You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergey Ushakov | -- | 3067 | 2023-06-01 02:26:28 | | | |
| 2 | Jessie Wu | Meta information modification | 3067 | 2023-06-01 02:36:41 | | | | |
| 3 | Jessie Wu | + 4 word(s) | 3071 | 2023-06-01 02:39:56 | | | | |
| 4 | Jessie Wu | -4 word(s) | 3067 | 2023-06-05 08:55:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ushakov, S.V.; Hong, Q.; Gilbert, D.A.; Navrotsky, A.; Walle, A.V.D. Thorium and Rare Earth Monoxides. Encyclopedia. Available online: https://encyclopedia.pub/entry/45086 (accessed on 21 December 2025).
Ushakov SV, Hong Q, Gilbert DA, Navrotsky A, Walle AVD. Thorium and Rare Earth Monoxides. Encyclopedia. Available at: https://encyclopedia.pub/entry/45086. Accessed December 21, 2025.
Ushakov, Sergey V., Qi-Jun Hong, Dustin A. Gilbert, Alexandra Navrotsky, Axel Van De Walle. "Thorium and Rare Earth Monoxides" Encyclopedia, https://encyclopedia.pub/entry/45086 (accessed December 21, 2025).
Ushakov, S.V., Hong, Q., Gilbert, D.A., Navrotsky, A., & Walle, A.V.D. (2023, June 01). Thorium and Rare Earth Monoxides. In Encyclopedia. https://encyclopedia.pub/entry/45086
Ushakov, Sergey V., et al. "Thorium and Rare Earth Monoxides." Encyclopedia. Web. 01 June, 2023.
Copy Citation
Thorium was a part of energy infrastructure in the 19th century due to the refractory and electronic properties of its dioxide. It will be a part of future energy infrastructure as the most abundant energy reserve based on nuclear fission.
thorium
thorium monoxide
rare earths
rare earth monoxides
1. Thorium and Rare Earth Rocksalt Phases
Rocksalt structure, also known as B1 or NaCl-type structure, can be described as two interpenetrating fcc sublattices of metal and non-metal atoms. Reported thorium rocksalt phases include ThO, ThC, ThN, ThS, ThAs, ThP, ThSb, and ThSe [1][2][3]. One could mistakenly conclude that such variety of rocksalt phases in thorium compounds is due to the fcc structure of thorium metal, but in fact, all other studied actinide monopnictides and monochalcogenides also form rocksalt phases, as well as rare earth monoxides, nitrides, and Ti, Zr, and Hf nitrides and carbides [4]. Most of these compounds have metallic conductivity but do not inherit ductility. Although they often have a range of possible stoichiometries, they are not interstitial Hagg phases [3], since most of these metals are not fcc and do experience changes in their positions to form the fcc sublattice in a rocksalt phase.
These compounds, in which metal electrons are drained from metallic bonding to form ionic/covalent bonds with non-metals, are fascinating from both fundamental and applied viewpoints. For thorium-rich phases, the changes in the role of 5f electrons with changes in the degree of metallic—ionic—covalent bonding is the fundamental aspect. The fact that these rocksalt phases retain metal-like thermal and electrical conductivity but gain remarkable increase in melting temperature and bulk modulus make them useful for a variety of applications from nuclear fuels to ultra-high temperature ceramics.
RE-N and Th-RE-N systems provide examples of continuous solid solutions between Th and RE nitrides in a rocksalt phase. Holleck [5][6] reported solid solutions in ThN-REN systems with Y and La-Nd. These findings suggest a possibility of complete miscibility in (Th,RE)O solid solutions. Extensive solubility is corroborated by continuous solid solutions between Th and RE metals in high-temperature bcc structure, with the exception of Yb and Eu, noted above.
Rocksalt Solid Solutions in Th-C-N-O Systems
Thorium monocarbide and thorium mononitride crystallize in NaCl-type rocksalt structure and form a complete solid solution. ThC is stable in rocksalt structure to ≈58 GPa [7]. The limits of oxygen solubility in thorium carbide at ambient pressure are unknown. Henney et al. [8] reported the synthesis of thorium carbide with carbon deficiency, ThC0.7 to ThC, with corresponding lattice parameters increasing from 5.295 Å to 5.344 Å, and stated that “circumstantial evidence indicates that oxygen solubility is responsible for the lower cell sizes.” ThN cell parameter is 5.160(2) Å, as measured for a well characterized carbon and oxygen free sample [9]. However, values from 5.159 to 5.196 Å have been reported in the literature [10]. The higher cell parameters are likely due to carbon impurity, since the nitridation of ThC is a common method to synthesize ThN [11]. The solubility of oxygen in ThN is unknown, but considered to be less than in ThC, at least at ambient pressure.
The Th-C binary was studied by several groups [12][13][14]. Th-N, Th-C-N, and Th-N-O systems were studied only by Benz [15][16][17] in Los Alamos. The congruently melting thorium carbide stoichiometry was reported to be ThC0.97, with a melting point of 2500 ± 35 °C. Below 1000 °C, ThN is essentially a line compound with negligible solubility of oxygen. ThN melts congruently at 2820 ± 30 °C at 2 Bar N2, and at 2810 ± 30 °C at 1 Bar. Benz [18] reported melting point maxima for rocksalt ThC-ThN solid solution in ThC0.35N0.65 composition with a melting temperature of 2910 ± 35 °C (Figure 1). It indicates a strong deviation from ideal solution in solid, liquid, or both. Interestingly, the increase in melting temperature correlates with the higher oxygen content reported in the analysis of melted samples (Figure 1).
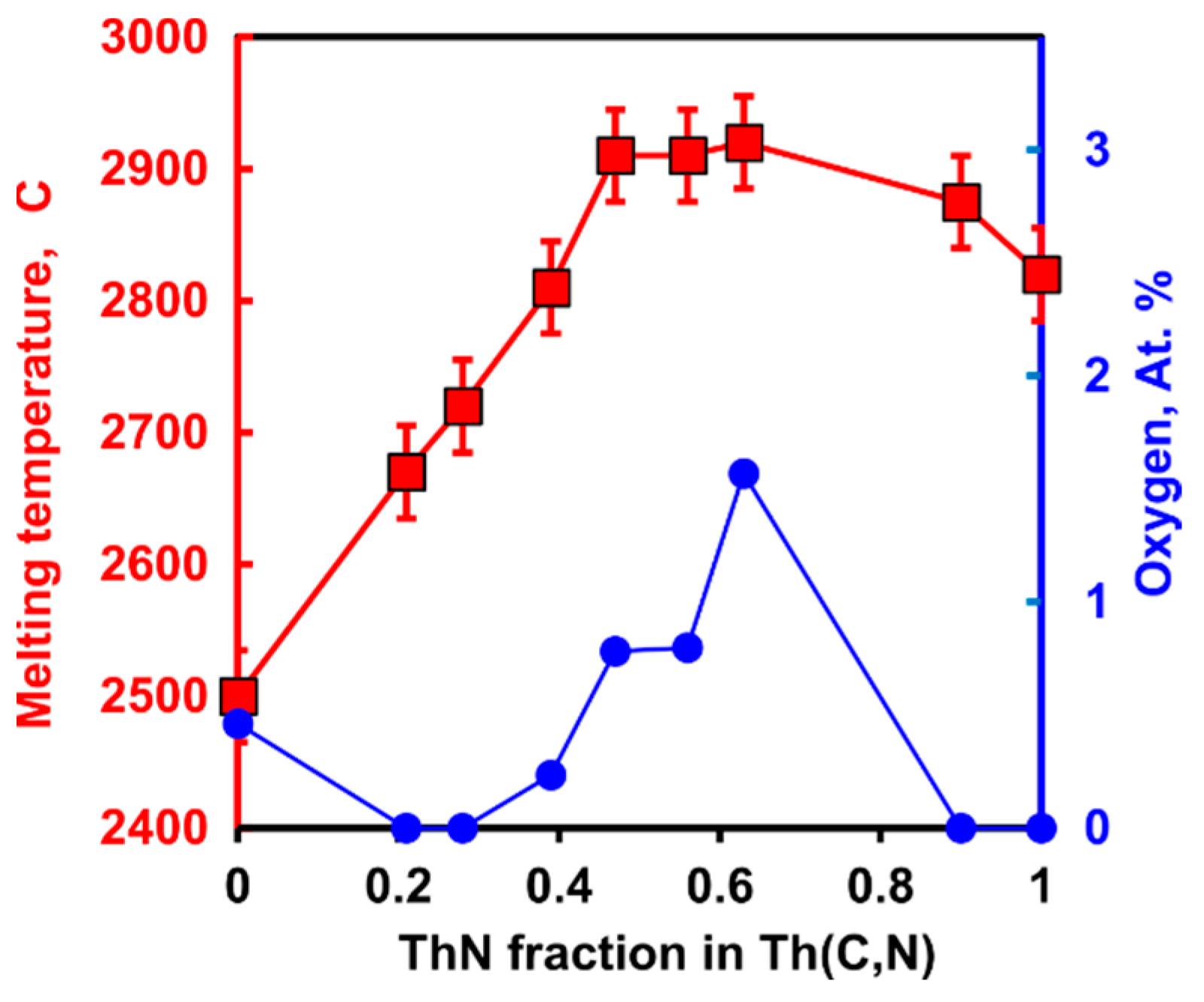
Figure 1. Melting temperature of Th(C,N) solid solutions and oxygen content in analyzed melted products after Benz (1968) [18].
2. Thorium Monoxide
The background on solid actinide monoxides is summarized succinctly by Petit et al. in a 2010 Physical Review publication: “There exists to date no convincing evidence that actinide oxides can form in the 1:1 stoichiometry” [19]. Petit et al. cited earlier reviews and some earlier reports on the synthesis of uranium monoxide, which were later proven to be uranium oxycarbide, oxynitride, or oxycarbonitride. They did not cite, however, experimental work on ThO and UO published by Ackerman and Rauh [20] from the Argonne National Laboratory. New experimental evidence of ThO formation in thin films at near-ambient conditions has been recently published [21], and bulk thorium monoxide was predicted to be stable in the NaCl structure at high pressure (Figure 1C) [22].
The stability of ThO, computed by density functional theory (DFT) methods, is attributed to the transfer of thorium’s 7s and 6d electrons to 5f orbitals and 6d -2p hybridization [22]. The computational prediction of the pressure-driven stabilization of PuO promptly followed [23]. These results challenge the current understanding of actinide chemistry, since, until this prediction, “the only hope of synthesis of actinide monoxides would appear to be the high-pressure route for AmO and CfO, an extremely demanding synthetic procedure” [24].
The experimental reports on ThO are summarized in Table 1, together with reference values for cell parameters for metallic Th and ThO2 phases. In most cases, ThO was reported to be prepared either on the decomposition of amorphous solids produced by the reaction of metallic Th with hydrochloric acid, or obtained on the surface of metallic Th.
Table 1. Unit cell parameters for Th and thorium oxides.
| Composition | a, Å | Notes | First Author, Year |
|---|---|---|---|
| Th fcc | 5.084 | fcc Th (Fm-3m) at RT | Hanawalt 1938 [25] |
| Th bcc | 4.11(1) | bcc Th (Im-3m) at 1500 °C | Chiotti 1954 [26] |
| ThO | 5.24 | film on the surface of Th metal | Rundle 1947 [3] |
| ThO [Th bcc] * | 4.31 * | (W)Th + ThO2 above ~1700 °C | Hoch 1954 [27] |
| ThO [ThO2] † | 5.63(1) † | 75 °C vacuum dry residual in HCl | Katzin 1958 [28] |
| ThO | 5.302(3) | 900 °C anneal of the residual in HCl | Ackerman 1973 [20] |
| ThO0.75 | 5.41 | 100 nm film on Th metal | He 2017 [21] |
| ThO2 flrt | 5.5997 | fluorite ThO2 (Fm-3m) at RT | Wyckoff 1963 [29] |
* likely high-temperature Th bcc structure, see text for details. † likely dioxide, compare lattice parameter with fluorite ThO2.
The only exception is the report from 1954 by Hoch and Johnston [27] on the formation of ThO above 1700 °C on the surface of thoriated tungsten cathodes. Based on high-temperature diffraction measurements, Hoch and Johnston reported ThO formation from liquid Th and ThO2 at high temperatures and its decomposition back to metal and dioxide on cooling. The possibility of ThO stability at high temperature was ruled out by Benz experiments in 1960s [30] on Th-O phase equilibria. The unit cell parameter for ThO reported by Hoch and Johnston [27] (4.31 Å) is much lower than in other reports (5.24–5.41 Å), and it is also lower than the room temperature lattice parameter for pure metallic Th in fcc structure. However, this lattice parameter is close to the value for the high-temperature Th bcc phase (4.11 Å) (Figure 2), not yet known at the time of the Hoch and Johnson [27] study. There are no experimental data on the Th-O system or the Th + ThO2 → 2ThO reaction at high pressure. The published work on ThO from low-temperature synthesis and growth as films on Th is discussed in detail in two separate sections below.
2.1. Residues from Th Dissolution in HCl
Metallic Th does not dissolve completely in hydrochloric acid, leaving a black residue. The hypothesis that this residue is composed of lower oxides of thorium was put forward more than 100 years ago by Werner von Bolton [31]. This incomplete dissolution of metallic thorium in HCl was rediscovered during its manufacturing for irradiation and U-233 separation [2]. The initial notion was that the residue is simply metal particles coated with ThO2 [32]. The study, performed at the Argonne National Laboratory by Katzin in 1958 [28], supported von Bolton’s hypothesis and concluded that the residue is basically ThO. Katzin reported that the ThO structure was clearly identified by neutron diffraction as ZnS type, with cell parameters similar to ThO2 (Table 1); however, the details of neutron diffraction measurements were not reported. In the same year, a study of the residue on Th dissolution in HCl was published by Karabash [33]. He reported that the black residue is thorium hydroxyhydride ThH(OH)2, which oxidizes to ThO2 on heating in air to ≈150 °C but appears stable in a vacuum. Four years later, another study on the residue was performed by Newbury and Searcy [34], defining its composition as ThO1.3Cl0.7H1.3.
The latest investigation on the decomposition of black Th residue was also performed in the Argonne National Laboratory, 15 years after Katzin’s experiments. Ackermann and Rauh [20] dissolved ≈1 g of metallic Th in concentrated HCl, obtained a black high surface area precipitate, and characterized its decomposition by X-ray diffraction (XRD) and thermogravimetry. They produced an empirical formula for the residue, ThO∙HCl∙H2O, and found that upon heating to 1200 °C in a vacuum, it decomposes to a mixture of rocksalt ThO, metallic Th, and fluorite ThO2 phases, with ThO disappearing at higher temperatures. Ackermann and Rauh [20] published an XRD pattern and reported the lattice constant for ThO to be 5.302 Å.
2.2. Rocksalt ThO Films on Metallic Th Surface
The first evidence on rocksalt ThO films was obtained at Ames during the Manhattan project and was included in Rundle’s 1947 paper [3]. Rundle refers to thorium monoxide in the scope of a new interpretation of structure—property relations in interstitial metallic carbides, nitrides, and oxides having the sodium chloride structure. Rundle does not explicitly report a lattice parameter for ThO, but does publish metal–metal distances which correspond to a lattice parameter 5.24 Å for rocksalt ThO, compared with 5.07 Å for fcc thorium metal, which is close to the currently accepted value (5.084 Å [29]). Rundle did not indicate how rocksalt ThO was obtained, but in reference to his work, Katzin [28] stated that it was “formed on the surface of freshly cleaned thorium metal”. Seventy years after Rundle’s [3] report, the growth of ThO thin films was reported by the Los Alamos Laboratory. He et al. [21] performed experiments on the controlled oxidation of metallic thorium with in situ neutron reflectometry measurements emerging from the study of oxidation of metallic Th using neutron reflectometry. ThO was formed after one hour of exposure of metallic Th to a ≈100 ppm O2 in an Ar mixture at 150 °C. From the modeling of the changes in experimentally measured neutron scattering length density (SLD) distribution, the composition can be derived as ThO0.75 with the cell parameter ≈5.41 Å. When the thickness of the oxide layer exceeded ≈100 nm, thorium dioxide was observed [21].
3. Rare Earth Monoxides
The monoxides of La, Ce, Nd, Sm, Eu, Y, and Yb with rocksalt structures were reported in the 1950s and 1960s [35][36]. They were synthesized by the reduction of sesquioxides with carbon or corresponding rare earth metals. However, it was later established that only divalent EuO can be synthesized at ambient pressure, and reported monoxides of other rare earth elements were, in fact, oxycarbides or oxynitrides [37].
Rocksalt EuO is the only rare earth monoxide known to be thermodynamically stable at ambient pressure (i.e., it will not decompose to Eu and Eu2O3, although it will oxidize in the presence of oxygen and, as all rare earth oxides, will react with water vapor, forming hydroxides). EuO has been synthesized by direct combustion of the metal, reaction of Eu with Eu2O3, and grown as single crystals with varying stoichiometry from Eu-Eu2O3 melt [38][39]. Close to stoichiometric EuO is a semiconductor, but EuO0.7 shows metallic conductivity. Melting temperatures of up to ≈2000 °C have been reported for oxygen-rich compositions. EuO is the only rare earth monoxide for which formation enthalpy [40][41] and heat capacity [42] have been measured [43].
3.1. Rocksalt Rare Earth Monoxides from High-Pressure Synthesis
YbO and lanthanide monoxides from La to Sm were synthesized at high pressure by Leger et al. [44]. La, Pr, Nd, and Sm monoxides were obtained at 4–5 GPa from stoichiometric mixtures of rare earth metals and sesquioxides in a compressed gasket (“belt-type”) apparatus. The reaction temperatures (800–1000 °C) were chosen to not exceed melting temperatures of corresponding lanthanide metals. The synthesis of pure YbO has been accomplished at pressures as low as 1 GPa [45]. Cerium monoxide was synthesized from metallic Ce and CeO2 at 1.5 GPa and 750 °C. CeO was only prepared in a mixture with Ce2O3 and unreacted metallic Ce; the other synthesized REO were reported to be single phases [46]. REO were not reported to decompose into metal and sesquioxide at ambient pressure [44]. Instead, hydroxide formation was observed, increasing from Sm to La, which is similar to the trend in rare earth sesquioxide affinity to reactions with water vapor [47]. The high-pressure syntheses were carried out in BN crucibles and reaction products were analyzed for H, C, and N to assure that no hydrides or carbonitrides were formed.
Yb and Eu are formally divalent in monoxides, have brown and red colors, and are semiconductors at ambient pressure; the rest of the synthesized REO have a metallic golden luster and the authors argued that the rare earth is trivalent [44]. The supposition of trivalent cations in the monoxide may seem bizarre, since researchers are used to the bonding in oxides being mostly ionic. In synthesized La-Sm monoxides, however, while overall formal oxidation state is +2, the RE is considered to be in trivalent state, with the third electron delocalized in metallic bonding; these compounds can be described as RE3+(O2−)(e−), according to Morss and Konings [48].
An ambiguity remained about SmO since Sm is divalent in the rocksalt monosulfide. SmS is a semiconductor but is known for its fascinating pressure-driven “black-golden” transition which can be induced by mere polishing [49]. It appears to be caused by Sm going into the trivalent state, switching SmS from semiconductor to metal, while still keeping the rocksalt structure [49]. Since golden SmO has metallic conductivity, it was surmised that Sm is mostly trivalent in the monoxide, which was confirmed by X-ray absorption spectroscopy [50]. Leger et al. [44] also attempted the synthesis of Gd, Dy, and Tm monoxides at 1 to 8 GPa and 600–1200 °C, but did not succeed.
Rocksalt CeO was further studied in a diamond anvil cell and found to be stable at least up to 25 GPa, which was the maximum pressure used in the experiments [51]. Rocksalt NdO was also obtained in shock compression experiments [52][53][54]. Comparing lattice parameters of NdO synthesized in shock compression with those under static 5 GPa loads (Table 2), the authors suggested that in NdO retrieved from explosions, the neodymium is partially divalent [53]. Researchers did not locate any other reports on high-pressure synthesis, thermodynamics, and property characterization of bulk rare earth monoxides.
Table 2. Lattice parameters reported for rocksalt-type (Fm3m) rare earth monoxides (REO) synthesized in thin films (TF) and in bulk.
| REO | a, Å (TF) * | Comment † | REO | a, Å * | Comment |
|---|---|---|---|---|---|
| LaO | 5.22–5.31 § | ~20 nm (0.98–1.02) [55] | LaO | 5.144 | golden, 4 GPa 900 °C [44] |
| CeO | 5.15 | ~6 nm (1.01) [56] | CeO ‡ | 5.089 | golden, 1.5 GPa 700 °C [46] |
| PrO | 5.054 | ~10 nm (1.02) [57] | PrO | 5.031 | golden, 5 GPa 800 °C [46] |
| NdO | 5.05–5.16 § | ~20-40 nm (1.01–0.99) [58] | NdO | 4.994 | golden, 5 GPa 1000 °C [44] |
| 5.086 | shock compression [54] | ||||
| SmO | 5.02 | ~70 nm (0.99) [59] | SmO | 4.943 | golden, 5 GPa 1000 °C [44] |
| EuO | 5.152 | ~100 nm (MBE) [60] | EuO | 5.144 | dark red, 0 GPa [61] |
| 5.12 | ~100 nm Eu0.9La0.1O [60] | 5.143 | EuO1.02 0 GPa [40] | ||
| GdO | 5.00–5.02 | ~50 nm (0.99) [62] | |||
| 5.03 | ~90 nm (1.00) Gd0.90La0.10O [62] | ||||
| TbO | 4.97 | ~90 nm (0.99) [63] | |||
| HoO | 5.04 | ~20-90 nm (0.97) [64] | |||
| YO | 4.92–4.98 § | ~90-200 nm (0.99) [65] | |||
| YbO | 4.87 | ~4-30 nm (0.99) [66] | YbO | 4.877 | 1-6 GPa 600–1400 °C [45] |
| LuO | 4.79 | ~150 nm (0.99) [67] |
* The lattice parameters are reported to the last significant digit. † The tetragonal distortion was induced by epitaxial growth (in plane lattice constant = a∙(k); the k values less than 1 correspond to compression, higher than 1 to tension); the films were grown by pulsed laser deposition (PLD), except EuO which was grown by molecular beam epitaxy (MBE). ‡ Synthesized by reaction of Ce with CeO2; Ce2O3 was also formed as a product. § The larger cell parameter may correspond to oxygen-deficient samples.
In a review on the thermochemistry of binary rare earth oxides, Morss and Konings stated that solution calorimetry experiments were performed on several NdO samples prepared by Leger’s group, “however their results were unusually exothermic, indicating that metallic Nd was present, and the non-reproducible measurements indicated that the samples were not sufficiently homogeneous to warrant further study” [48].
3.2. Rocksalt Rare Earth Monoxides Films from Pulsed Laser Deposition
Experiments on the high-pressure synthesis of rare earth monoxides described above were performed more than 40 years ago and were never repeated. However, in the last decade, many rare earth monoxides were synthesized as epitaxial thin films at low pressures, motivated by their potential use in spintronics. Most of the experiments were performed by a group in Tohoku University using pulsed laser deposition (PLD) [55][58][59][62][65][67]. The films were prepared in an ultra-high vacuum on a variety of substrates including CaF2, LaAlO3, and YAlO3, typically held at temperatures 200–400 °C. The oxygen pressure varied from less than 10−9 to 10−7 Torr, depending on whether metallic or oxide targets were used for evaporation. The thickness of synthesized REO films varied from 6 nm for CeO to 200 nm for YO (Table 2).
The discovery of superconductivity in LaO films [55] and ferromagnetism in semiconducting GdO films [62] prompted a number of experiments on the growth of films on different substrates and background oxygen pressures to evaluate their effects on critical superconductivity and Curie temperatures. Thin films were grown both in compressive and tensile epitaxial strain, which may indicate that epitaxial growth is not critical for the low-pressure synthesis of REO, and they can potentially be synthesized as separate grains or nanoparticles. In oxygen deficient rocksalt REO1−x, the cell parameters were found to increase with decreasing oxygen content.
In Figure 2, lattice cell parameters of rocksalt REO grown as thin films are compared with those for bulk monoxides from high-pressure synthesis and with EuO, prepared at ambient pressure. For monoxides from lanthanum to samarium, for which data both from thin films and bulk are available, cell parameters from the thin films are larger than from the bulk. La to Sm monoxides were reported to be metallic both in bulk and in thin films.
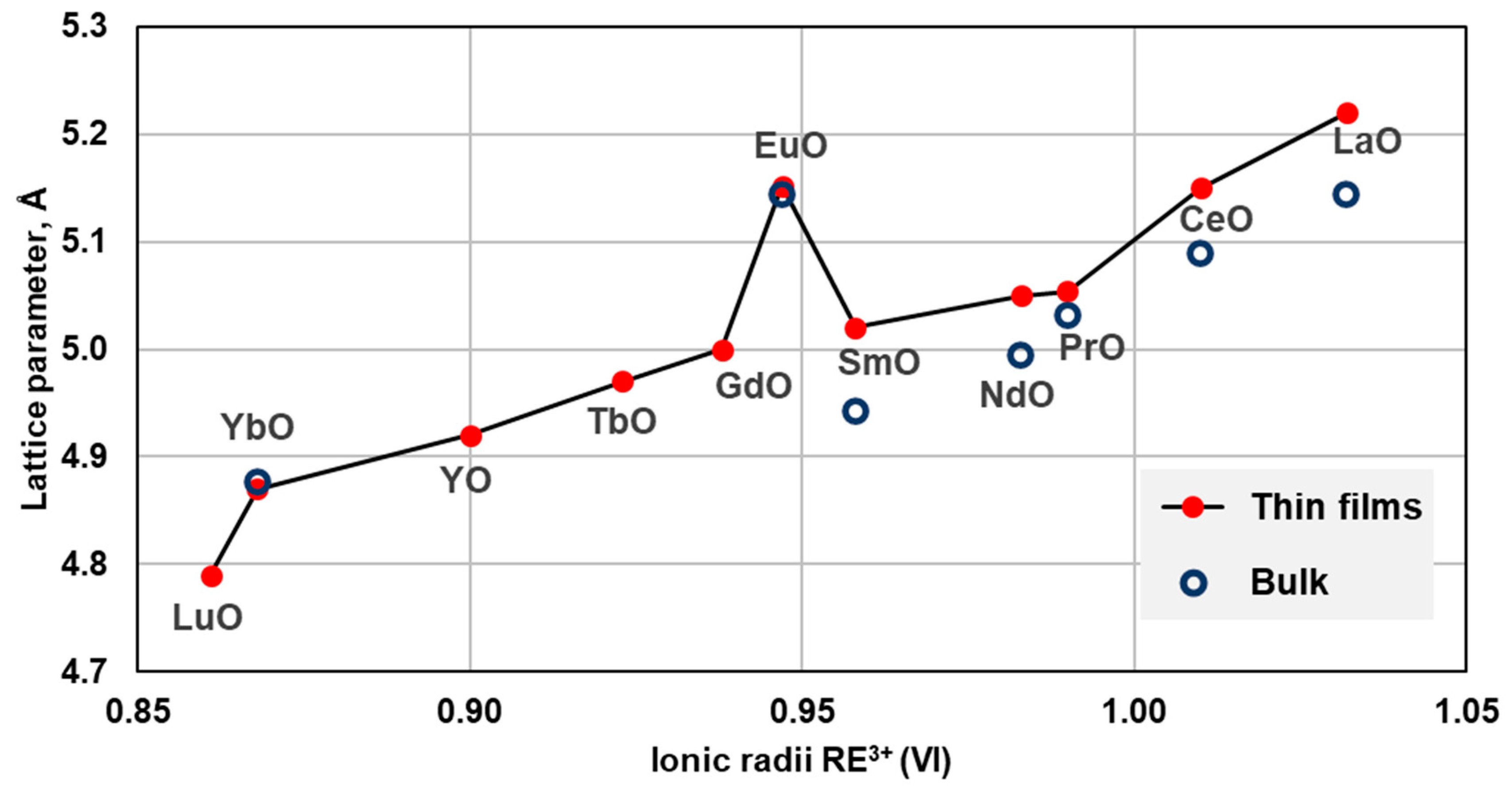
Figure 2. Cell parameters of rocksalt-type REO from high-pressure synthesis and thin films. The symbol sizes exceed reported uncertainties. Reported cell parameter for HoO film (a = 5.04 Å) is not included in the plot as a possible outlier. The references are listed in Table 2.
YO and heavy lanthanide monoxides from Gd to Lu were synthesized only in thin films. They were found to be semiconductors, similar to EuO and YbO, which are semiconductors in bulk and in thin films. From X-ray photoelectron spectroscopy (XPS) measurements, the authors concluded that rare earths from Gd to Lu are in the divalent state in monoxides [65][68]. This is in stark contrast with metallic monoxides from La to Sm, in which the rare earths are considered to be trivalent. The excellent agreement between lattice constants for Yb2+ and Eu2+ monoxides in bulk and in thin films prompts the question whether larger lattice constants of REO from La to Sm in thin films vs. bulk indicate mixed valence states in these REO as well.
References
- McTaggart, F.K.; Wadsley, A. The sulphides, selenides, and tellurides of titanium, zirconium, hafnium, and thorium. I. Preparation and characterization. Aust. J. Chem. 1958, 11, 445–457.
- Daane, A.H.; Seaborg, G.T.; Katzin, L.I. Production and Separation of U233: Survey; Technical Information Service Extension; U.S. Atomic Energy Commission: Washington, DC, USA, 1951.
- Rundle, R.E. A new interpretation of interstitial compounds: Metallic carbides, nitrides, and oxides of composition MX. Acta Crystallogr. 1948, 1, 180–187.
- Ushakov, S.V.; Navrotsky, A.; Hong, Q.-J.; van de Walle, A. Carbides and Nitrides of Zirconium and Hafnium. Materials 2019, 12, 2728.
- Holleck, H.; Smailos, E. Mixed nitrides of thorium with rare earths. J. Nucl. Mater. 1980, 91, 237–239.
- Holleck, H. Ternary phase equilibriums in the systems actinide-transition metal-carbon and actinide-transition metal-nitrogen. Mater. Sci. 1975, 2, 213–264.
- Yu, C.; Lin, J.; Huai, P.; Guo, Y.; Ke, X.; Yu, X.-h.; Yang, K.; Li, N.; Yang, W.; Sun, B.; et al. Structural Phase Transition of ThC Under High Pressure. Sci. Rep. 2017, 7, 96.
- Henney, J.; Jones, J.W.S.; Hill, N.A. Cell size variations in thorium monocarbide. Carbides Nucl. Energy Proc. Symp. Harwell Engl. 1964, 1, 69–70.
- Parker, S.S.; White, J.T.; Hosemann, P.; Nelson, A.T. Thermophysical properties of thorium mononitride from 298 to 1700 K. J. Nucl. Mater. 2019, 526, 151760.
- Auskern, A.B.; Aronson, S. Electrical properties of thorium nitrides. J. Phys. Chem. Solids 1967, 28, 1069–1071.
- Parkison, A.J.; Parker, S.S.; Nelson, A.T. Fabrication of ThN Using a Carbothermic Reduction to Nitridation Process. J. Am. Ceram. Soc. 2016, 99, 3909–3914.
- Takeuchi, S.; Homma, T.; Satow, T.; Hirai, T. Th-ThC phase diagram. Trans. Jpn. Inst. Met. 1966, 7, 59–67.
- Wilhelm, H.A.; Chiotti, P. Thorium-carbon system. Trans. Am. Soc. Met. 1950, 42, 1295–1310.
- Manara, D.; De Bruycker, F.; Sengupta, A.K.; Agarwal, R.; Kamath, H.S. Thermodynamic and Thermophysical Properties of the Actinide Carbides; Elsevier: Amsterdam, The Netherlands, 2012; pp. 87–137.
- Benz, R.; Troxel, J.E. Thorium-carbon-nitrogen phase diagram. High Temp. Sci. 1971, 3, 422–432.
- Benz, R. Thorium-nitrogen-oxygen phase diagram. J. Nucl. Mater. 1972, 43, 1–7.
- Benz, R.; Hoffman, C.G.; Rupert, G.N. Some phase equilibria in the thorium-nitrogen system. J. Am. Chem. Soc. 1967, 89, 191–197.
- Benz, R. Melting point maxima of thorium carbide-thorium nitride and of uranium carbide-uranium nitride solid solutions. J. Nucl. Mater. 1969, 31, 93–98.
- Petit, L.; Svane, A.; Szotek, Z.; Temmerman, W.M.; Stocks, G.M. Electronic structure and ionicity of actinide oxides from first principles. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 045108.
- Ackermann, R.J.; Rauh, E.G. The preparation and characterization of the metastable monoxides of thorium and uranium. J. Inorg. Nucl. Chem. 1973, 35, 3787–3794.
- He, H.; Majewski, J.; Allred, D.D.; Wang, P.; Wen, X.; Rector, K.D. Formation of solid thorium monoxide at near-ambient conditions as observed by neutron reflectometry and interpreted by screened hybrid functional calculations. J. Nucl. Mater. 2017, 487, 288–296.
- Sun, W.; Ahuja, R.; Sun, W.; Luo, W.; Ahuja, R. Stability of a new cubic monoxide of Thorium under pressure. Sci. Rep. 2015, 5, 13740.
- Qiu, R.; Zhang, Y.; Ao, B. Stability and optical properties of plutonium monoxide from first-principle calculation. Sci. Rep. 2017, 7, 12167.
- Konings, R.J.M.; Morss, L.R.; Fuger, J. Thermodynamic properties of actinides and actinide compounds. In The Chemistry of the Actinide and Transactinide Elements; Morss, L.R., Edelstein, N.M., Fuger, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 52–134.
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-ray Diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512.
- Chiotti, P. High temperature crystal structure of thorium. J. Electrochem. Soc. 1954, 101, 567.
- Hoch, M.; Johnston, H.L. The reaction occurring on thoriated cathodes. J. Am. Chem. Soc. 1954, 76, 4833–4835.
- Katzin, L.I. Concerning a Lower Oxide of Thorium and Related Compounds of the Last Row Elements1,2. J. Am. Chem. Soc. 1958, 80, 5908–5910.
- Wyckoff, R.W.G. Crystal Structures; Interscience Publishers: New York, NY, USA, 1963; Volume 1.
- Benz, R. Thorium-thorium dioxide phase equilibria. J. Nucl. Mater. 1969, 29, 43–49.
- Bolton, W.v. Über das Thorium. Z. Für Elektrochem. Und Angew. Phys. Chem. 1908, 14, 768–770.
- Rodden, C.J.; Warf, J.C. Analytical Chemistry of the Manhattan Project. NNES Div. VIII, Vol. 1, Chap. 2. Thorium; National Bureau of Standards: Washington, DC, USA, 1949.
- Karabash, A.G. Several chemical properties of thorium and uranium. Zh. Neorg. Khim. 1958, 3, 986–995.
- Newbury, R.S.; Searcy, A.W. The Composition and Properties of the Solid Produced by Reaction of Thorium with Hydrochloric Acid. Inorg. Chem. 1962, 1, 794–798.
- Butherus, A.D.; Eick, H.A. Preparation and some properties of the lanthanide oxide carbides, Ln4O3C. J. Amer. Chem. Soc. 1968, 90, 1715–1718.
- Eick, H.A.; Baenziger, N.C.; Eyring, L. Lower oxides of samarium and europium. The preparation and crystal structure of SmO0.4-0.6, SmO, and EuO. J. Am. Chem. Soc. 1956, 78, 5147–5149.
- Eyring, L. Chapter 27 The binary rare earth oxides. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1979; Volume 3, pp. 337–399.
- Shafer, M.W.; Torrance, J.B.; Penney, T. Relation of crystal growth parameters of the stoichiometry of europium(II) oxide as determined by ir and conductivity measurements. J. Phys. Chem. Solids 1972, 33, 2251–2266.
- Matsukura, F.; Ohno, H. 15—Magnetic Semiconductors. In Handbook of Crystal Growth (Second Edition), 2nd ed.; Kuech, T.F., Ed.; North-Holland: Boston, MA, USA, 2015; pp. 649–682.
- Burnett, J.L. The Thermochemistry of di- and Tri-Valent Europium. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1964. (ucrl-11850).
- Huber, E.J., Jr.; Holley, C.E., Jr. Enthalpy of formation of europium monoxide. J. Chem. Thermodyn. 1969, 1, 301–304.
- McMasters, O.D.; Gschneidner, K.A.; Kaldis, E.; Sampietro, G. High-temperature enthalpies and standard Gibbs free energies of formation of the europium chalcogenides: EuO, EuS, EuSe, and EuTe. J. Chem. Thermodyn. 1974, 6, 845–857.
- Konings, R.J.M.; Beneš, O.; Kovács, A.; Manara, D.; Sedmidubský, D.; Gorokhov, L.; Iorish, V.S.; Yungman, V.; Shenyavskaya, E.; Osina, E. The Thermodynamic Properties of the f-Elements and their Compounds. Part 2. The Lanthanide and Actinide Oxides. J. Phys. Chem. Ref. Data 2014, 43, 013101.
- Leger, J.M.; Yacoubi, N.; Loriers, J. Synthesis of rare earth monoxides. J. Solid State Chem. 1981, 36, 261–270.
- Leger, J.M.; Loriers, C.; Albert, L.; Achard, J.C. Synthesis of ytterbium monoxide under high pressure. High Press. Sci. Technol. AIRAPT Conf. 1979, 1, 1021–1025.
- Leger, J.M.; Yacoubi, N.; Loriers, J. Synthesis of cerium and praseodymium monoxides. Mater. Res. Bull. 1979, 14, 1431–1436.
- Adachi, G.; Imanaka, N.; Kang, Z.C. Binary Rare Earth Oxides; Springer: Dordrecht, The Netherlands, 2006.
- Morss, L.R.; Konings, R.J.M. Thermochemistry of Binary Rare Earth Oxides. In Binary Rare Earth Oxides; Adachi, G., Imanaka, N., Kang, Z.C., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 163–188.
- Sousanis, A.; Smet, P.F.; Poelman, D. Samarium Monosulfide (SmS): Reviewing Properties and Applications. Materials 2017, 10, 953.
- Krill, G.; Ravet, M.F.; Kappler, J.P.; Abadli, L.; Leger, J.M.; Yacoubi, N.; Loriers, C. Magnetic properties of some rare earth monoxides LnO (Ln = praseodymium, neodymium, samarium) mixed valence state of samarium(II) oxide. Solid State Commun. 1980, 33, 351–353.
- Vedel, I.; Redon, A.M.; Leger, J.M. Pressure-induced electronic instability in CeO. J. Physics. C Solid State Phys. 1986, 19, 3549–3554.
- Batsanov, S.S. Inorganic chemistry of high dynamic pressures. Russ. Chem. Rev. 1986, 55, 579–607.
- Batsanov, S.S.; Dorogova, G.V.; Kopaneva, L.I.; Temnitskii, I.N. Shock wave thermal synthesis of neodymium oxide. Izv. Akad. Nauk SSSR Ser. Khim. 1985, 11, 2656.
- Batsanov, S.S.; Dorogova, G.V.; Kopaneva, L.I. Effects of an explosion on neodymium oxide. Izv. Akad. Nauk SSSR Neorg. Mater. 1980, 16, 549–550.
- Kaminaga, K.; Oka, D.; Hasegawa, T.; Fukumura, T. Superconductivity of rock-salt structure LaO epitaxial thin film. J. Am. Chem. Soc. 2018, 140, 6754–6757.
- Abe, N.; Oka, D.; Kaminaga, K.; Shiga, D.; Saito, D.; Yamamoto, T.; Kimura, N.; Kumigashira, H.; Fukumura, T. Rocksalt CeO epitaxial thin film as a heavy-fermion system transiting from p-type metal to partially compensated n-type metal by 4f delocalization. Phys. Rev. B 2022, 106, 125106.
- Shimizu, H.; Oka, D.; Kaminaga, K.; Saito, D.; Yamamoto, T.; Abe, N.; Kimura, N.; Shiga, D.; Kumigashira, H.; Fukumura, T. Rocksalt-type PrO epitaxial thin film as a weak ferromagnetic Kondo lattice. Phys. Rev. B 2022, 105, 014442.
- Saito, D.; Kaminaga, K.; Oka, D.; Fukumura, T. Itinerant ferromagnetism in rocksalt NdO epitaxial thin films. Phys. Rev. Mater. 2019, 3, 064407.
- Uchida, Y.; Kaminaga, K.; Fukumura, T.; Hasegawa, T. Samarium monoxide epitaxial thin film as a possible heavy-fermion compound. Phys. Rev. B 2017, 95, 125111/1–125111/4.
- Miyazaki, H.; Im, H.J.; Terashima, K.; Yagi, S.; Kato, M.; Soda, K.; Ito, T.; Kimura, S. La-doped EuO: A rare earth ferromagnetic semiconductor with the highest Curie temperature. Appl. Phys. Lett. 2010, 96, 232503/1–232503/3.
- Wachter, P. Chapter 19 Europium chalcogenides: EuO, EuS, EuSe and EuTe. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1979; Volume 2, pp. 507–574.
- Yamamoto, T.; Kaminaga, K.; Saito, D.; Oka, D.; Fukumura, T. Rock salt structure GdO epitaxial thin film with a high ferromagnetic Curie temperature. Appl. Phys. Lett. 2020, 117, 052402.
- Sasaki, S.; Oka, D.; Kaminaga, K.; Saito, D.; Yamamoto, T.; Abe, N.; Shimizu, H.; Fukumura, T. A high-TC heavy rare earth monoxide semiconductor TbO with a more than half-filled 4f orbital. Dalton Trans. 2022, 51, 16648–16652.
- Amrillah, T.; Oka, D.; Shimizu, H.; Sasaki, S.; Saito, D.; Kaminaga, K.; Fukumura, T. Rock salt-type HoO epitaxial thin film as a heavy rare-earth monoxide ferromagnetic semiconductor with a Curie temperature above 130 K. Appl. Phys. Lett. 2022, 120, 082403.
- Kaminaga, K.; Sei, R.; Hayashi, K.; Happo, N.; Tajiri, H.; Oka, D.; Fukumura, T.; Hasegawa, T. A divalent rare earth oxide semiconductor: Yttrium monoxide. Appl. Phys. Lett. 2016, 108, 122102.
- Yamamoto, T.; Kaminaga, K.; Saito, D.; Oka, D.; Fukumura, T. High electron mobility with significant spin-orbit coupling in rock-salt YbO epitaxial thin film. Appl. Phys. Lett. 2019, 114, 162104/1–162104/4.
- Kaminaga, K.; Oka, D.; Hasegawa, T.; Fukumura, T. New lutetium oxide: Electrically conducting rock-salt LuO epitaxial thin film. ACS Omega 2018, 3, 12501–12504.
- Kaminaga, K.; Fukumura, T. New development of divalent rare earth ion compounds—From complexes to inorganic solids. Kagaku 2018, 73, 70–71.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
4 times
(View History)
Update Date:
05 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




