1. Plant-Fiber Components
Plant fibers are found as structural elements in agricultural crops and in their botanical parts, such as nuts, grains, or seeds (beans, split peas, soybeans, corn, sunflowers, barley, oats, wheat, almonds, pumpkins, etc.); lentils, legumes, or vegetables (cauliflower, carrots, broccoli, celery, cabbage, turnip greens, brussels sprouts, potatoes, artichokes, eggplants, beets, cauliflower, endives, turnips, fennel, onions, leeks, rutabagas, etc.); and fruits (guavas, mangoes, strawberries, pomegranates, bananas, prunes, apples, raspberries, pears, avocados, blackberries, oranges, pineapples, etc.). Plant fibers have a complex structure that is really made up of a cell wall and a central lumen channel. The middle lamella, the primary wall, and the secondary wall are the three components that make up a cell wall [
71]. The primary wall is made up of disorganized cellulose in a pectin, hemicellulose, and lignin matrix. The secondary wall is composed of crystalline cellulose and is separated into three sections: the exterior, middle, and interior secondary walls [
72]. The chemical components of plant fibers, including cellulose, lignin, hemicellulose, pectin, and wax, can be different depending on their sources and origins [
71]. In food science, cellulose, lignin, pectin, and hemicellulose derived or extracted from fibrous plant foods or contained in plant foods or plant-fiber matrices are designated as cellulose fiber, lignin fiber, pectin fiber, and hemicellulose fiber, respectively.
The main component of plant cells is generally cellulose, which is arranged in microfibrils and surrounded by hemicellulose, which includes xylans, mannans, glucomannans, galactans, and arabinogalactans as well as lignin, pectin, and trace amounts of protein [
59,
73]. Fibers include functional groups such as carboxyl, phenolic, lactonic, and hydroxyl groups that bind to metals and remove them from aqueous environments. These functional groups interact with metal ions and act as hydrogen-ion replacements. Over a wide pH range, the process includes electrostatic and dispersive interactions between cations and the acidic surface area [
66]. Feng and Guo [
74] showed how Pb
2+, Cd
2+, and Ni
2+ ions were attached to modified orange peel by inclusion of carboxyl and hydroxyl groups. The constancy of metal-fiber complexes varies with the type of metal, the experimental settings, the fiber sources, and other factors, according to published studies [
59,
66]. Al-Ghouti and Li [
75] revealed that raw date pits may be utilized to remove Cu
2+ and Cd
2+ through the processes of complexation, coordination, chelation, ion exchange, and adsorption.
2. Fibrous Plant-Based Biomass Parts
The different parts of fibrous plant-based biomasses, considered low-cost potential metal biosorbents, are leaves, stems, stalks, roots, bagasse, seeds, shells, peels, husks, bark, and fibers [
46,
76,
77]. Various plant fiber-based biomasses have been widely used as natural materials, pretreated or chemically modified, for heavy-metal removal from aqueous media, including wastewater and aqueous solutions. These are carrot residue [
78]; potato peel [
79]; sunflower stalks and leaves [
80]; coconut shells [
81]; seed shells [
82]; coffee husks [
83]; sugar-beet pulp [
84,
85]; crude olive stones [
86]; olive-oil waste and hydrolyzed olive cake [
87,
88]; apple peel beads [
89]; citrus peels [
90], the shells of hazelnuts and almonds [
91]; physic seed hulls [
92]; rice husks [
93]; neem bark [
94]; tea waste [
95]; sunflower, potato, canola and walnut shells [
80]; sugarcane bagasse [
96]; bamboo charcoal [
97]; pistachio-hull waste [
98]; cashew-nut shells [
99]; agave bagasse [
100];
Rosa damascena leaf powder [
101]; ajwa date pits [
102]; chemically modified orange peel [
74]; banana peel and chemically modified banana peel [
103]; orange and potato immobilized on sodium alginate beads [
104]; olive-oil waste and hydrolyzed olive cake [
87,
88]; banana thrunk fibers [
105]; and cellulose fibers extracted from pineapple leaves [
106] (
Table 1).
3. Factors Influencing the Sorption Efficiencies of Fibrous Plants
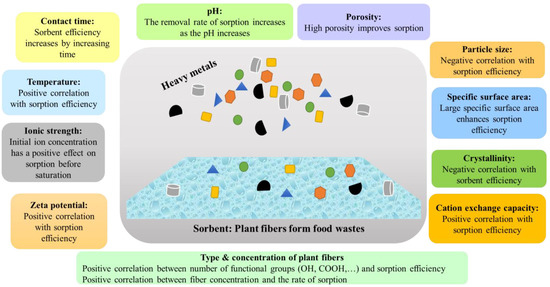
The capacities of biowaste-derived sorbents for metal-ion sorption are, however, dependent on the physicochemical properties of the prepared sorbents. The most important properties of these sorbents are cation exchange capacity (CEC), pore distribution, porosity, specific surface area, surface functional groups, etc. [
113,
114]. Lyu and Wang [
115] stated that larger specific surface areas led to higher metal (Cd
2+, Cu
2+, Pb
2+, and Zn
2+) sorption from their aqueous solutions using insoluble fiber from soybean dregs (okara). They described that the smaller particle size of insoluble okara fiber demonstrated a higher oil-holding capacity (OHC), CEC, and sorption capacity of heavy metals. In another study, ultramicro-grinding of insoluble fiber from carrot pomace decreased the particle size of the total fiber and increased its Brunauer–Emmett–Teller surface area from 0.374 to 1.835 m
2/g, leading to an increase in the water-holding capacity (WHC), swelling capacity (SC), and OHC, as well as the nitrite- and Pb
2+-ion-adsorbing capacities [
116]. Furthermore, Al-Ghouti and Li [
75] discovered that the volume of solute (Cu
2+ and Cd
2+) adsorbed increases as the particle size of the adsorbents decreases. The crystallinity of the cellulosic structure also affects sorption kinetics [
117]. Amorphous regions have a positive correlation, while crystalline structures have a negative correlation with heavy-metal sorption [
118]. With a rise in pH, the negative charge density of a fiber surface improves, which leads to an increase in sorption of heavy metals [
119]. Higher Cd
2+, Cu
2+, and Pb
2+ bind to biosorbents by having more acidic functional groups and negative zeta potential [
120]. Similarly, Wang and Yang [
121] observed that there is a positive correlation between efficiency of removing heavy metals and pH, and that removal efficiency improved when the pH was increased to 7.
Nevertheless, the biological origins of plants and types of processing have a considerable influence on sorption properties. For instance, beet pectin demonstrated a higher affinity for Pb
2+ and Cu
2+, while citrus pectin did so for Ni
2+ and apple pectin did so for Co
2+ [
122]. Requena and González [
123] showed that CEC significantly varies depending on the source of fiber; for example, CEC values of 3.5, 4.1, 2.6, 2.7, 2.6, and 1.3 meq/g were reported for ashen agave bagasse, green agave bagasse, cabuche, prickly pear peel, palm flowers, and the leaves of smooth amaranth, respectively. Nevertheless, sorption capacity largely depends on solution ion strength [
124]. The higher charge density of Cu
2+ (116 C mm
−3) results in increased ion sorption compared to Pb
2+ (32 C mm
−3) and Cd
2+ (59 C mm
−3) [
120]. Several critical factors affecting sorption efficiency of heavy metals are presented in
Figure 1.
Figure 1. Critical factors affecting sorption efficiency of heavy metals from aqueous media.
Fiber concentration also possesses substantial effects on sorption efficiency; efficiency of ion sorption increases linearly with increasing concentrations of fiber due to concurrent production of more active sites (OH and COOH) on macromolecules. Sorption efficacy may decrease beyond the optimal sorbent dose due to increased aggregation of adsorbent molecules [
120]. Pb
2+ sorption increased for 21.78, 23.41, and 26.98 mg/L at various polymer concentrations, of 1, 2, and 3%, respectively, and at a pH of 5.0, according to Basiri and Shekarforoush [
125]. Additionally, it was discovered that sorption effectiveness changes with temperature; it increases with rising temperatures before dropping after a period of time. Guiza [
126] studied Cu-ion sorption from aqueous solutions by cellulose from waste orange peel. That team observed that sorption was dependent on solution pH, adsorbent dosage, contact time, metal-ion concentration, and agitation speed. According to Pal and Giri [
127], the sorption efficiency of guar gum increased at up to a temperature of 40 °C and then decreased at 40 °C. The efficiency increased due to the possibility that a temperature increase would enhance the ions’ mobility and mobilize more Pb
2+ towards the giant adsorbent molecules, which would then increase their contact with the surfaces of the adsorbents. However, the efficiency dropped beyond 40 °C due to dominant desorption of Pb
2+ because of the increased Brownian movement [
128].
4. Different Modification Technologies for Enhancing Sorption Efficiency
The ability of fibrous plants and their components, such as cellulose, hemicellulose, and lignin, to remove heavy metals from effluents has been intensively studied. Cellulose, as a plant-fiber component, works as a skeleton of natural plant-cell walls, whereas lignin and hemicellulose are distributed in the fibrous plant matrix, resulting in poor functionalities of fibers [
129]. Subsequently, it is imperative to develop a suitable method for increasing the functionalities of fibers to enhance usage of plant byproducts, such as biosorbents [
130]. The effectiveness of these materials can be enhanced through various types of modification techniques. Several technical approaches, including mechanical, chemical, enzymatic, and/or biological technologies, have been developed to disrupt plant cellular integrity and isolate fibers with altered structural, physicochemical, and functional characteristics to enhance sorption efficiency [
68,
69].
Physical modification involves altering the physical structures of fibrous plant materials, such as pore size and surface area, to increase their sorption capacities. Methods such as grinding, milling, and sieving can be used to affect physical changes. Huang and Liao [
131] demonstrated that homogenization via mechanical shearing resulted in damage to the cellulose and crystallization regions of citrus peels, as well as an increase in the specific surface area and the total number of charged ions. Similarly, the molecules of cellulose and lignin were destroyed and transformed into tiny molecules during a steam explosion, increasing the sorption capacities for heavy metals [
118]. Xu and Wang [
132] showed that high hydrostatic pressure may significantly enhance the ability of insoluble fibers for water retention and swelling, oil holding and cation exchange, and glucose adsorption. Meanwhile, the twin-screw extrusion treatment has been shown to reduce the OHC of orange-peel fiber and increase the lead-binding ability of garlic-skin fiber [
133].
Chemical modification involves treating fibrous plants with chemical reagents to introduce functional groups to their surfaces, thereby enhancing their sorption capacities. Using oxidizing compounds such as sodium chlorite and sodium periodate, for example, it is possible to introduce carboxylic and hydroxyl groups. Following a chemical treatment, Wang and Li [
134] found that kiwifruit fiber treated with NaOH has greater thermal stability, but kiwifruit fiber treated with citric acid delivers higher sorption capacities for water, oil, bile acid, nitrite ions, and glucose. In this regard, Adegoke and Akinnawo [
135] employed numerous surface-modification treatments, such as acid, alkaline, magnetic, and grafting modifications, for improving sorption of heavy metals, including As, Cd, Cr, Cu, Co, Fe, Hg, Mn, Ni, Pb, and Zn. They revealed that acidic treatment mostly favors the sorption process. It has been proven that some pretreatments, such as hydrochloric acid, tartaric acid, sodium carbonate, and sodium hydroxide, can effectively increase the rate of heavy-metal sorption by rice husks [
136,
137].
Biological modification entails treating fibrous plant materials with microorganisms or enzymes to alter their surface properties, such as charge and hydrophobicity, thereby increasing their sorption capacities. As a biological surface functionalization, cationic surfactant can be applied to remediate heavy metals in wastewater, in which case, a cationic surfactant could change the negative surface charge of a biosorbent to a positive surface charge and would have the profound ability to uptake metal anions rather than cations. Rastogi and Tiwari [
138] used agroindustrial waste to synthesize a biosurfactant via submerged fermentation using
Bacillus haynesii, and the biosurfactant could significantly remediate Pb
2+. In addition, Dong and Du [
139] implied that modified wheat straw with polyethylenimine (a highly branched molecule containing amine groups) has a paramount impact on Cu
2+ purification from aqueous solutions. Furthermore, Chu and Zhao [
140] used
Bacillus natto to ferment millet bran. As a result of degradation of cellulose and hemicellulose by fermentation, the modified millet bran fiber developed more porous and loose structures, which increased its sorption capacity.
In conclusion, the relationship between various types of modification techniques and the respective components of the plant-fiber sorbent materials used for heavy-metal sorption is complex and dependent on the specific modification technique employed, the type of plant fiber used, and the degree of modification. The selection of an effective modification technique for fibrous plant-based sorbent materials used for heavy-metal sorption requires careful consideration of these factors.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28104205