Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nandini Vishwakarma | -- | 2199 | 2023-05-30 18:30:24 | | | |
| 2 | Camila Xu | Meta information modification | 2199 | 2023-05-31 02:20:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Greenberg, G.C.; Vishwakarma, N.; Tirupattur, M.P.; Sprague, H.M.; Katwa, L.C. COVID-19 Infection on Pregnancy Maternal and Fetal Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/45023 (accessed on 08 February 2026).
Greenberg GC, Vishwakarma N, Tirupattur MP, Sprague HM, Katwa LC. COVID-19 Infection on Pregnancy Maternal and Fetal Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/45023. Accessed February 08, 2026.
Greenberg, Grace C., Nandini Vishwakarma, Myna Prakash Tirupattur, Hannah M. Sprague, Laxmansa C. Katwa. "COVID-19 Infection on Pregnancy Maternal and Fetal Health" Encyclopedia, https://encyclopedia.pub/entry/45023 (accessed February 08, 2026).
Greenberg, G.C., Vishwakarma, N., Tirupattur, M.P., Sprague, H.M., & Katwa, L.C. (2023, May 30). COVID-19 Infection on Pregnancy Maternal and Fetal Health. In Encyclopedia. https://encyclopedia.pub/entry/45023
Greenberg, Grace C., et al. "COVID-19 Infection on Pregnancy Maternal and Fetal Health." Encyclopedia. Web. 30 May, 2023.
Copy Citation
When compared with uninfected mothers, pregnant women with a COVID-19 infection are at a higher risk for maternal mortality. With the additional difficulties of the COVID-19 infection, pregnant persons also have higher hospitalization and ICU admission rates, increased risk of requiring ventilation, and elevated mortality rate than non-pregnant women who are infected, according to the Centers for Disease Control and Prevention (CDC) COVID-19 surveillance system.
COVID-19
pregnancy
vaccination
cardiovascular disease
1. Introduction
Initially, it was unclear the impact a COVID-19 infection would have on pregnant individuals and their offspring. There has been evidence of past outbreaks of viral infections, specifically respiratory illnesses, such as the Middle East respiratory syndrome, ending poorly for both mother and baby [1]. There is also evidence to support that pregnant women are at an increased risk for more severe infections of the influenza virus, hepatitis E virus, herpes simplex virus, as well as malaria parasites [2]. At first, it was believed that because of the weakened immune system during pregnancy, they are more at risk for encountering COVID-19 [3]. However, it has been indicated that pregnancy does not raise the risk of contracting the disease, but it has the possibility to result in acute illness [4][5]. The CDC states that if an individual is pregnant or was recently pregnant, they are more likely to become severely ill from COVID-19 compared with those who are not pregnant, meaning that the individual may need hospitalization, admission into an ICU, and potentially a ventilator or other device to help them breathe [6][7][8].
Some earlier observations during the pandemic, including one published in November 2020, concluded that a COVID-19 infection in pregnancy was not associated with adverse maternal and fetal health outcomes [7]. Although, over the last three years, more data has been collected and published as the virus has evolved, that conclude that COVID-19 could very well be correlated with dire consequences in pregnancy. A recent meta-analysis published concluded that infection of SARS-CoV-2 in pregnancy at any time leads to an increased incidence of maternal mortality, maternal morbidities, and adverse newborn outcomes. It was found that those with an infection had a higher risk for several fatal effects, such as hypertensive disorders of pregnancy, pre-eclampsia or eclampsia, pre-term labor, and thromboembolic disease. The analysis included data from 12 studies and included 13,136 pregnant individuals from 12 different countries [9]. A systematic review also found that an increased risk of pre-eclampsia and pre-term birth is associated with a COVID-19 infection [8]. Pre-eclampsia has been associated with cardiovascular sequelae, such as hypertension and altered vascular function in the neonate. It is also a major cause of maternal morbidity and is connected to fetal health concerns [10]. More information on COVID-19 and pre-eclampsia is needed as it is a concern both for mother and baby, and COVID-19 infections could be contributing to increased incidence of developing the condition.
Furthermore, the gestational age can vary the turnout of COVID-19 infection in pregnancy. There are varying data on whether the incidence of adversities is higher in those at a lower gestational age or a higher one. A retrospective cohort study conveyed that a first- or second-trimester infection accounted for an increase in pre-term labor and stillbirth. The results concluded that regardless of the severity of the COVID-19 infection, pregnant people are at an increased risk for pre-term birth. The infected individuals were unvaccinated, highlighting a need for information on maternal infections in vaccinated mothers [11]. However, a different investigation concluded otherwise: that an infection in the first trimester showed no evidence of increased complications in the pregnancy [12]. The outcomes of COVID-19 in pregnancy should be further examined based on gestational age to understand how the two are correlated.
2. Risk of Stillbirth or Miscarriage
According to the CDC, a miscarriage is defined as the loss of a baby before 20 weeks of gestation or before viability, and stillbirth is defined as a loss of a fetus after 20 weeks of gestation [13]. Approximately 1 in 100 pregnancies at or after 20 weeks gestation is impacted by stillbirth [14][15]. There are some circumstances that can greatly higher the risk of an individual having a stillbirth, including being overweight or obese, being 35 years of age or older, and having pre-gestational and gestational medical disorders, such as hypertension and diabetes [16]. In total, 15.3% of pregnancies end in miscarriage, and risk factors for miscarriage include age, body mass index (BMI), and ethnicity [17]. Maternal infection in previous coronavirus epidemics has been linked with a higher incidence of pregnancy losses and miscarriage [18]. Furthermore, it has been a question since the beginning of the pandemic whether the same applies to COVID-19. As of today, COVID-19 has been proven to potentially have adverse effects on pregnant women and their fetuses, but there are still gaps in the literature to conclude whether infection with COVID-19 may or may not have a causal or correlational relationship with stillbirth or miscarriage.
Studies have been conducted to determine how infection of COVID-19 plays a role in the incidence of miscarriage, but the results are varied. COVID-19 appears to increase the risk of delivering a stillborn infant, according to the CDC [19][20]. However, a study published in early 2021 concluded that there was no difference in the cumulative incidence of miscarriage between those infected with the virus and the control group [21]. Eleven percent of the cases of infection ended in first-trimester pregnancy loss, and 9.6% of the control cases ended in a first-trimester loss and concluded that SARS-CoV-2 infection in the first trimester does not appear to cause early pregnancy loss as the difference was not considered significant [21]. Similarly, another article demonstrated that pregnant women with a self-reported COVID-19 infection had a 14% rate of miscarriage, compared to 5% in a group who were uncertain of their infection status and 8% in the presumed uninfected group [20]. The risk of early miscarriage did appear to be higher among women with a self-reported infection than those without an infection of COVID-19, but this data was not concluded to be statistically significant [20]. It was concluded by an analysis performed in 2021 that the miscarriage rates of pregnant people with COVID-19 seemed to be in the range of the rates in negative pregnant people. However, these results were limited by a small number of cases and lacked proper controls. It also concluded that the presence of symptoms during the acute phase of COVID-19, plasma viral load, severity, and obstetrical risk factors seem to increase the prevalence of miscarriage for women with a SARS-CoV-2 infection [21].
3. Postpartum Difficulties
Furthermore, investigating the effects of the infection during pregnancy in the postpartum period is important as well because although the pregnancy has ended, the mother may still be dealing with the ramifications of the infection. Because long-term outcomes of COVID-19 infections are not fully known at this point, as the pandemic began only three years ago, this makes determining the effects on the mother immediately after birth and forward more complex. Continued research, including long-term studies, is needed to determine the postpartum risks of a COVID-19 infection in pregnancy. Controversy has arisen as to whether COVID-19 is spreading through breast milk from mother to baby. However, it is not apparent that transmission of COVID-19 occurs through breast milk [22]. Having to stop breastfeeding abruptly can be a challenging and distressing experience for new mothers. While breastfed infants have tested positive for COVID-19, the mode of infection cannot be determined, and it is not recommended to interrupt breastfeeding.
There are other potential long-term complications for the mother after giving birth. Circulation by AHA found that women who had at least one pregnancy before menopause and those who experienced pre-eclampsia during their pregnancy were at a higher risk of stroke later in their lifetime [22][23]. With the prevalence of pre-eclampsia in COVID-19-infected mothers, these statistics raise a concern about the long-term effects of the infection on not just the neonate but also the mother.
4. Potential Adverse Effects on Fetus
There is little evidence to support whether mothers infected by COVID-19 transmit the virus to the fetus in utero. It has been demonstrated by the Boston University School of Medicine that Angiotensin-Converting-Enzyme 2 (ACE2) is found in lower concentrations in the placentas of those infected by COVID-19 compared to those pregnancies without infection [24]. It is currently known that the SARS-CoV-2 virus binds to ACE2 using a spike-like protein, acting as a cellular doorway for the virus [25][26]. Hence, because the placenta sheds these ACE2 receptors, it can be interpreted that the body performs this to protect the fetus from the virus. However, this phenomenon needs to be investigated deeper to form a solid conclusion.
Vertical transmission is defined as the possibility of an infection transmitting to the fetus from the mother before or during delivery or to the neonate after the delivery [20]. Furthermore, Figure 1 demonstrates the three possible ways that vertical transmission may occur. Vertical transmission is a rare incidence of COVID-19 infections, but there has been some evidence that it occurs in some cases, and the literature has no consensus on if it occurs and how it occurs. According to a systematic review conducted in 2021, vertical transmission of COVID-19 was found in 3.2% of the cases by infant nasopharyngeal swab testing. From this data, it is evident that vertical transmission is possible and does occur, albeit rarely [27]. This study highlighted the need for information on how the gestational age of the pregnancy plays a role in the incidence and severity of a case of vertical transmission. On reviewing the effect of infection on fetal health, vertical transmission was of high concern, especially during the beginning of the pandemic. Long-term investigations would be needed to explore the possible effects of infection during pregnancy.
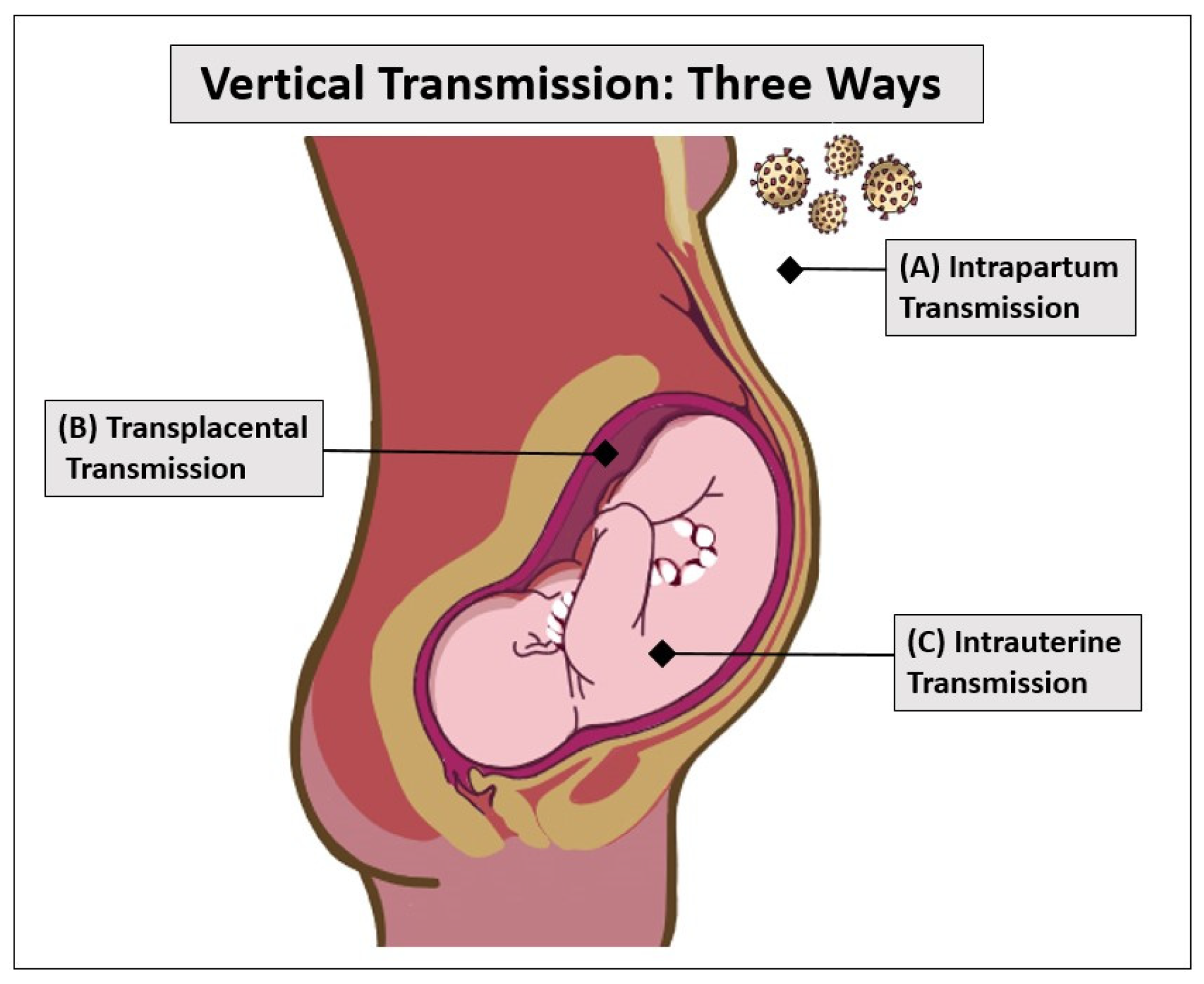
Figure 1. With most infectious diseases, as well as COVID-19, vertical transmission can occur in three ways: intrapartum transmission (top), transplacental transmission (middle), and intrauterine transmission (bottom). Intrapartum transmission (A) happens when neonates are exposed to maternal blood or an environment containing the COVID-19 virus. Transplacental transmission (B) occurs when the virus crosses the protective placental barrier and infects the fetus. Intrauterine transmission (C) happens when the virus is swallowed by the fetus through amniotic fluid.
When assessing a group of COVID-19-positive mothers in 2020, 6.6% of neonates were found also to be COVID-19 positive; however, biological samples from the time of birth, such as cord blood, placental membrane, vaginal fluid, amniotic fluid, and peritoneal fluid from C-sections all tested positive, and so the study concluded that vertical transmission was not a possibility [28]. As mentioned in Figure 1, intrapartum transmission may have occurred after birth, where the babies may have been exposed to the virus after birth. However, there is no evidence of how the vertical transmission may have occurred. Conclusively, the neonates were discharged after two weeks with all routine immunizations, having shown no severe reactions to infection [28]. In yet another case, a baby tested positive 36 h after birth to an asymptomatic mother, and it was concluded that in utero transmission must have occurred [29].
While vertical transmission of the virus has not been observed in an overwhelming number of cases, there is still potential for maternal immune activation [30]. When maternal immune activation occurs, it has been stated that an impact on the developing fetal brain is likely. A case report published in April showed that two infants born to mothers who had COVID-19 during the second trimester were born with fatal brain injuries [31]. One infant was born prematurely and later died from an episode of asystole cardiac arrest. The other infant was born at full term and tested positive for SARS-CoV-2 at birth. The infant has suffered from clinical seizures, microcephaly, significant neurological delay, and multiple respiratory infections and is now in hospice [31]. Though cases like these are rare occurrences, they still raise a concern about the exact mechanisms under which they may have occurred at all. As discussed in earlier sections, the underlying conditions, ethnicity, associated factors, genetics, and vaccination status of the mother could also play a role since the mothers were of a minority ethnicity [27]. Another study concluded that among the offspring exposed to COVID-19 in utero, there was a higher risk for male offspring to have a neurodevelopmental diagnosis one year after birth. This is an association that needs to be further examined on a larger scale, as this was limited by a smaller cohort of infants [32]. Aside from neurological concerns, it has also been reported that COVID-19 infection in the third trimester could cause fetal kidney developmental injury [33].
In a contradictory observation, intrauterine vertical transmission was found to be a rare issue in COVID-19 during pregnancy which may lead to placental dysfunction. Upon assessing neonatal outcomes, the study concluded that the results were not severe [34]. Clinical observation also confirms this finding, where there were no serious effects on the neonate [35]. Comparing these cases above shows two potential ramifications: one where vertical transmission has led to dire consequences for the baby and one where the effects are not grave. Most literature shows that vertical transmission of COVID-9 is rare [36], but if it is transpiring and has a possibility to affect future generations, then it should certainly be investigated. Despite these concerns, many studies show evidence that the transplacental transfer of antibodies occurs between the mother and the fetus, whether it is in the form of infection or vaccination [37][38][39]. Notably, the role of vertical transmission in fetal health is clearly undescribed and needs to be researched further.
References
- Rasmussen, S.A.; Kelley, C.F.; Horton, J.P.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) Vaccines and Pregnancy. Obstet. Gynecol. 2021, 137, 408–414.
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and Infection. N. Engl. J. Med. 2014, 370, 2211–2218.
- Phoswa, W.N.; Khaliq, O.P. Is pregnancy a risk factor of COVID-19? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 605–609.
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Galang, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., III; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. 2020. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e3.htm (accessed on 5 March 2023).
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul-Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynecol. Obstet. 2020, 151, 7–16.
- CDC. People with Certain Medical Conditions; Center for Disease Control and Prevention: Atlanta, GA, USA, 2023.
- Capretti, M.G.; Marsico, C.; Gabrielli, L.; Vocale, C.; Arcuri, S.; Simonazzi, G.; Piccinini, A.R.; Brandolini, C.; Lazzarotto, T.; Corvaglia, L.T. Infants Born Following SARS-CoV-2 Infection in Pregnancy. Pediatrics 2022, 150, e2022056206.
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med. Assoc. J. 2021, 193, E540–E548.
- Smith, E.R.; Oakley, E.; Grandner, G.W.; Ferguson, K.; Farooq, F.; Afshar, Y.; Ahlberg, M.; Ahmadzia, H.; Akelo, V.; Aldrovandi, G.; et al. Adverse maternal, fetal, and newborn outcomes among pregnant women with SARS-CoV-2 infection: An individual participant data meta-analysis. BMJ Glob. Health 2023, 8, e009495.
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625.
- Piekos, S.N.; Roper, R.T.; Hwang, Y.M.; Sorensen, T.; Price, N.D.; Hood, L.; Hadlock, J.J. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: A retrospective multicentre cohort study. Lancet Digit. Health 2022, 4, e95–e104.
- Trilla, C.; Mora, J.; Crovetto, F.; Crispi, F.; Gratacós, E.; Llurba, E. First-Trimester SARS-CoV-2 Infection: Clinical Presentation, Inflammatory Markers, and Obstetric Outcomes. Fetal Diagn. Ther. 2022, 49, 67–76.
- CDC. What Is Stillbirth? Center for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Centers for Disease Control and Prevention. Pregnancy and Infant Loss. Available online: https://www.cdc.gov/ncbddd/stillbirth/features/pregnancy-infant-loss.html (accessed on 5 March 2023).
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667.
- Flenady, V.; Koopmans, L.; Middleton, P.; Froen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major risk factors for stillbirth in high-income countries: A systematic review and meta-analysis. Lancet 2011, 377, 1331–1340.
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297.
- Desisto, C.L. Morbidity and Mortality Weekly Report Risk for Stillbirth among Women with and without COVID-19 at Delivery Hospitalization—United States, March 2020–September 2021. Available online: https://msdh.ms.gov/msdhsite/_static/23,23645,341.html (accessed on 5 March 2023).
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Carosso, M.; Bovetti, M.; Filippini, C.; D’avolio, A.; Ghisetti, V.; Di Perri, G.; et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: A case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2020, 224, 391.e1–391.e7.
- Balachandren, N.; Davies, M.C.; A Hall, J.; Stephenson, J.M.; David, A.L.; Barrett, G.; O’neill, H.C.; Ploubidis, G.B.; Yasmin, E.; Mavrelos, D. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: A UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum. Reprod. 2022, 37, 1126–1133.
- Cavalcante, M.B.; Cavalcante, C.T.D.M.B.; Cavalcante, A.N.M.; Sarno, M.; Barini, R.; Kwak-Kim, J. COVID-19 and miscarriage: From immunopathological mechanisms to actual clinical evidence. J. Reprod. Immunol. 2021, 148, 103382.
- de Havenon, A.; Delic, A.; Stulberg, E.; Sheibani, N.; Stoddard, G.; Hanson, H.; Theilen, L. Association of Preeclampsia with Incident Stroke in Later Life among Women in the Framingham Heart Study. JAMA Netw. Open 2021, 4, e215077.
- Peng, Z.; Zhang, J.; Shi, Y.; Yi, M. Research progress in vertical transmission of SARS-CoV-2 among infants born to mothers with COVID-19. Futur. Virol. 2022, 17, 211–214.
- Bushnell, C.; Chireau, M. Preeclampsia and Stroke: Risks during and after Pregnancy. Stroke Res. Treat. 2011, 2011, 858134.
- Taglauer, E.S.; Wachman, E.M.; Juttukonda, L.; Klouda, T.; Kim, J.; Wang, Q.; Ishiyama, A.; Hackam, D.J.; Yuan, K.; Jia, H. Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding. bioRxiv 2021, preprint.
- What Is the ACE2 Receptor, How Is It Connected to Coronavirus and Why Might It Be Key to Treating COVID-19? The Experts Explain. 2020. Available online: https://theconversation.com/what-is-the-ace2-receptor-how-is-it-connected-to-coronavirus-and-why-might-it-be-key-to-treating-covid-19-the-experts-explain-136928 (accessed on 5 March 2023).
- Katwa, L.C.; Mendoza, C.; Clements, M. CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts. Cells 2022, 11, 1316.
- Benny, M.; Bandstra, E.S.; Saad, A.G.; Lopez-Alberola, R.; Saigal, G.; Paidas, M.J.; Jayakumar, A.R.; Duara, S. Maternal SARS-CoV-2, Placental Changes and Brain Injury in 2 Neonates. Pediatrics 2023, 151, e2022058271.
- Sharma, R.; Seth, S.; Sharma, R.; Yadav, S.; Mishra, P.; Mukhopadhyay, S. Perinatal outcome and possible vertical transmission of coronavirus disease 2019: Experience from North India. Clin. Exp. Pediatr. 2021, 64, 239–246.
- Mendoza-Hernández, M.; de Rivera, I.H.-N.; Yoldi-Negrete, M.; Saviñon-Tejeda, P.; Franco-Cendejas, R.; López-Jácome, L.E.; Navarro-Castellanos, I. Probable Case of Vertical Transmission of SARS-CoV-2 in a Newborn in Mexico. Neonatology 2021, 118, 364–367.
- Shook, L.L.; Sullivan, E.L.; Lo, J.O.; Perlis, R.H.; Edlow, A.G. COVID-19 in pregnancy: Implications for fetal brain development. Trends Mol. Med. 2022, 28, 319–330.
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 during Pregnancy. JAMA Netw. Open 2022, 5, e2215787.
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Haneuse, S.; Kaimal, A.J.; Perlis, R.H. Sex-Specific Neurodevelopmental Outcomes among Offspring of Mothers with SARS-CoV-2 Infection during Pregnancy. JAMA Netw. Open 2023, 6, e234415.
- He, Z.; Fang, Y.; Zuo, Q.; Huang, X.; Lei, Y.; Ren, X.; Liu, D. Vertical transmission and kidney damage in newborns whose mothers had coronavirus disease 2019 during pregnancy. Int. J. Antimicrob. Agents 2020, 57, 106260.
- Zaigham, M.; Holmberg, A.; Karlberg, M.; Lindsjö, O.; Jokubkiene, L.; Sandblom, J.; Strand, A.; Andersson, O.; Hansson, S.; Nord, D.; et al. Intrauterine vertical SARS-CoV-2 infection: A case confirming transplacental transmission followed by divergence of the viral genome. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1388–1394.
- Thapa, B.; Acharya, S.; Karki, S. Vertical Transmission of COVID-19: A Case Report and Review of Literature. J. Nepal Health Res. Counc. 2021, 19, 203–205.
- Yuan, J.; Qian, H.; Cao, S.; Dong, B.; Yan, X.; Luo, S.; Zhou, M.; Zhou, S.; Ning, B.; Zhao, L. Is there possibility of vertical transmission of COVID-19: A systematic review. Transl. Pediatr. 2021, 10, 423–434.
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e154834.
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021, 175, 594–600.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
31 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




