Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga Krasnovskaya | -- | 2574 | 2023-05-31 13:33:37 | | | |
| 2 | Sirius Huang | Meta information modification | 2574 | 2023-06-02 09:53:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Krasnovskaya, O.O.; Abramchuck, D.; Erofeev, A.; Gorelkin, P.; Kuznetsov, A.; Shemukhin, A.; Beloglazkina, E.K. Copper-Based Radiopharmaceuticals for Radioimmunotherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/45058 (accessed on 07 February 2026).
Krasnovskaya OO, Abramchuck D, Erofeev A, Gorelkin P, Kuznetsov A, Shemukhin A, et al. Copper-Based Radiopharmaceuticals for Radioimmunotherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/45058. Accessed February 07, 2026.
Krasnovskaya, Olga O., Daniil Abramchuck, Alexander Erofeev, Peter Gorelkin, Alexander Kuznetsov, Andrey Shemukhin, Elena K. Beloglazkina. "Copper-Based Radiopharmaceuticals for Radioimmunotherapy" Encyclopedia, https://encyclopedia.pub/entry/45058 (accessed February 07, 2026).
Krasnovskaya, O.O., Abramchuck, D., Erofeev, A., Gorelkin, P., Kuznetsov, A., Shemukhin, A., & Beloglazkina, E.K. (2023, May 31). Copper-Based Radiopharmaceuticals for Radioimmunotherapy. In Encyclopedia. https://encyclopedia.pub/entry/45058
Krasnovskaya, Olga O., et al. "Copper-Based Radiopharmaceuticals for Radioimmunotherapy." Encyclopedia. Web. 31 May, 2023.
Copy Citation
Copper-64 (T1/2 = 12.7 h) is a positron and beta-emitting isotope, with decay characteristics suitable for both positron emission tomography (PET) imaging and radiotherapy of cancer. Copper-67 (T1/2 = 61.8 h) is a beta and gamma emitter, appropriate for radiotherapy β-energy and with a half-life suitable for single-photon emission computed tomography (SPECT) imaging. The chemical identities of 64Cu and 67Cu isotopes allow for convenient use of the same chelating molecules for sequential PET imaging and radiotherapy.
copper-64
copper-67
PET
SPECT
radiotherapy
radioimmunotherapy
1. Introduction
Nuclear medicine is based on the use of radiochemical properties of isotopes for the therapy and diagnosis of various diseases. Radiotherapy accompanies almost 50% of the use of chemotherapy, thus being an extremely important treatment modality not only in the treatment of tumor diseases but also in the palliative care of inoperable patients [1]. Since radiation therapy affects cancer tissues via DNA damaging, targeted, and strictly localized effects of radiation, preliminary imaging of the biodistribution of radiopharmaceuticals using theranostic pairs is extremely important [2].
A classical targeted radiopharmaceutical is a tissue-affine molecule, conjugated with chelator, which is further radiolabeled with radioactive isotope. Depending on the type of radiation emitted, imaging or therapeutic capability is assigned to the radiopharmaceutical category. Positron-emitting isotopes are used for PET imaging, and gamma-emitting radioisotopes are used for SPECT visualization; for radiotherapy, α-, β-, and Auger electron emitters are considered [3][4]. However, the design of radiopharmaceuticals is based not only on the emission properties of the isotope but also on the method of its synthesis, and the ability to produce the isotope in sufficient quantity, purity, and specific activity is extremely important. Radionuclides can be produced via nuclear reactors, linear accelerators, and medical cyclotrons, and can also be conveniently eluted from portable nuclide generators. However, the production of nuclides via nuclear reactors is a rather difficult task due to the large amount of radioactive waste produced along the way; moreover, the transportation of nuclear waste is a public safety issue. The use of cyclotrons allows the production of high-quality nuclides; however, a limited number of cyclotrons poses logistical problems for the delivery of short-lived radionuclides. The use of linear accelerators does not always make it possible to obtain a radionuclide of sufficient purity and activity [5][6][7].
2. Copper-Based Radiopharmaceuticals Based on Peptides
2.1. Octreotate
Somatostatin (SST) is a small peptide that regulates both cell growth and hormone secretion. Somatostatin receptors (SSTRs) are a common target for the treatment of neuroendocrine tumors (NETs) [8]. Octreotate is a peptide capable of SSTRs binding, which is used worldwide for the targeted delivery of radioactive isotopes to NETs [9][10].
Detectnet (Copper 64Cu-dotatate) is a PET-imaging agent for the localization of SSTR-positive NETs in adult patients, which was approved by the Food and Drug Administration (FDA) in 2020 [11]. One year before, in 2019, a similar 68Ga-based radiopharmaceutical [68Ga]Ga-DOTA-TOC was approved by FDA as the first 68Ga-radiopharmaceutical for imaging of SSTR-positive gastroenteropancreatic NETs [12]. Both radiopharmaceuticals are based on tyrosine-octreotate, conjugated with DOTA (tetraxetan) as a metal chelator (Figure 2).
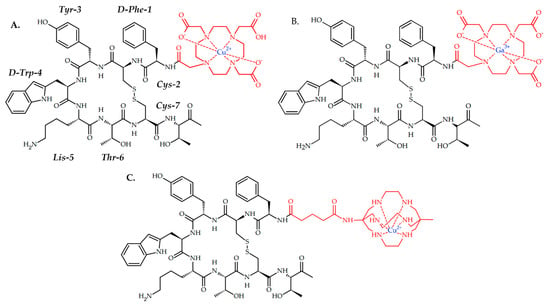
Figure 2. (A) Detectnet, 64Cu-based radiopharmaceutical for PET imaging of SSTR positive NETs; (B) [68Ga]Ga-DOTA-TOC, 68Ga-based radiopharmaceutical for PET imaging of SSTR positive NETs in adult and pediatric patients. (C) CuSarTATE, 67Cu-based radiopharmaceutical for radionuclide therapy of neuroblastoma in pediatric patients.
A direct comparison of Detectnet and [68Ga]Ga-DOTA-TOC, which was provided in 59 patients with NETs, showed undeniable advantages in lesion detection in NET patients of Detectnet over 68Ga-DOTATOC [13]. Recently, Song et al. reported a 64Cu-DOTATATE uptake in a 43-year-old woman with a slowly enlarging pulmonary nodule [14].
Cullinane et al. reported a preclinical investigation of a similar (Tyr3)-octreotate-based radiopharmaceutical, with MeCOSar as copper chelator, CuSarTATE (Figure 2) [15]. Two injections of [67Cu]CuSarTATE (15-MBq fractions two weeks apart) showed good antitumor efficacy in vivo on BALB/C mice with AR42J tumor, similar with [177Lu]LuTATE. Currently, [67Cu]CuSarTATE is ongoing clinical trials as drug for radionuclide therapy of neuroblastoma in pediatric patients (NCT04023331).
2.2. PSMA
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that consists of 750 amino acids, that are overexpressed in tumor tissues 100- to 1000-fold higher than that in normal ones. The commonly used substrate of PSMA is a urea-based peptide with a C-terminal glutamate, capable of active site binding [16][17]. In 2022, Debnath et al. summarized PSMA-targeting and theranostic agents [18]. In 2022, Jeitner et al. also summarized advances in PSMA theranostics [19].
Gallium (68Ga) gozetotide is a clinically used drug for PET prostate cancer imaging, which was approved in the United States in December 2020 [20] and in the European Union in December 2022 [21].
Despite FDA approval of (68Ga) gozetotide for prostate cancer imaging, several benefits of 64Cu over 68Ga arouse interest in 64Cu-based radiopharmaceuticals for PET imaging of prostate cancer [22]. Additionally, 64CuCl2 salt showed promising therapeutic efficacy on the 3D prostate cancer model [23].
In 2018, Umbricht et al. reported a successful PET visualization of PSMA-positive PC-3 PIP/flu tumor with 64Cu-based PSMA conjugates capable of albumin binding [24].
PSMA-specific uptake for both radiolabeled ligands was confirmed on PSMA-positive (PC-3 PIP) and PSMA-negative (PC-3 flu) tumor cells. Biodistribution and PET/CT imaging studies performed on PC-3 PIP/flu tumor-bearing mice proved the ability of 64Cu−PSMA−ALB-89 to accumulate in PSMA-positive tumors. Even though 64Cu−PSMA−ALB-56 showed lower tumor uptake than 64Cu−PSMA−ALB-89, almost no retention of 64Cu−PSMA−ALB-56 was detected in the kidneys, which is critical to avoid false positive PET results.
In 2020, Kelly et al. reported RPS-085 ligand, capable of both PSMA and serum albumin binding, and a theranostic approach based on 64Cu/67Cu pair for prostate cancer therapy and imaging [24].
RPS-085 was designed based on previously reported ligand RPS-063 with DOTA chelator [25]. Metal-free RPS-085 showed the ability for both PSMA inhibition and human serum albumin binding. After ligand radiolabeling, LNCaP was successfully visualized with [64Cu]Cu-RPS-085. Importantly, the biodistribution of [67Cu]Cu-RPS-085 closely mimics that of [64Cu]Cu-RPS-085, thus confirming the possibility of pre-imaging when using the theranostic 64Cu/67Cu pair. Although studies of the therapeutic efficacy of [67Cu]Cu-RPS-085 have not been conducted, the principal possibility of pre-imaging with copper-64 before copper-67 radiotherapy has been proven.
In 2019, Zia et al. reported two sarcophagine ligands with one or two PSMA-targeting moieties [26]. The cell surface binding and internalization were evaluated in LNCaP cells and [64Cu]CuSarbisPSMA displayed higher cell surface binding and internalization, which is evidence that two target-binding vectors yield better results than one.
To access imaging properties, PET images of PSMA-positive LNCaP-tumor-bearing NSG mice were obtained at 0.5, 2 and 22 h p.i., and significant tumor uptake of both radioligands was evident. Expectedly, bivalent [64Cu]CuSarbisPSMA showed higher tumor uptake and retention when compared to the monomer, which was confirmed in vivo biodistribution studies. However, [64Cu]CuSarPSMA showed better tumor uptake than clinically used 68Ga-PSMA (Ga-PSMA-11) at 1 h p.i.
Since 64Cu-CuSarbisPSMA showed both an excellent uptake and retention in LNCaP tumors, the suggestion that the 67Cu variant may be suited to PSMA-targeted radiotherapy is relevant. Thus, in 2021, McInnes et al. reported a therapeutic potential of 67Cu-CuSarbisPSMA [27]. Expectedly, [67Cu]CuSarbisPSMA and [177Lu]LuPSMA provided similar tumor inhibition and survival extension at equivalent administered activities, since the energy from the β emissions from 67Cu and 177Lu are similar. However, the shorter half-life of 67Cu than of 177Lu (61.9 h vs. 6.7 days) shortens radiotherapy while maintaining its efficiency. 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are currently in clinical trials as drugs for identification and treatment of PSMA-expressing metastatic castrate resistant prostate cancer (NCT04868604).
2.3. Other Peptides
In 2018, Sarkar et al. reported five bifunctional chelators, conjugated with arginyl glycyl aspartic acid (RGD peptide), and radiolabeled them with 64Cu. To evaluate the effects of the chelator’s nature on the pharmacokinetics of 64Cu-radiopharmateutical, five different chelators were used [28].
Three 64Cu-labeled cross-bridged chelators showed better in vivo stability compared to the two non-cross-bridged chelators and 64Cu-labeled PCB-TE2A-Bn-NCS proved to be the most stable. PET imaging in glioma U87MG tumor-bearing mice was obtained, and two 64Cu-labeled PCB-TE2A conjugates exhibited higher tumor uptake compared with others. 64Cu-PCB-TE2A-Bn-NCS-c(RGDyK) also showed 4-fold lower demetallation in blood compared with the others.
The melanocortin-1 receptors (MC1Rs) are a group of G protein-coupled receptors, which are overexpressed in human melanomas. MC1Rs can bind with alpha-melanocyte-stimulating hormone (α-MSH) peptides [29].
In 2022, Qiao et al. demonstrated the ability of copper-based radiopharmaceuticals to visualize melanoma. Two theranostic 64Cu-radiolabeled α-MSH peptides were designed as potential agents for melanoma PET imaging [30].
Radiolabeled peptide 64Cu-NOTA-PEG2Nle-CycMSHhex showed both high MC1R binding affinity on B16/F10 cells and MC1R-specific cellular uptake on B16/F10 cells. Good tumor uptake of 64Cu-NOTA-PEG2Nle-CycMSHhex on B16/F10 melanoma-bearing mice was demonstrated by PET.
Gastrin-releasing peptide receptor (GRPR) is overexpressed on the surface of different cancers. GRPR can bind with high affinity to bombesin, a 14-amino acid peptide. Since bombesin itself exhibits low stability, its analogs have been investigated as GRPR-targeted ligands for the diagnosis and therapy of GRPR-positive tumors [31].
In 2022, Huynh et al. reported successful radiotherapy of GRPR-positive PC-3 tumor with 67Cu-labeled bombesin antagonist [32]. 67Cu-radiolabeled GRPR-targeted peptide [67Cu]Cu-SAR-BBN was designed.
[67Cu]Cu-SAR-BBN showed the ability to accumulate well in the GRPR-positive PC-3 cell line. Administration of six doses of 24 MBq of [67Cu]Cu-SAR-BBN resulted in inhibited PC-3 tumor growth with a 93.3% reduction in tumor volume, with no significant weight loss.
3. Copper-Based Radiopharmaceuticals for Radioimmunotherapy
3.1. Direct Conjugation of Radiolabeled Chelator and Antibody
Radioimmunotherapy (RIT) is a subtype of radiotherapy, that uses monoclonal antibodies as a delivery agent for radionuclides. Antibodies labeled with positron-emitting radionuclides are used for PET imaging and dosimetry, while radioimmunoconjugates labeled with therapeutic nuclides are used for therapy [33][34][35].
PD-1/PD-L1 inhibitors are a class of anticancer drugs, capable of blocking the activity of PD-1 and PDL1 immune checkpoint proteins [36]. Thus, anti-PD-1 or anti-PD-L1 antibodies are clinically used, and non-invasive imaging of PD-L1 expression levels in malignant tumors is of interest [37].
In 2018, Xu et al. reported the successful immunotherapy of a PD-L1 positive MC38 tumor with an anti-PD-L1 antibody, which was preliminary radiolabeled with copper-64, and its tumor accumulation was confirmed using PET [38].
When PET imaging of MC38 and 4T1 tumor grafts in vivo were performed, only the PD-L1 positive MC38 tumor was visualized by radiolabeled antibody [64Cu]Cu-NOTA-MX001. Immunotherapy studies provided in mice bearing MC38 tumor with MX001 antibody resulted in tumor growth suppression. In contrast, low anti-tumor efficacy of MX001 on 4T1 tumor was revealed, thereby proving the effectiveness and specificity of immunotherapy. Thus, an antibody may be successfully visualized with 64Cu before immunotherapy.
Trastuzumab is a human epidermal growth factor receptor protein (HER-2)-affine monoclonal antibody, clinically used in the treatment of (HER-2)+ metastatic breast cancer [39].
In 2021, Lee et al. reported a visualization of a NIH3T6.7 tumor with a 64Cu-radiolabeled trastuzumab antibody. In addition, a novel conjugation approach based on click reaction was proposed [40]. For the chemical binding of the antibody with a radiolabeled chelator, Tz/TCO click reaction was used. Tz/TCO is a bio-orthogonal inverse electron-demand Diels–Alder click reaction between trans-cyclooctene (TCO) and tetrazine (Tz).
Copper-catalyzed azide–alkyne cycloaddition is usually not used in the synthesis of copper-chelating conjugates, due to it chelating the catalyst with reagents and the subsequent failure of the reaction. However, Lee et al. succeeded in choosing the conditions for the click reaction in which the catalytic agent is not chelated and the fast and quantitative click conjugation of the chelator and linker occurs. Since a cross-bridged chelator is not prone to complexation with Cu(II) ions at a lower temperature, Cu(I)-catalyzed alkyne−azide cycloaddition was used for conjugation of chelator and linker.
Both 64Cu-radiolabeled trastuzumab conjugates showed in vivo stability, and tumor accumulation and proved the ability to visualize a HER-2 positive NIH3T6.7 tumor. However, the conjugate with a PEG linker demonstrated fast body clearance.
Pertuzumab is another anti-HER-2 humanized monoclonal antibody that is used in combination with Trastuzumab in the therapy of HER-2-positive breast cancers [41].
In 2021, Hao et al. reported successful radioimmunotherapy of HER-2 positive HCC1954 tumor with radiolabeled 67Cu-Pertuzumab [42]. [67Cu]Cu-NOTA-Pertuzumab was obtained by conjugation of p-SCN-Bn-NOTA to pertuzumab and further radiolabeling. The efficacy of radioimmunotherapy was assessed on mice xenografts bearing a HER2 positive HCC1954 tumor. During the therapy, a dose-dependent tumor growth inhibition was observed even with the low dose of [67Cu]Cu-NOTA-Pertuzumab.
A theranostic potential of 67Cu was confirmed via registration of SPECT/CT imaging after the injection of [67Cu]Cu-NOTA-Pertuzumab. Tumors were successfully visualized by SPECT, thereby confirming the possibility of the successful use of 67Cu radiopharmaceuticals as theranostic agents.
CD4+ T cells are inflammatory mediators of autoimmune rheumatoid arthritis [43]. In 2022, Clausen et al. reported a visualization of rheumatoid arthritis with 64Cu-labeled radiotracer [44].
The F(ab)’2 fragments of R-anti-mouse CD4 antibodies were conjugated to NOTA and radiolabeled with 64Cu. PET/CT images of a mouse with collagen-induced arthritis at different time points were obtained. Despite the drug accumulation in various organs, an increased accumulation of [64Cu]Cu-NOTA-IgG2b in joints with pronounced arthritis was revealed. Additionally, a decrease in tracer accumulation after dexamethasone injection confirmed a correlation of [64Cu]Cu-NOTA-CD4 accumulation with arthritic inflammation levels.
3.2. Pretargeting Approach in Conjugation of Radiolabeled Chelator and Antibody
One of the main disadvantages of radioimmunotherapy is the fact that it can take several days for antibodies after administration to accumulate in their therapeutic target (tumor tissue). Thus, if antibodies are used as delivery agents for therapeutic radionuclides, only long-lived radionuclides should be used, which can lead to high radiation doses to healthy tissues. To solve this problem, in vivo pretargeting approach was suggested based on injecting the two components separately. An antibody is given several hours (or days) to accumulate in the tumor and clear from the blood. Then, the radiolabeled small molecule, capable of chemical binding with the antibody, is administered [45].
In 2020, Keinänen et al. reported an in vivo pretargeting with 64Cu/67Cu theranostic pair, with both PET imaging and subsequent radioimmunotherapy of SW1222 human colorectal carcinoma [46].
Two radioligands, [64Cu]CuMeCOSar-Tz and [67Cu]Cu-MeCOSar-Tz, as well as TCO-conjugated huA33 antibody, capable of targeting the A33 antigen, which is expressed in >95% colorectal cancers, were designed.
Xenografts grafted with SW1222 human colorectal carcinoma (A33 antigen-positive) were administered with huA33-TCO, and after 24 or 72 h [64Cu]Cu-MeCOSar-Tz was injected. In the absence of huA33-TCO, [64Cu]Cu-MeCOSar-Tz showed negligible tumoral uptake, while in the mice treated with both huA33-TCO and [64Cu]CuMeCOSar-Tz, clear tumor PET imaging was registered.
As for therapeutic efficacy, various strategies of longitudinal therapy have been tried to find the optimal dose and interval. As a result, HuA33-TCO and [67Cu]CuMeCOSar-Tz were injected 72 h apart, and a dose-dependent therapeutic response was observed. Importantly, PET images registered after injection of [64Cu]Cu-MeCOSar-Tz accurately predicted the efficacy of the [67Cu]Cu-MeCOSar-Tz, which was injected later, which is direct evidence of the effectiveness of the theranostic couple concept.
In 2022, Jallinoja et al. reported another pretargeting approach, with novel ferrocene-based radioligands ([64Cu]Cu-NOTA-PEG3-Fc and [64Cu]Cu-NOTA-PEG7-Fc) [47]. To conjugate antibodies with a chelator, a host–guest chemistry between a cucurbit [7] uril (CB7) and a ferrocene (Fc) was used [48].
M5A, CB7-M5A antibody can bind carcinoembryonic antigen (CEA), which is expressed in several cancers, such as colorectal, gastric and pancreatic cancers, and also in some breast cancer and non-small-cell lung cancer [49]. The antibody was modified with dibenzocyclooctyne. Both radioligands showed good in vitro stability and had similar in vivo profiles in healthy mice, with relatively slow excretion through the gastrointestinal tract. The pretargeting approach has been investigated with a time interval of 120 h, and radioligands showed specific tumor uptake. In addition, a pretargeting approach with an extended time interval of up to 9 days still showed good tumor localization.
References
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24.
- Nickoloff, J.; Sharma, N.; Taylor, L. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Genes 2013, 11, 99.
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT imaging: A literature review over the last decade. Int. J. Mol. Sci. 2022, 23, 5023.
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. 2014, 15, 845–863.
- Boschi, A.; Martini, P.; Costa, V.; Pagnoni, A.; Uccelli, L. Interdisciplinary tasks in the cyclotron production of Radiometals for medical applications. The case of 47Sc as example. Molecules 2019, 24, 444.
- Currie, G.M.; Wheat, J.M.; Davidson, R.; Kiat, H. Radionuclide production. Radiographer 2011, 58, 46–52.
- Onda, Y.; Taniguchi, K.; Yoshimura, K.; Kato, H.; Takahashi, J.; Wakiyama, Y.; Coppin, F.; Smith, H. Radionuclides from the Fukushima Daiichi nuclear power plant in terrestrial systems. Nat. Rev. Earth Environ. 2020, 1, 644–660.
- He, Z.; Jia, H.; Zheng, M.; Wang, H.; Yang, W.; Gao, L.; Zhang, Z.; Xue, J.; Xu, B.; Yang, W.; et al. Trp2 peptide-assembled nanoparticles with intrinsically self-chelating 64Cu properties for PET imaging tracking and dendritic cell-based immunotherapy against melanoma. ACS Appl. Bio Mater. 2021, 4, 5707–5716.
- Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447.
- Sanli, Y.; Garg, I.; Kandathil, A.; Kendi, T.; Zanetti, M.J.; Kuyumcu, S.; Subramaniam, R.M. Neuroendocrine tumor diagnosis and management: 68Ga-DOTATATE PET/CT. Am. J. Roentgenol. 2018, 211, 267–277.
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A prospective study of 59 patients with neuroendocrine tumors. J. Nucl. Med. 2017, 58, 451–457.
- Urso, L.; Nieri, A.; Uccelli, L.; Castello, A.; Artioli, P.; Cittanti, C.; Marzola, M.C.; Florimonte, L.; Castellani, M.; Bissoli, S.; et al. Lutathera® Orphans: State of the Art and Future Application of Radioligand Therapy with 177Lu-DOTATATE. Pharmaceutics 2023, 15, 1110.
- Hennrich, U.; Benešová, M. Ga-DOTA-TOC: The first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceutics 2020, 13, 38.
- Cullinane, C.; Jeffery, C.M.; Roselt, P.D.; Dam, E.M.; Jackson, S.; Kuan, K.; Jackson, P.; Binns, D.; van Zuylekom, J.; Harris, M.J.; et al. Peptide receptor radionuclide therapy with 67Cu-CuSarTATE is highly efficacious against a somatostatin-positive neuroendocrine tumor model. J. Nucl. Med. 2020, 61, 1800–1805.
- Song, H.; Guja, K.E.; Yang, E.J.; Guo, H.H. 64Cu-DOTATATE Uptake in a Pulmonary Hamartoma. Clin. Nucl. Med. 2022, 10, 1097.
- Vahidfar, N.; Farzanehfar, S.; Abbasi, M.; Mirzaei, S.; Delpassand, E.S.; Abbaspour, F.; Salehi, Y.; Biersack, H.J.; Ahmadzadehfar, H. Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of Cu-DOTA-TOC. Cancers 2022, 14, 1914.
- Machulkin, A.E.; Shafikov, R.R.; Uspenskaya, A.A.; Petrov, S.A.; Ber, A.P.; Skvortsov, D.A.; Nimenko, E.A.; Zyk, N.U.; Smirnova, G.B.; Pokrovsky, V.S.; et al. Synthesis and biological evaluation of PSMA ligands with aromatic residues and fluorescent conjugates based on them. J. Med. Chem. 2021, 64, 4532–4552.
- Machulkin, A.E.; Ivanenkov, Y.A.; Aladinskaya, A.V.; Veselov, M.S.; Aladinskiy, V.A.; Beloglazkina, E.K.; Koteliansky, V.E.; Shakhbazyan, A.G.; Sandulenko, Y.B.; Majouga, A.G. Small-molecule PSMA ligands. Current state, SAR and perspectives. J. Drug Target. 2016, 24, 679–693.
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-targeting imaging and theranostic agents—Current status and future perspective. Int. J. Mol. Sci. 2022, 23, 1158.
- Jeitner, T.M.; Babich, J.W.; Kelly, J.M. Advances in PSMA theranostics. Trans. Oncol. 2022, 22, 101450.
- U.S. Food and Drug Administration. Drug Approval Package: Illuccix; U.S. Food and Drug Administration (FDA): Washington, DC, USA, 2022.
- Locametz EPAR. European Medicines Agency (EMA). 12 October 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/locametz (accessed on 19 May 2023).
- Milot, M.C.; Benesty, O.B.; Dumulon-Perreault, V.; Ait-Mohand, S.; Richard, P.O.; Rousseau, É.; Guérin, B. 64Cu-DOTHA2-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window. Pharmaceutics 2022, 15, 996.
- Kelly, J.M.; Ponnala, S.; Amor-Coarasa, A.; Zia, N.A.; Nikolopoulou, A.; Williams, C., Jr.; Schlyer, D.J.; DiMagno, S.G.; Donnelly, P.S.; Babich, J.W. Preclinical evaluation of a high-affinity sarcophagine-containing PSMA ligand for 64Cu/67Cu-based theranostics in prostate cancer. Mol. Pharm. 2020, 17, 1954–1962.
- Kelly, J.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Schlyer, D.; Zhao, Y.; Kim, D.; Babich, J.W. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1841–1851.
- Zia, N.; Cullinane, C.; Zuylekom, K.; Waldeck, K.; McInnes, E.; Buncic, G.; Haskali, M.; Roselt, P.; Hicks, R.; Donnelly, P. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew. Chem. Int. Ed. 2019, 58, 14991–14994.
- McInnes, L.E.; Cullinane, C.; Roselt, P.D.; Jackson, S.; Blyth, B.J.; van Dam, E.M.; Zia, N.A.; Harris, M.J.; Hicks, R.J.; Donnelly, P.S. Therapeutic efficacy of a bivalent inhibitor of prostate-specific membrane antigen labeled with 67Cu. J. Nucl. Med. 2021, 62, 829–832.
- Sarkar, S.; Bhatt, N.; Ha, Y.S.; Huynh, P.T.; Soni, N.; Lee, W.; Lee, Y.J.; Kim, J.Y.; Pandya, D.N.; An, G.I.; et al. High in vivo stability of 64Cu-labeled cross-bridged chelators is a crucial factor in improved tumor imaging of RGD peptide conjugates. J. Med. Chem. 2018, 61, 385–395.
- Yang, J.; Xu, J.; Gonzalez, R.; Lindner, T.; Kratochwil, C.; Miao, Y. 68Ga-DOTA-GGNle-CycMSHhex targets the melanocortin-1 receptor for melanoma imaging. Sci. Trans. Med. 2018, 10, eaau4445.
- Qiao, Z.; Xu, J.; Gonzalez, R.; Miao, Y. Novel 64Cu-labeled NOTA-conjugated lactam-cyclized alpha-melanocyte-stimulating hormone peptides with enhanced tumor to kidney uptake ratios. Mol. Pharm. 2022, 19, 2535–2541.
- Rurarz, B.P.; Bukowczyk, M.; Gibka, N.; Piastowska-Ciesielska, A.W.; Karczmarczyk, U.; Ulański, P. Nanostrategies for Therapeutic and Diagnostic Targeting of Gastrin-Releasing Peptide Receptor. Int. J. Mol. Sci. 2023, 24, 3455.
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceutics 2022, 15, 728.
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjugate Chem. 2009, 20, 825–841.
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.V.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360.
- Bailly, C.; Cléry, P.F.; Faivre-Chauvet, A.; Bourgeois, M.; Guérard, F.; Haddad, F.; Bodet-Milin, C. Immuno-PET for Clinical Theranostic Approaches. Int. J. Mol. Sci. 2016, 18, 57.
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front. Pharm. 2021, 12, 731798.
- De Sousa Linhares, A.; Battin, C.; Jutz, S.; Leitner, J.; Hafner, C.; Tobias, J.; Wiedermann, U.; Kundi, M.; Zlabinger, G.J.; Grabmeier-Pfistershammer, K.; et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci. Rep. 2019, 9, 11472.
- Xu, M.; Han, Y.; Liu, G.; Xu, Y.; Duan, D.; Liu, H.; Du, F.; Luo, P.; Liu, Z. Preclinical study of a fully human anti-PD-L1 antibody as a theranostic agent for cancer immunotherapy. Mol. Pharm. 2018, 15, 4426–4433.
- Vu, T.; Claret, F.X. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012, 2, 62.
- Lee, W.; Sarkar, S.; Pal, R.; Kim, J.Y.; Park, H.; Huynh, P.T.; Bhise, A.; Bobba, K.N.; Kim, K.I.; Ha, Y.S.; et al. Successful Application of CuAAC Click Reaction in Constructing 64Cu-Labeled Antibody Conjugates for Immuno-PET Imaging. ACS Appl. Bio Mater. 2021, 4, 2544–2557.
- Capelan, M.; Pugliano, L.; De Azambuja, E.; Bozovic, I.; Saini, K.S.; Sotiriou, C.; Piccart-Gebhart, M.J. Pertuzumab: New hope for patients with HER2-positive breast cancer. Ann. Oncol. 2012, 24, 273–282.
- Hao, G.; Mastren, T.; Silvers, W.; Hassan, G.; Öz, O.; Sun, X. Copper-67 radioimmunotheranostics for simultaneous immunotherapy and immuno-SPECT. Sci. Rep. 2021, 11, 3622.
- Yap, H.Y.; Tee, S.; Wong, M.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161.
- Clausen, A.S.; Christensen, C.; Christensen, E.; Cold, S.; Kristensen, L.K.; Hansen, A.E.; Kjaer, A. Development of a 64Cu-labeled CD4+ T cell targeting PET tracer: Evaluation of CD4 specificity and its potential use in collagen-induced arthritis. EJNMMI Res. 2022, 12, 62.
- Van de Watering, F.C.J.; Rijpkema, M.; Robillard, M.; Oyen, W.J.G.; Boerman, O.C. Pretargeted Imaging and Radioimmunotherapy of Cancer Using Antibodies and Bioorthogonal Chemistry. Front. Med. 2014, 1, 44.
- Keinänen, O.; Fung, K.; Brennan, J.M.; Zia, N.; Harris, M.; van Dam, E.; Biggin, C.; Hedt, A.; Stoner, J.; Donnelly, P.S.; et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 28316–28327.
- Jallinoja, V.I.; Carney, B.D.; Bhatt, K.; Abbriano, C.H.; Schlyer, D.J.; Yazaki, P.J.; Houghton, J.L. Investigation of Copper-64-Based Host–Guest Chemistry Pretargeted Positron Emission Tomography. Mol. Pharm. 2022, 19, 2268–2278.
- Jeon, W.S.; Moon, K.; Park, S.H.; Chun, H.; Ko, Y.H.; Lee, J.Y.; Kim, K. Complexation of Ferrocene Derivatives by the Cucurbituril Host: A Comparative Study of the Cucurbituril and Cyclodextrin Host Families. J. Am. Chem. Soc. 2005, 127, 12984–12989.
- Mohajershojai, T.; Jha, P.; Boström, A.; Frejd, F.Y.; Yazaki, P.J.; Nestor, M. In Vitro Characterization of 177Lu-DOTA-M5A Anti-Carcinoembryonic Antigen Humanized Antibody and HSP90 Inhibition for Potentiated Radioimmunotherapy of Colorectal Cancer. Front. Oncol. 2022, 12, 849338.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
799
Revisions:
2 times
(View History)
Update Date:
02 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




