
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seddik Khalloufi | -- | 2227 | 2023-05-31 15:44:02 | | | |
| 2 | Jessie Wu | Meta information modification | 2227 | 2023-06-01 04:42:09 | | |
Video Upload Options
“Heavy metals” are associated with environmental pollution, food contamination, and toxicity and have adverse effects on terrestrial and aquatic ecosystems and animal and human health. Hazardous heavy metals and metalloids, such as arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), and mercury (Hg), and several essential heavy metals, such as copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn), above threshold levels, have been identified as priority contaminants and one of the key environmental issues of global concern due to their mobility in terrestrial and natural aquatic ecosystems and their carcinogenic nature. Use of inexpensive and abundantly available biosorbents generated from fibrous plant-based food-waste materials to remove heavy metals has garnered considerable research attention.
1. Plant-Fiber Components
2. Fibrous Plant-Based Biomass Parts
| Sorbents | Heavy Metals Removed | Sorption Conditions |
Modification Method | Mechanisms of Action |
Sorption Capacity (mg/g) | References |
|---|---|---|---|---|---|---|
| Artocarpus nobilis (Peel) | Ni2+ | pH of 4 90 min 175 °C |
HNO3 | Ion exchange | Ni2+: 0.012 | [40] |
| Black Oak (Bark) | Hg2+ | pH of 2–10 20–150 min Adsorption dose of 20–60 mg/L |
None | Complexation, adsorption on surface, diffusion, and ion exchange | Hg2+: 400 | [14] |
| Coconut (Shell) | Cd2+, Pb2+ | pH of 2–10 20–150 min Adsorption dose of 20–60 mg/L |
None | Complexation, adsorption on surface, diffusion, and ion exchange | Cd2+: 285 Pb2+: 263 |
[14] |
| Cantaloupe (Peel) | Cd2+, Cu2+, Pb2+ | pH of 5–7 | Acrylic acid | Ion exchange and complexation | Cd2+: 45.4 Cu2+: 33.1 Pb2+: 143.3 |
[41] |
| Carrot (Residue) | Cr3+, Cu2+, Zn2+ | pH of 4 Initial ion concentration of 20 to 500 mg/L |
None | Ion exchange | Cr3+: 1.65 Cu2+: 1.82 Zn2+: 1.45 |
[11] |
| Lemon (Peel) | Cd2+ | pH of 5 45 min Initial ion concentration of 45 mg/L Particle size of 0.24–0.42 mm |
Protonation and HNO3 | Ion exchange | Cd2+: 32.5 | [42] |
| Potato (Peel) | Cu2+ | pH of 6 20 min 30 °C Initial ion concentration of 150 mg/L Particle size of 0.2 mm |
None | Surface complexation and ion exchange | Cu2+: 0.15 | [43] |
| Potato (Shell) | Cu2+, Cd2+ | pH of 6.8 200 min (Cd) 50 min (Cu) |
None | Electrostatic interaction | Cd2+: 90 Cu2+: 41.7 |
[13] |
| Soybean (Straw) | Cu2+ | pH of 6 60 min |
Citric acid | Ion exchange | Cu2+: 48.2–48.8 | [44] |
| Sunflower (Stalk and Leaves) | Cd2+, Cu2+ | pH of 6 120 min (Cd) 50 min (Cu) |
None | Electrostatic interaction | Cd2+: 63.3 Cu2+: 30.3 |
[13] |
| Tangerine (Peel) | Cd2+, Co2+, Cr3+, Cu2+, Mn2+, Ni2+, Pb2+, Zn2+ | pH of 5 20 min Room temperature Adsorbent dose of 1–4 g/L |
Nitric acid | Ion exchange | Cd2+: 0.003 Co2+: 0.01 Cr3+: 0.01 Cu2+: 0.002 Mn2+: 0.01 Ni2+: 0.01 Pb2+: 0.002 Zn2+: 0.003 |
[45] |
| Wheat (Bran) | Cr6+ | pH of 2–10 20–150 min Adsorption dose of 20–60 mg/L |
None | Complexation, adsorption on surface, diffusion, and ion exchange | Cr6+: 310 | [14] |
| Wheat (Bran) | As3+, Cd2+, Hg2+, Pb2+ | pH of 7 37 ℃ |
None | Ion exchange | As3+: 0.98 Cd2+: 36.1 Hg2+: 39.6 Pb2+: 58.2 |
[46] |
3. Factors Influencing the Sorption Efficiencies of Fibrous Plants
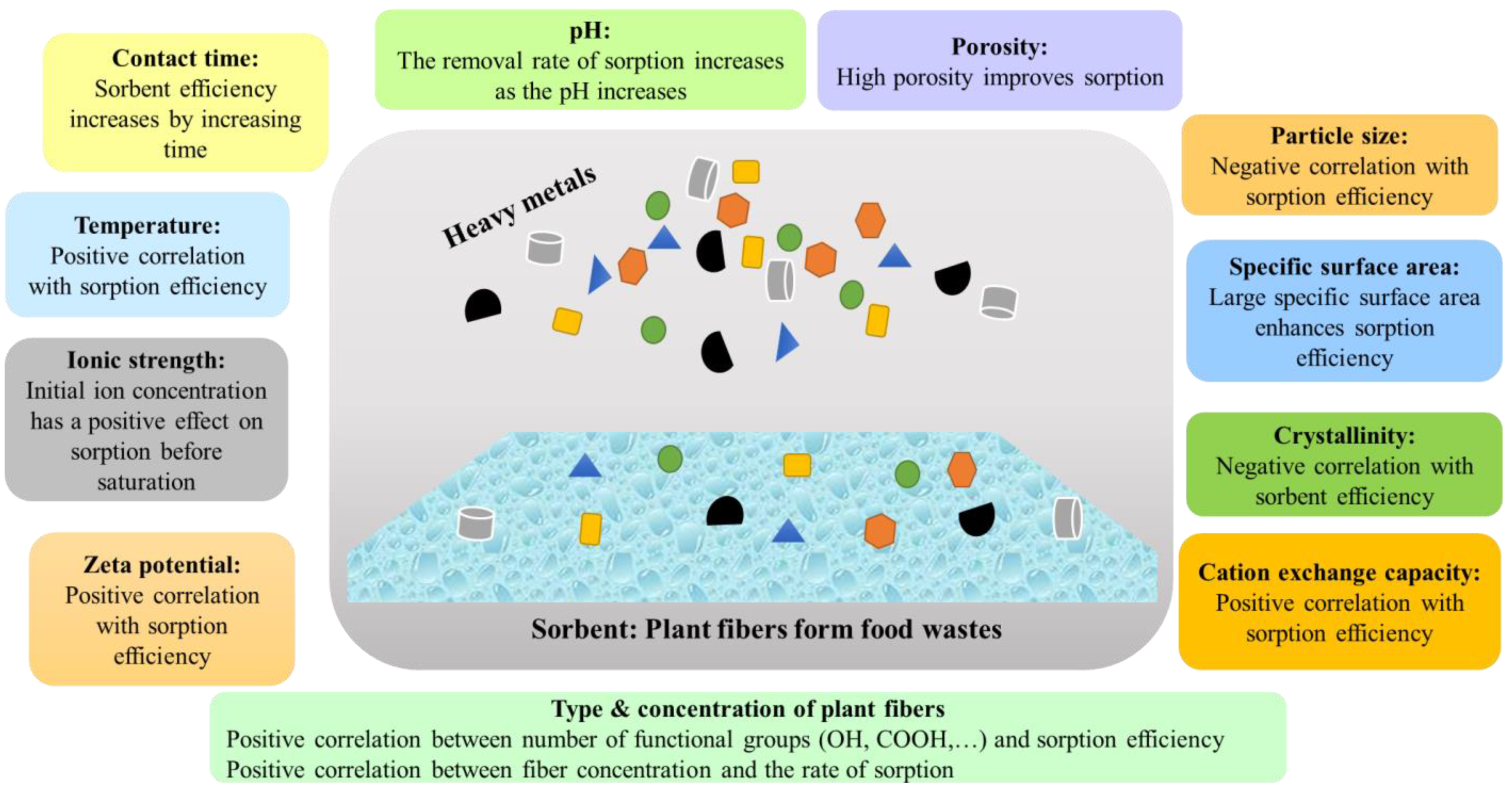
4. Different Modification Technologies for Enhancing Sorption Efficiency
References
- Latif, R.; Wakeel, S.; Zaman Khan, N.; Noor Siddiquee, A.; Lal Verma, S.; Akhtar Khan, Z. Surface treatments of plant fibers and their effects on mechanical properties of fiber-reinforced composites: A review. J. Reinf. Plast. Compos. 2019, 38, 15–30.
- Fidelis, M.E.A.; Pereira, T.V.C.; Gomes, O.d.F.M.; de Andrade Silva, F.; Toledo Filho, R.D. The effect of fiber morphology on the tensile strength of natural fibers. J. Mater. Res. Technol. 2013, 2, 149–157.
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229.
- Al-Asheh, S.; Duvnjak, Z. Sorption of cadmium and other heavy metals by pine bark. J. Hazard. Mater. 1997, 56, 35–51.
- Nawirska, A. Binding of heavy metals to pomace fibers. Food Chem. 2005, 90, 395–400.
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54.
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520.
- Malik, D.; Jain, C.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136.
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877.
- Paranjape, P.; Sadgir, P. Heavy Metal Removal Using Plant Origin Biomass and Agricultural Waste-Derived Biomass from Aqueous Media: A Review. Water Conserv. Sci. Eng. 2023, 8, 9.
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process Biochem. 2005, 40, 1319–1322.
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett–Burman design. J. Chem. 2019, 2019, 4926240.
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136.
- Alalwan, H.A.; Kadhom, M.A.; Alminshid, A.H. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Supply Res. Technol.-Aqua 2020, 69, 99–112.
- Maina, I.W.; Obuseng, V.; Nareetsile, F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (Morula) nut shells for removal of heavy metals from wastewater and borehole water. J. Chem. 2016, 2016, 9312952.
- Thi Quyen, V.; Pham, T.-H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Jung, S.H.; Kim, T. Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 2021, 284, 131312.
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J. Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal. J. Hazard. Mater. 2010, 178, 243–248.
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn (II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866.
- Nieto, L.M.; Alami, S.B.D.; Hodaifa, G.; Faur, C.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Adsorption of iron on crude olive stones. Ind. Crop. Prod. 2010, 32, 467–471.
- Petrella, A.; Spasiano, D.; Acquafredda, P.; De Vietro, N.; Ranieri, E.; Cosma, P.; Rizzi, V.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention (Pb (II), Cd (II), Ni (II)) from single and multimetal solutions by natural biosorbents from the olive oil milling operations. Process Saf. Environ. Prot. 2018, 114, 79–90.
- Fernández-González, R.; Martín-Lara, M.Á.; Blázquez, G.; Pérez, A.; Calero, M. Recovering Metals from aqueous solutions by biosorption onto hydrolyzed olive cake. Water 2019, 11, 2519.
- Singh, R.; Martin, C.; Barr, D.; Rosengren, R. Immobilised apple peel bead biosorbent for the simultaneous removal of heavy metals from cocktail solution. Cogent Environ. Sci. 2019, 5, 1673116.
- Šabanović, E.; Memić, M.; Sulejmanović, J.; Selović, A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Pol. J. Chem. Technol. 2020, 22, 46–53.
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41.
- Mohammad, M.; Maitra, S.; Ahmad, N.; Bustam, A.; Sen, T.; Dutta, B.K. Metal ion removal from aqueous solution using physic seed hull. J. Hazard. Mater. 2010, 179, 363–372.
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961.
- Maheshwari, U.; Gupta, S. Removal of Cr (VI) from wastewater using activated neem bark in a fixed-bed column: Interference of other ions and kinetic modelling studies. Desalination Water Treat. 2016, 57, 8514–8525.
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. Bioremediat. Biodegrad. 2015, 6, 1000315.
- Chao, H.-P.; Chang, C.-C.; Nieva, A. Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. J. Ind. Eng. Chem. 2014, 20, 3408–3414.
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306.
- Moussavi, G.; Barikbin, B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900.
- SenthilKumar, P.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Sivanesan, S. Removal of copper (II) ions from aqueous solution by adsorption using cashew nut shell. Desalination 2011, 266, 63–71.
- Cholico-González, D.; Ortiz Lara, N.; Fernández Macedo, A.M.; Chavez Salas, J. Adsorption Behavior of Pb(II), Cd(II), and Zn(II) onto Agave Bagasse, Characterization, and Mechanism. ACS Omega 2020, 12, 3302–3314.
- Fawzy, M.A.; Al-Yasi, H.M.; Galal, T.M.; Hamza, R.Z.; Abdelkader, T.G.; Ali, E.F.; Hassan, S.H. Statistical optimization, kinetic, equilibrium isotherm and thermodynamic studies of copper biosorption onto Rosa damascena leaves as a low-cost biosorbent. Sci. Rep. 2022, 12, 8583.
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Heavy metal ions removal from aqueous solutions by treated ajwa date pits: Kinetic, isotherm, and thermodynamic approach. Polymers 2022, 14, 914.
- Massocatto, C.; Paschoal, E.; Buzinaro, N.; Oliveria, T.; Tarley, C.; Caetano, J.; Gonçalves Jr, A.; Dragunski, D.; Diniz, K. Preparation and evaluation of kinetics and thermodynamics studies of lead adsorption onto chemically modified banana peels. Desalination Water Treat. 2013, 51, 5682–5691.
- Nathan, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Simultaneous removal of heavy metals from drinking water by banana, orange and potato peel beads: A study of biosorption kinetics. Appl. Water Sci. 2021, 11, 28.
- Sathasivam, K.; Haris, M.R.H.M. Banana trunk fibers as an efficient biosorbent for the removal of Cd(II), Cu(II), Fe(II) and Zn(II) from aqueous solutions. J. Chil. Chem. 2010, 55, 278–282.
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296.
- Priyantha, N.; Kotabewatta, P. Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni (II) sorption under static and dynamic conditions. Appl. Water Sci. 2019, 9, 37.
- Tran, H.N.; Chao, H.-P. Adsorption and desorption of potentially toxic metals on modified biosorbents through new green grafting process. Environ. Sci. Pollut. Res. 2018, 25, 12808–12820.
- Schiewer, S.; Patil, S.B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903.
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf. B Biointerfaces 2008, 63, 116–121.
- Zhu, B.; Fan, T.; Zhang, D. Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J. Hazard. Mater. 2008, 153, 300–308.
- Abdić, Š.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: Tangerine peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518.
- Zhang, N.; Huang, C.; Ou, S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J. Hazard. Mater. 2011, 186, 236–239.
- Almendros, A.; Martín-Lara, M.; Ronda, A.; Pérez, A.; Blázquez, G.; Calero, M. Physico-chemical characterization of pine cone shell and its use as biosorbent and fuel. Bioresour. Technol. 2015, 196, 406–412.
- Pathirana, C.; Ziyath, A.M.; Jinadasa, K.; Egodawatta, P.; Goonetilleke, A. Mathematical modelling of the influence of physico-chemical properties on heavy metal adsorption by biosorbents. Chemosphere 2020, 255, 126965.
- Lyu, B.; Wang, H.; Swallah, M.S.; Fu, H.; Shen, Y.; Guo, Z.; Tong, X.; Li, Y.; Yu, H.; Jiang, L. Structure, properties and potential bioactivities of high-purity insoluble fibre from soybean dregs (Okara). Food Chem. 2021, 364, 130402.
- Ma, S.; Ren, B.; Diao, Z.; Chen, Y.; Qiao, Q.; Liu, X. Physicochemical properties and intestinal protective effect of ultra-micro ground insoluble dietary fibre from carrot pomace. Food Funct. 2016, 7, 3902–3909.
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233.
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98.
- Song, Y.; Su, W.; Mu, Y.C. Modification of bamboo shoot dietary fiber by extrusion-cellulase technology and its properties. Int. J. Food Prop. 2018, 21, 1219–1232.
- Pathirana, C.; Ziyath, A.M.; Jinadasa, K.; Egodawatta, P.; Sarina, S.; Goonetilleke, A. Quantifying the influence of surface physico-chemical properties of biosorbents on heavy metal adsorption. Chemosphere 2019, 234, 488–495.
- Wang, Z.; Yang, L.; Wu, J.; Zhang, H.; Zhu, L.; Zhan, X. Potential application of a low-viscosity and high-transparency xanthan gum produced from Xanthomonas campestris CCTCC M2015714 in foods. Prep. Biochem. Biotechnol. 2018, 48, 402–407.
- Kartel, M.T.; Kupchik, L.A.; Veisov, B.K. Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere 1999, 38, 2591–2596.
- Requena, M.C.; González, C.N.A.; Barragán, L.A.P.; Correia, T.; Esquivel, J.C.C.; Herrera, R.R. Functional and physico-chemical properties of six desert-sources of dietary fiber. Food Biosci. 2016, 16, 26–31.
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: A review. Sci. Total Environ. 2019, 646, 265–279.
- Basiri, S.; Shekarforoush, S.S.; Mazkour, S.; Modabber, P.; Kordshouli, F.Z. Evaluating the potential of mucilaginous seed of psyllium (Plantago ovata) as a new lead biosorbent. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100242.
- Guiza, S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 2017, 99, 134–140.
- Pal, A.; Giri, A.; Bandyopadhyay, A. Influence of hydrodynamic size and zeta potential of a novel polyelectrolyte poly (acrylic acid) grafted guar gum for adsorption of Pb (II) from acidic waste water. J. Environ. Chem. Eng. 2016, 4, 1731–1742.
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr (VI), Cu (II) and Cd (II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74.
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401.
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872.
- Berglund, L.; Breedveld, L.; Oksman, K. Toward eco-efficient production of natural nanofibers from industrial residue: Eco-design and quality assessment. J. Clean. Prod. 2020, 255, 120274.
- Fayaz, G.; Soleimanian, Y.; Mhamadi, M.; Turgeon, S.L.; Khalloufi, S. The applications of conventional and innovative mechanical technologies to tailor structural and functional features of dietary fibers from plant wastes: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2149–2199.
- Huang, J.; Liao, J.; Qi, J.; Jiang, W.; Yang, X. Structural and physicochemical properties of pectin-rich dietary fiber prepared from citrus peel. Food Hydrocoll. 2021, 110, 106140.
- Xu, G.; Wang, L.; Xie, Y.; Tao, M.; Zhang, W. Highly selective and efficient adsorption of Hg2+ by a recyclable aminophosphonic acid functionalized polyacrylonitrile fiber. J. Hazard. Mater. 2018, 344, 679–688.
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Pérez-Carrillo, E.; Serna-Saldívar, S.O.; Campanella, O.H.; Welti-Chanes, J. Functional and compositional changes of orange peel fiber thermally-treated in a twin extruder. LWT 2019, 111, 673–681.
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162.
- Adegoke, K.A.; Akinnawo, S.O.; Ajala, O.A.; Adebusuyi, T.A.; Maxakato, N.W.; Bello, O.S. Progress and challenges in batch and optimization studies on the adsorptive removal of heavy metals using modified biomass-based adsorbents. Bioresour. Technol. Rep. 2022, 19, 101115.
- Bhattacharya, A.; Mandal, S.; Das, S. Adsorption of Zn (II) from aqueous solution by using different adsorbents. Chem. Eng. J. 2006, 123, 43–51.
- Yakout, S.; Daifullah, A.; El-Reefy, S. Adsorption of naphthalene, phenanthrene and pyrene from aqueous solution using low-cost activated carbon derived from agricultural wastes. Adsorpt. Sci. Technol. 2013, 31, 293–302.
- Rastogi, S.; Tiwari, S.; Ratna, S.; Kumar, R. Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its synergistic application in biosorption of Pb2+. Bioresour. Technol. Rep. 2021, 15, 100706.
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102.
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86.




