoplasmic function sigma factor cotranscribed with its cognate anti-sigma factor confers tolerance to NaCl, ethanol and methylene blue in Azospirillum brasilense Sp7. Microbiology 2011, 157, 988–999.
- Thirunavukkarasu, N.; Mishra, M.N.; Spaepen, S.; Vanderleyden, J.; Gross, C.A.; Tripathi, A.K. An extra-cytoplasmic function sigma factor and anti-sigma factor control carotenoid biosynthesis in Azospirillum brasilense. Microbiology 2008, 154, 2096–2105.
- Rai, A.K.; Dubey, A.P.; Kumar, S.; Dutta, D.; Mishra, M.N.; Singh, B.N.; Tripathi, A.K. Carotenoid biosynthetic pathways are regulated by a network of multiple cascades of alternative sigma factors in Azospirillum brasilense Sp7. J. Bacteriol. 2016, 198, 2955–2964.
- Kumar, S.; Rai, A.K.; Mishra, M.N.; Shukla, M.; Singh, P.K.; Tripathi, A.K. RpoH2 sigma factor controls the photooxidative stress response in a non-photosynthetic rhizobacterium, Azospirillum brasilense Sp7. Microbiology 2012, 158, 2891–2902.
- Gupta, N.; Gupta, A.; Kumar, S.; Mishra, R.; Singh, C.; Tripathi, A.K. Cross-talk between cognate and noncognate RpoE sigma factors and Zn2+-binding anti-sigma factors regulates photooxidative stress response in Azospirillum brasilense. Antioxid. Redox Signal. 2014, 20, 42–59.
- Singh, V.S.; Dubey, B.K.; Pandey, P.; Rai, S.; Tripathi, A.K. Cometabolism of ethanol in Azospirillum brasilense Sp7 is mediated by fructose and glycerol and regulated negatively by an alternative sigma factor RpoH2. J. Bacteriol. 2021, 203, e00269-21.
- Pandey, P.; Dubey, A.P.; Mishra, S.; Singh, V.S.; Singh, C.; Tripathi, A.K. β-lactam resistance in Azospirillum baldaniorum Sp245 is mediated by lytic transglycosylase and β-lactamase and regulated by a cascade of RpoE7 → RpoH3 sigma factors. J. Bacteriol. 2022, 204, e00010-22.
- Rai, A.K.; Singh, S.; Dwivedi, S.K.; Srivastava, A.; Pandey, P.; Kumar, S.; Singh, B.N.; Tripathi, A.K. Catalase expression in Azospirillum brasilense Sp7 Is regulated by a network consisting of OxyR and two RpoH paralogs and including an RpoE1 → RpoH5 regulatory cascade. Appl. Environ. Microbiol. 2018, 84, e01787-18.
- García, J.E.; Labarthe, M.M.; Pagnussat, L.A.; Amenta, M.; Creus, C.M.; Maroniche, G.A. Signs of a phyllospheric lifestyle in the genome of the stress-tolerant strain Azospirillum brasilense Az19. Syst. Appl. Microbiol. 2020, 43, 126130.
- Ayotamuno, J.M.; Kogbara, R.B. Determining the tolerance level of Zea mays (maize) to a crude oil polluted agricultural soil. Afr. J. Biotechnol. 2007, 6, 1332–1337.
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public Health 2019, 16, 2474.
- Viñas, M.; Sabaté, J.; Espuny, M.J.; Solanas, A.M. Bacterial community dynamics and polycyclic aromatic hydrocarbon degradation during bioremediation of heavily creosote-contaminated soil. Appl. Environ. Microbiol. 2005, 71, 7008–7018.
- Muratova, A.I.; Turkovskaia, O.V.; Antoniuk, L.P.; Makarov, O.E.; Pozdniakova, L.I.; Ignatov, V.V. Oil-oxidizing potential of associative rhizobacteria of the genus Azospirillum. Mikrobiologiia 2005, 74, 248–254.
- Barkay, T.; Navon-Venezia, S.; Ron, E.Z.; Rosenberg, E. Enhancement of solubilization and biodegradationof polyaromatic hydrocarbons by the bioemulsifier alasan. Appl. Environ. Microbiol. 1999, 65, 2697–2702.
- Furtak, K.; Gawryjołek, K.; Gałązka, A.; Grządziel, J. The Response of Red Clover (Trifolium pratense L.) to Separate and Mixed Inoculations with Rhizobium leguminosarum and Azospirillum brasilense in Presence of Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2020, 17, 5751.
- Muratova, A.Y.; Bondarenkova, A.D.; Panchenko, L.V.; Turkovskaya, O.V. Use of integrated phytoremediation for cleaning-up of oil-sludge-contaminated soil. Appl. Biochem. Microbiol. 2010, 46, 789–794.
- Huang, X.D.; El-Alawi, Y.; Penrose, D.M.; Glick, B.R.; Greenberg, B.M. A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ. Pollut. 2004, 130, 465–476.
- Król, M.J.; Perzynski, A. Wykorzystanie pirenu jako jedynego zrodla wegla w wiazaniu wolnego azotu przez bakterie z rodzaju Azospirillum. Pamiętnik Puławski 2004, 137, 95–105.
- Miranda-Martínez, M.R.; Delgadillo-Martínez, J.; Alarcón, A.; Ferrera-Cerrato, R. Degradación de fenantreno por microorganismos en la rizósfera del pasto alemán. Terra Latinoam. 2007, 25, 25–33.
- Eckford, R.; Cook, F.D.; Saul, D.; Aislabie, J.; Foght, J. Free-living heterotrophic nitrogen-fixing bacteria isolated from fuel-contaminated Antarctic soils. Appl. Environ. Microbiol. 2002, 68, 5181–5185.
- Al-Mailem, D.M.; Kansour, M.K.; Radwan, S.S. Cross-bioaugmentation among four remote soil samples contaminated with oil exerted just inconsistent effects on oil-bioremediation. Front. Microbiol. 2019, 10, 2827.
- Saeed, M.; Ilyas, N.; Arshad, M.; Sheeraz, M.; Ahmed, I.; Bhattacharya, A. Development of a plant microbiome bioremediation system for crude oil contamination. J. Environ. Chem. Eng. 2021, 9, 105401.
- Young, C.C.; Hupfer, H.; Siering, C.; Ho, M.J.; Arun, A.B.; Lai, W.A.; Rekha, P.D.; Shen, F.T.; Hung, M.H.; Chen, W.M.; et al. Azospirillum rugosum sp. nov., isolated from oil-contaminated soil. Int. J. Syst. Evol. Microbiol. 2008, 58, 959–963.
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66.
- Varjani, S.J.; Singh, K.V. Plant growth-promoting rhizobacteria and its role in sustainable agriculture. In Probiotics in Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2017; pp. 195–206.
- Ismail, M.; Prasad, R.; Ibrahim, A.I.M.; Ahmed, A.I.S. Modern prospects of nanotechnology in plant pathology. In Nanotechnology; Springer: Singapore, 2017; pp. 305–317.
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239.
- Sayyed, R.Z.; Patel, P.R.; Shaikh, S.S. Plant growth promotion and root colonization by EPS producing Enterobacter sp. RZS5 under heavy metal contaminated soil. Indian J. Exp. Biol. 2015, 53, 116–123.
- Fazeli-Nasab, B.; Sayyed, R.Z.; Mojahed, L.S.; Rahmani, A.F.; Ghafari, M.; Antonius, S. Biofilm production: A strategic mechanism for survival of microbes under stress conditions. Biocatal. Agric. Biotechnol. 2022, 42, 102337.
- Sheikh, T.; Hamid, B.; Baba, Z.; Iqbal, S.; Yatoo, A.; Fatima, S.; Nabi, A.; Kanth, R.; Dar, K.; Hussain, N.; et al. Extracellular polymeric substances in psychrophilic cyanobacteria: A potential bioflocculant and carbon sink to mitigate cold stress. Biocatal. Agric. Biotechnol. 2022, 42, 102375.
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W.; et al. The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 2022, 133, 2717–2741.
- Vezza, M.E.; Olmos Nicotra, M.F.; Agostini, E.; Talano, M.A. Biochemical and molecular characterization of arsenic response from Azospirillum brasilense Cd, a bacterial strain used as plant inoculant. Environ. Sci. Pollut. Res. 2020, 27, 2287–2300.
- Kaur, P.; Singh, S.; Kumar, V.; Singh, N.; Singh, J. Effect of rhizobacteria on arsenic uptake by macrophyte Eichhornia crassipes (Mart.) Solms. Int. J. Phytoremediat. 2018, 20, 114–120.
- Armendariz, A.L.; Talano, M.A.; Oller, A.L.W.; Medina, M.I.; Agostini, E. Effect of arsenic on tolerance mechanisms of two plant growth-promoting bacteria used as biological inoculants. J. Environ. Sci. 2015, 33, 203–210.
- Arora, K.; Sharma, S.; Monti, A. Bio-remediation of Pb and Cd polluted soils by switchgrass: A case study in India. Int. J. Phytoremediation 2016, 18, 704–709.
- Muratova, A.Y.; Lyubun, E.V.; Golubev, S.N.; Turkovskaya, O.V. Effect of copper ions on the associations of Azospirillum bacteria with wheat seedlings (Triticum aestivum L.). Vavilov J. Genet. Breed. 2022, 26, 477.
- Peralta, J.M.; Bianucci, E.; Romero-Puertas, M.C.; Furlan, A.; Castro, S.; Travaglia, C. Targeting redox metabolism of the maize-Azospirillum brasilense interaction exposed to arsenic-affected groundwater. Physiol. Plant. 2021, 173, 1189–1206.
- Peng, H.; De-Bashan, L.E.; Higgins, B.T. Azospirillum brasilense reduces oxidative stress in the green microalgae Chlorella sorokiniana under different stressors. J. Biotechnol. 2021, 325, 179–185.
- Housh, A.B.; Benoit, M.; Wilder, S.L.; Scott, S.; Powell, G.; Schueller, M.J.; Ferrieri, R.A. Plant-growth-promoting bacteria can impact zinc uptake in Zea mays: An examination of the mechanisms of action using functional mutants of Azospirillum brasilense. Microorganisms 2021, 9, 1002.
- Rajwade, J.M.; Chikte, R.G.; Paknikar, K.M. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biotechnol. 2020, 104, 1437–1461.
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Arch. Microbiol. 2011, 193, 275–286.
- Meyer, J.M.; Azelvandre, P.; Georges, C. Iron metabolism in Pseudomonas: Salicylic acid, a siderophore of Pseudomonas fluorescens CHAO. BioFactors 1992, 4, 23–27.
- Delaney, T.P.; Uknes, S.; Vernooij, B.; Friedrich, L.; Weymann, K.; Negrotto, D.; Gaffney, T.; Gut-Rella, M.; Kessmann, H.; Ward, E.; et al. A central role of salicylic acid in plant disease resistance. Science 1994, 266, 1247–1250.
- Ross, A.F. Systemic acquired resistance induced by localized virus infections in plants. Virology 1961, 14, 340–358.
- Kloepper, J.W.; Tuzun, S.; Kuć, J.A. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992, 2, 349–351.
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386.
- Pieterse, C.M.; Van Wees, S.C.; Hoffland, E.; Van Pelt, J.A.; Van Loon, L.C. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 1996, 8, 1225–1237.
- Thomma, B.P.H.J.; Eggermont, K.; Penninckx, I.A.M.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.A.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111.
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863.
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170.
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349.
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725.
- Elías, J.M.; Guerrero-Molina, M.F.; Martínez-Zamora, M.G.; Díaz-Ricci, J.C.; Pedraza, R.O. Role of ethylene and related gene expression in the interaction between strawberry plants and the plant growth-promoting bacterium Azospirillum brasilense. Plant Biol. 2018, 20, 490–496.
- Kusajima, M.; Shima, S.; Fujita, M.; Minamisawa, K.; Che, F.S.; Yamakawa, H.; Nakashita, H. Involvement of ethylene signaling in Azospirillum sp. B510-induced disease resistance in rice. Biosci. Biotechnol. Biochem. 2018, 82, 1522–1526.
- Yasuda, M.; Isawa, T.; Shinozaki, S.; Minamisawa, K.; Nakashita, H. Effects of colonization of a bacterial endophyte, Azospirillum sp. B510, on disease resistance in rice. Biosci. Biotechnol. Biochem. 2009, 73, 2595–2599.
- Drogue, B.; Sanguin, H.; Chamam, A.; Mozar, M.; Llauro, C.; Panaud, O.; Prigent-Combaret, C.; Picault, N.; Wisniewski-Dyé, F. Plant root transcriptome profiling reveals a strain-dependent response during Azospirillum-rice cooperation. Front. Plant Sci. 2014, 5, 607.
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of A rabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol. 2014, 201, 850–861.
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 1–13.
- Jarosz, L. Growing inequality: Agricultural revolutions and the political ecology of rural development. Int. J. Agric. Sustain. 2012, 10, 192–199.
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112.
- Giménez–Moolhuyzen, M.; van der Blom, J.; Lorenzo–Mínguez, P.; Cabello, T.; Crisol–Martínez, E. Photosynthesis inhibiting effects of pesticides on sweet pepper leaves. Insects 2020, 11, 69.
- Vitkalova, I.Y.; Gureev, A.P.; Shaforostova, E.A.; Boyko, O.N.; Igamberdiev, A.U.; Popov, V.N. The effect of pesticides on the mtDNA integrity and bioenergetic properties of potato mitochondria. Pestic. Biochem. Physiol. 2021, 172, 104764.
- Liu, R.; Li, J.; Zhang, L.; Feng, T.; Zhang, Z.; Zhang, B. Fungicide difenoconazole induced biochemical and developmental toxicity in wheat (Triticum aestivum L.). Plants 2021, 10, 2304.
- Jakl, M.; Kovač, I.; Zeljković, S.Ć.; Dytrtová, J.J. Triazole fungicides in soil affect the yield of fruit, green biomass, and phenolics production of Solanum lycopersicum L. Food Chem. 2021, 351, 129328.
- Singh, S.; Gupta, R.; Kumari, M.; Sharma, S. Nontarget effects of chemical pesticides and biological pesticide on rhizospheric microbial community structure and function in Vigna radiata. Environ. Sci. Pollut. Res. 2015, 22, 11290–11300.
- Gomez, F.; Salmeron, V.; Rodelas, B.; Martinez-Toled, M.V.; Gonzalez-Lopez, J. Response of Azospirillum brasilense to the pesticides bromopropylate and methidathion on chemically defined media and dialysed-soil media. Ecotoxicology 1998, 7, 43–47.
- Bashan, Y.; Holguin, G.; De-Bashan, L.E. Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances (1997–2003). Can. J. Microbiol. 2004, 50, 521–577.
- Raji, P.; Pillai, M.V. Effect of plant protection chemicals on Azospirillum in cowpea . Legume Res. Int. J. 2000, 23, 177–179.
- Foster, L.J.R.; Kwan, B.H.; Vancov, T. Microbial degradation of the organophosphate pesticide, Ethion. FEMS Microbiol. Lett. 2004, 240, 49–53.
- Santos, M.S.; Rondina, A.B.; Nogueira, M.A.; Hungria, M. Compatibility of Azospirillum brasilense with pesticides used for treatment of maize seeds. Int. J. Microbiol. 2020, 2020, 8833879.
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Response of soil microorganisms and enzymes to the foliar application of Helicur 250 EW fungicide on Horderum vulgare L. Chemosphere 2020, 242, 125163.
- Takahashi, W.Y.; Galvão, C.W.; Urrea-Valencia, S.; Gonçalves, D.R.P.; Hyeda, D.; Caires, E.F.; Etto, R.M. Impact of seed-applied fungicide and insecticide on Azospirillum brasilense survival and wheat growth-promoting ability. Lett. Appl. Microbiol. 2022, 74, 604–612.
- Acevedo-Barrios, R.; Olivero-Verbel, J. Perchlorate Contamination: Sources, Effects, and Technologies for Remediation. Rev. Environ. Contam. Toxicol. Vol. 2021, 256, 103–120.
- Andraski, B.J.; Jackson, W.A.; Welborn, T.L.; Böhlke, J.K.; Sevanthi, R.; Stonestrom, D.A. Soil, plant, and terrain effects on natural perchlorate distribution in a desert landscape. J. Environ. Qual. 2014, 43, 980–994.
- Xie, Y.; Tao, G.; Chen, Q.; Tian, X. Effects of perchlorate stress on growth and physiological characteristics of rice (Oryza sativa L.) seedlings. Water Air Soil Pollut. 2014, 225, 1–8.
- He, H.; Gao, H.; Chen, G.; Li, H.; Lin, H.; Shu, Z. Effects of perchlorate on growth of four wetland plants and its accumulation in plant tissues. Environ. Sci. Pollut. Res. 2013, 20, 7301–7308.
- Acevedo-Barrios, R.; Sabater-Marco, C.; Olivero-Verbel, J. Ecotoxicological assessment of perchlorate using in vitro and in vivo assays. Environ. Sci. Pollut. Res. 2018, 25, 13697–13708.
- Butler, C.S.; Clauwaert, P.; Green, S.J.; Verstraete, W.; Nerenberg, R. Bioelectrochemical perchlorate reduction in a microbial fuel cell. Environ. Sci. Technol. 2010, 44, 4685–4691.
- Bardiya, N.; Bae, J.H. Dissimilatory perchlorate reduction: A review. Microbiol. Res. 2011, 166, 237–254.
- Coates, J.D.; Achenbach, L.A. Microbial perchlorate reduction: Rocket-fuelled metabolism. Nat. Rev. Microbiol. 2004, 2, 569–580.
- Ju, X.; Field, J.A.; Sierra-Alvarez, R.; Salazar, M.; Bentley, H.; Bentley, R. Chemolithotrophic perchlorate reduction linked to the oxidation of elemental sulfur. Biotechnol. Bioeng. 2007, 96, 1073–1082.
- Nerenberg, R.; Rittmann, B.E.; Najm, I. Perchlorate reduction in a hydrogen-based membrane–biofilm reactor. J. Am. Water Work. Assoc. 2002, 94, 103–114.
- Luo, Y.H.; Chen, R.; Wen, L.L.; Meng, F.; Zhang, Y.; Lai, C.Y.; Rittmann, B.E.; Zhao, H.P.; Zheng, P. Complete perchlorate reduction using methane as the sole electron donor and carbon source. Environ. Sci. Technol. 2015, 49, 2341–2349.
- Lv, P.L.; Shi, L.D.; Wang, Z.; Rittmann, B.; Zhao, H.P. Methane oxidation coupled to perchlorate reduction in a membrane biofilm batch reactor. Sci. Total Environ. 2019, 667, 9–15.
- Xie, T.; Yang, Q.; Winkler, M.K.; Wang, D.; Zhong, Y.; An, H.; Chen, F.; Yao, F.; Wang, X.; Wu, J.; et al. Perchlorate bioreduction linked to methane oxidation in a membrane biofilm reactor: Performance and microbial community structure. J. Hazard. Mater. 2018, 357, 244–252.
- Wan, D.; Liu, Y.; Niu, Z.; Xiao, S.; Li, D. Perchlorate reduction by hydrogen autotrophic bacteria and microbial community analysis using high-throughput sequencing. Biodegradation 2016, 27, 47–57.
- Coates, J.; Jackson, W.A. Principles of perchlorate treatment. In Situ Bioremediation of Perchlorate in Groundwater; Stroo, H.F., Ward, C.H., Eds.; Springer: New York, NY, USA, 2009; pp. 29–53.
- Xu, X.; Gao, B.; Jin, B.; Zhen, H.; Wang, X.; Dai, M. Study of microbial perchlorate reduction: Considering of multiple pH, electron acceptors and donors. J. Hazard. Mater. 2015, 285, 228–235.
- Shrout, J.D.; Scheetz, T.E.; Casavant, T.L.; Parkin, G.F. Isolation and characterization of autotrophic, hydrogen-utilizing, perchlorate-reducing bacteria. Appl. Microbiol. Biotechnol. 2005, 67, 261–268.
- Acevedo-Barrios, R.; Bertel-Sevilla, A.; Alonso-Molina, J.; Olivero-Verbel, J. Perchlorate-reducing bacteria from hypersaline soils of the Colombian Caribbean. Int. J. Microbiol. 2019, 2019, 6981865.
- Waller, A.S.; Cox, E.E.; Edwards, E.A. Perchlorate-reducing microorganisms isolated from contaminated sites. Environ. Microbiol. 2004, 6, 517–527.
- Coates, J.D.; Michaelidou, U.; Bruce, R.A.; O’Connor, S.M.; Crespi, J.N.; Achenbach, L.A. Ubiquity and diversity of dissimilatory (per) chlorate-reducing bacteria. Appl. Environ. Microbiol. 1999, 65, 5234–5241.
- Nozawa-Inoue, M.; Scow, K.M.; Rolston, D.E. Reduction of perchlorate and nitrate by microbial communities in vadose soil. Appl. Environ. Microbiol. 2005, 71, 3928–3934.
- Zhu, J.K.; Hasegawa, P.M.; Bressan, R.A.; Bohnert, H.J. Molecular aspects of osmotic stress in plants. Crit. Rev. Plant Sci. 1997, 16, 253–277.
- Hoekstra, F.A.; Golovina, E.A.; Tetteroo, F.A.; Wolkers, W.F. Induction of desiccation tolerance in plant somatic embryos: How exclusive is the protective role of sugars? Cryobiology 2001, 43, 140–150.
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703.
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171.
- Gorham, J. Betaines in higher plants-biosynthesis and role in stress metabolism. Semin. Ser. Soc. Exp. Biol. 1995, 56, 173.
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216.
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689.
- Riou, N.; Le Rudulier, D. Osmoregulation in Azospirillum brasilense: Glycine betaine transport enhances growth and nitrogen fixation under salt stress. Microbiology 1990, 136, 1455–1461.
- Madkour, M.A.; Smith, L.T.; Smith, G.M. Preferential osmolyte accumulation: A mechanism of osmotic stress adaptation in diazotrophic bacteria. Appl. Environ. Microbiol. 1990, 56, 2876–2881.
- Riou, N.; Poggi, M.C.; Le Rudulier, D. Characterization of an osmoregulated periplasmic glycine betaine-binding protein in Azospirillum brasilense sp7. Biochimie 1991, 73, 1187–1193.
- Tripathi, A.K.; Mishra, B.M. Isolation and cloning of a Azospirillum lipoferum locus that complements Escherichia coli proU mutant. FEMS Microbiol. Lett. 1998, 162, 241–247.
- Chowdhury, P.S.; Nagarajan, T.; Tripathi, R.; Mishra, M.N.; Le Rudulier, D.; Tripathi, A.K. Strain-specific salt tolerance and osmoregulatory mechanisms in Azospirillum brasilense. FEMS Microbiol. Lett. 2007, 267, 72–79.
- Nagarajan, T.; Vanderleyden, J.; Tripathi, A.K. Identification of salt stress inducible genes that control cell envelope related functions in Azospirillum brasilense Sp7. Mol. Genet. Genom. 2007, 278, 43–51.
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33.
- Caravaca, F.; Alguacil, M.M.; Hernández, J.A.; Roldán, A. Involvement of antioxidant enzyme and nitrate reductase activities during water stress and recovery of mycorrhizal Myrtus communis and Phillyrea angustifolia plants. Plant Sci. 2005, 169, 191–197.
- Ali, S.Z.; Sandhya, V.; Venkateswar Rao, L. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 2014, 64, 493–502.
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24.
- Mariotti, L.; Scartazza, A.; Curadi, M.; Picciarelli, P.; Toffanin, A. Azospirillum baldaniorum Sp245 induces physiological responses to alleviate the adverse effects of drought stress in purple basil. Plants 2021, 10, 1141.
- Akhtar, N.; Ilyas, N.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol. Biochem. 2021, 166, 160–176.
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694.
- Creus, C.M.; Graziano, M.; Casanovas, E.M.; Pereyra, M.A.; Simontacchi, M.; Puntarulo, S.; Barassi, C.A.; Lamattina, L. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 2005, 221, 297–303.
- Molina-Favero, C.; Creus, C.M.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Aerobic nitric oxide production by Azospirillum brasilense Sp245 and its influence on root architecture in tomato. Mol. Plant-Microbe Interact. 2008, 21, 1001–1009.
- Tiwari, S.; Lata, C.; Singh Chauhan, P.; Prasad, V.; Prasad, M. A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr. Genom. 2017, 18, 469–482.
- Kudoyarova, G.R.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Yu Veselov, S. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66, 2133–2144.
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462.
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in arabidopsis plants. Plant Growth Regul. 2008, 54, 97–103.
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Rhizobacteria inoculation effects on phytohormone status of potato microclones cultivated in vitro under osmotic stress. Biomolecules 2020, 10, 1231.
- Bashan, Y.; Alcaraz-Melendez, L.; Toledo, G. Responses of soybean and cowpea root membranes to inoculation with Azospirillum brasilense. Symbiosis 1992, 13, 217–228.
- Pereyra, M.A.; Zalazar, C.A.; Barassi, C.A. Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress. Plant Physiol. Biochem. 2006, 44, 873–879.
- Rodríguez-Salazar, J.; Suarez, R.; Caballero-Mellado, J.; Iturriaga, G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009, 296, 52–59.
- Cassan, F.; Maiale, S.; Masciarelli, O.; Vidal, A.; Luna, V.; Ruiz, O. Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur. J. Soil Biol. 2009, 45, 12–19.
- Tiepo, A.N.; Hertel, M.F.; Rocha, S.S.; Calzavara, A.K.; De Oliveira, A.L.M.; Pimenta, J.A.; Oliveira, H.C.; Bianchini, E.; Stolf-Moreira, R. Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol. Biochem. 2018, 130, 277–288.
- Muhammad, F.; Raza, M.A.S.; Iqbal, R.; Zulfiqar, F.; Aslam, M.U.; Yong, J.W.H.; Altaf, M.A.; Zulfiqar, B.; Amin, J.; Ibrahim, M.A. Ameliorating Drought Effects in Wheat Using an Exclusive or Co-Applied Rhizobacteria and ZnO Nanoparticles. Biology 2022, 11, 1564.
- Tiepo, A.N.; Constantino, L.V.; Madeira, T.B.; Gonçalves, L.S.A.; Pimenta, J.A.; Bianchini, E.; de Oliveira, A.L.M.; Oliveira, H.C.; Stolf-Moreira, R. Plant growth-promoting bacteria improve leaf antioxidant metabolism of drought-stressed Neotropical trees. Planta 2020, 251, 1–11.
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61.
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130.
- Konnova, S.A.; Brykova, O.S.; Sachkova, O.A.; Egorenkova, I.V.; Ignatov, V.V. Protective role of the polysaccharide-containing capsular components of Azospirillum brasilense. Microbiology 2001, 70, 436–440.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651.
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol. Biochem. 2014, 83, 126–133.
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884.
- Najafi, S.; Nazari Nasi, H.; Tuncturk, R.; Tuncturk, M.; Sayyed, R.Z.; Amirnia, R. Biofertilizer application enhances drought stress tolerance and alters the antioxidant enzymes in medicinal pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021, 7, 588.
- Sudha, A.; Durgadevi, D.; Archana, S.; Muthukumar, A.; Suthin Raj, T.; Nakkeeran, S.; Poczai, P.; Nasif, O.; Sayyed, R.Z. Unraveling the tripartite interaction of volatile compounds of Streptomyces rochei with grain mold pathogens infecting sorghum. Front. Microbiol. 2022, 13, 923360.
- Rivarola, V.; Castro, S.; Mori, G.; Jofré, E.; Fabra, A.; Garnica, R.; Balegno, H. Response of Azospirillum brasilense Cd to sodium chloride stress. Antonie Leeuwenhoek 1998, 73, 255–261.
- Saleena, L.M.; Rangarajan, S.; Nair, S. Diversity of Azospirillum strains isolated from rice plants grown in saline and nonsaline sites of coastal agricultural ecosystem. Microb. Ecol. 2002, 44, 271–277.
- Rabie, G.H.; Aboul-Nasr, M.B.; Al-Humiany, A. Increased salinity tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungus Glomus clarum and a nitrogen-fixer Azospirillum brasilense. Mycobiology 2005, 33, 51–60.
- Del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2011, 39, 82–90.
- Khalifa, T.; Elbagory, M.; Omara, A.E.D. Salt stress amelioration in maize plants through phosphogypsum application and bacterial inoculation. Plants 2021, 10, 2024.
- Powell, A.; Wilder, S.L.; Housh, A.B.; Scott, S.; Benoit, M.; Powell, G.; Waller, S.; Guthrie, J.M.; Schueller, M.J.; Ferrieri, R.A. Examining effects of rhizobacteria in relieving abiotic crop stresses using carbon-11 radiotracing. Physiol. Plant. 2022, 174, e13675.
- Checchio, M.V.; de Cássia Alves, R.; de Oliveira, K.R.; Moro, G.V.; Santos, D.M.M.D.; Gratão, P.L. Enhancement of salt tolerance in corn using Azospirillum brasilense: An approach on antioxidant systems. J. Plant Res. 2021, 134, 1279–1289.
- Alamri, S.A.; Mostafa, Y.S. Effect of nitrogen supply and Azospirillum brasilense Sp-248 on the response of wheat to seawater irrigation. Saudi J. Biol. Sci. 2009, 16, 101–107.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
640
Revisions:
2 times
(View History)
Update Date:
02 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




