Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga Senko | -- | 4215 | 2023-05-30 15:59:18 | | | |
| 2 | Rita Xu | Meta information modification | 4215 | 2023-05-31 03:06:45 | | | | |
| 3 | Rita Xu | -1 word(s) | 4214 | 2023-06-01 08:48:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Efremenko, E.; Senko, O.; Stepanov, N.; Aslanli, A.; Maslova, O.; Lyagin, I. Quorum Sensing. Encyclopedia. Available online: https://encyclopedia.pub/entry/45016 (accessed on 07 February 2026).
Efremenko E, Senko O, Stepanov N, Aslanli A, Maslova O, Lyagin I. Quorum Sensing. Encyclopedia. Available at: https://encyclopedia.pub/entry/45016. Accessed February 07, 2026.
Efremenko, Elena, Olga Senko, Nikolay Stepanov, Aysel Aslanli, Olga Maslova, Ilya Lyagin. "Quorum Sensing" Encyclopedia, https://encyclopedia.pub/entry/45016 (accessed February 07, 2026).
Efremenko, E., Senko, O., Stepanov, N., Aslanli, A., Maslova, O., & Lyagin, I. (2023, May 30). Quorum Sensing. In Encyclopedia. https://encyclopedia.pub/entry/45016
Efremenko, Elena, et al. "Quorum Sensing." Encyclopedia. Web. 30 May, 2023.
Copy Citation
Quorum sensing (QS) of various microorganisms (bacteria, fungi, microalgae) today attracts the attention of researchers mainly from the point of view of clarifying the biochemical basics of this general biological phenomenon, establishing chemical compounds that regulate it, and studying the mechanisms of its realization. Today the particular attention focused on biotechnological sides of QS application in the elaboration of various prospective biocatalytic systems for different processes carried out under aerobic and anaerobic conditions (synthesis of enzymes, polysaccharides, organic acids, etc.).
quorum sensing
triggering factors
biocatalysts
yeast cells
1. Introduction
A number of biotechnological processes based on the use of highly concentrated populations of cells of various microorganisms in order to intensify them and improve the main characteristics (productivity and stability of functioning) of biotechnological processes are known today [1][2][3][4]. At the same time, highly concentrated cell populations are considered as effective biocatalysts (BCs) of various biotechnological processes that allow a variety of target products (polysaccharides, organic solvents and acids, enzymes, biogas, vitamins, etc.) to be obtained (Figure 1). The reason for the interest in such BCs lies in the manifestation by cells in such populations of properties characterized as the state of quorum sensing (QS). QS consists in changing the biochemical status of cells in comparison with single cells of the same microorganism due to the built-in genetic program, which is implemented at a certain concentration per unit volume under the influence of a total concentration of substances called quorum inducers. To date, the appearance of QS is known as a general mechanism of cell-to-cell chemical communication dependent on population density revealed for different microorganisms (bacteria, microalgae, yeast, filamentous fungi), including those that are part of complex consortia [2][3][5][6][7]. Initially (50 years ago) QS was discovered as a mechanism responsible for cellular communication in populations of Gram-negative bacteria Vibrio fischeri [8].
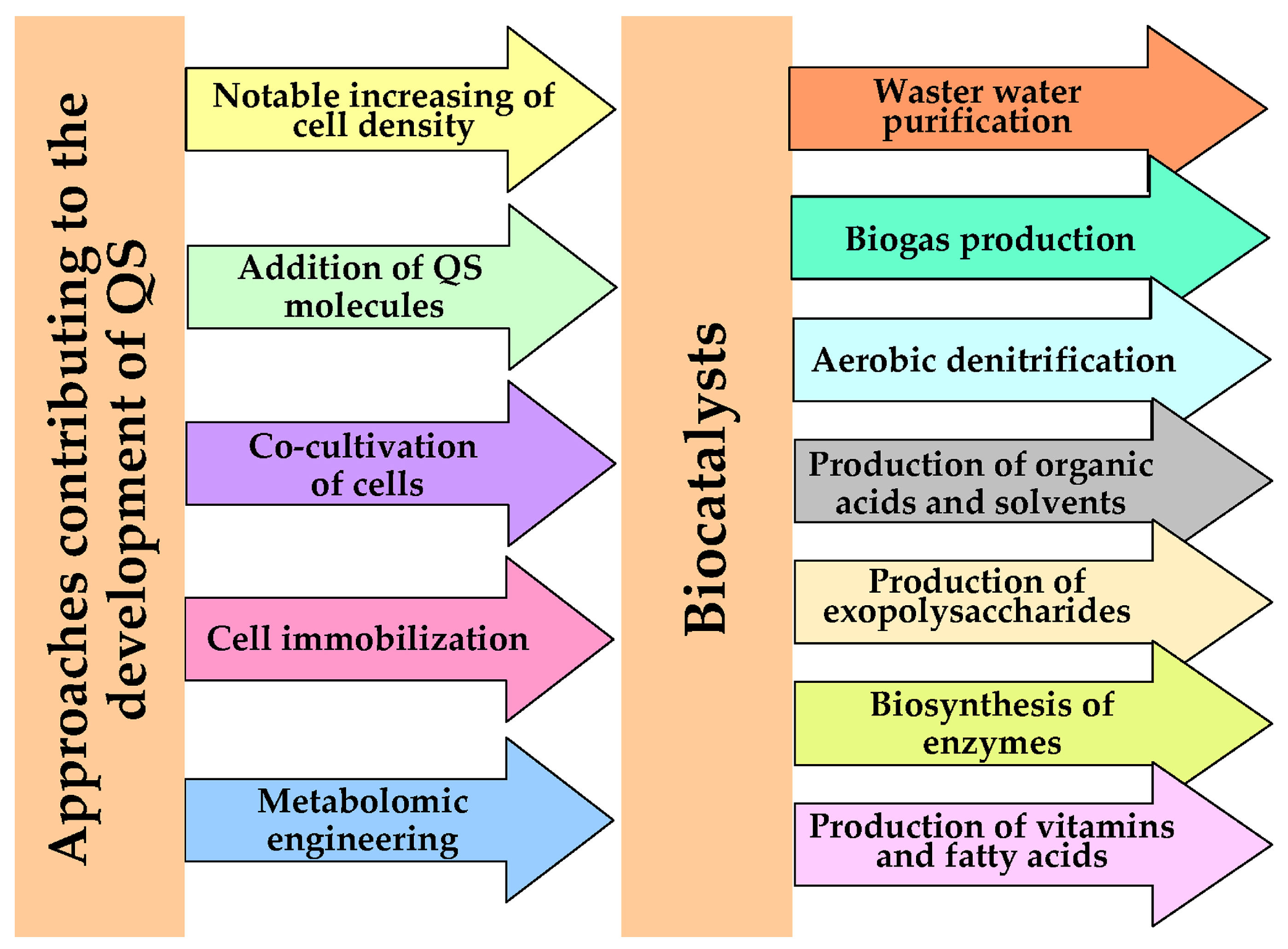
Figure 1. The factors used as triggers of QS as a regulator of improved properties of BCs catalyzing various biotechnological processes.
It is well known today that cells of microorganisms used in various biotechnological processes as biocatalysts and composed populations in QS state have a number of advantages over single cells [9]:
- -
-
Increased resistance to negative external influences (pH, temperature, culture components, the presence of toxins, antimicrobial substances, etc.);
- -
-
The ability to long-term preservation of metabolic activity during storage and repeated use in biotechnological processes;
- -
-
Increased total productivity of the processes in which they are used as biocatalysts due to the possibility of maintaining high concentrations of cells per unit volume in the absence of noticeable cell lysis.
Usually, one of the most important characteristics of cells of different microorganisms that transform into a state of QS is the synchronization of their functional activity [10], which leads to the simultaneous implementation of the same biochemical metabolic pathways by the majority of cells in such highly concentrated populations (“mainstreams”). This allows cells to increase the yields of certain metabolites and obtain the necessary energy reserve in the form of adenosine triphosphate (ATP) [9]. This reduces the variability (narrowing the range) of the byproducts with a simultaneous decrease in their concentrations, which contributes to the production of a biotechnological culture with fewer by-products and simplifies the separation of target low-molecular metabolites from them [10][11].
2. General Approaches to the Regulation of QS in Bacteria Used as BCs in Various Biotechnological Processes
For the development of QS, bacteria use various signaling molecules, autoinducers (AI), which bind to cytoplasmic receptors in bacterial cells and activate the coordinated expression of target genes when the population reaches the quorum concentration. The most common signaling molecules of QS used by most Gram-negative and Gram-positive bacteria are N-acyl homoserine lactones (AHL) and modified oligopeptides (autoinducing peptides, AIP), respectively. To date, various small cyclic furanone compounds (autoinducers-2, AI-2), widely distributed among both Gram-positive and Gram-negative bacteria, are also known, which provide intra- and interspecies communication between them [1].
The Lux-type QS system (LuxR/LuxI circuit), which is mediated by AHL molecules, is common in many Gram-negative bacteria. The LuxI and LuxR are important proteins for QS regulation of bioluminescence in V. fischeri, where LuxI (AHL synthase) catalyzes the synthesis of autounducer, which is then detected by transcription factor LuxR (cytoplasmic receptor). LuxR-type proteins consist of an N-terminal AHL-binding domain and a C-terminal DNA-binding domain. The binding of AHLs to LuxR results in the formation of LuxR-AHL complex, which binds “lux box” sequence and activates the expression of target genes [12].
Pairs of LuxR/LuxI homologues, which are critical for regulating diverse physiological functions (bioluminescence, biofilm formation, etc.), have been identified in many bacterial species. Two different homologues of Lux-type QS systems such as Las (LasR/LasI) and Rhl(RhlR/RhlI) regulate the cascade of virulence regulators in Pseudomonas aeruginosa [13], including exoprotease secretion, toxin production, motility, and biofilm formation [14].
There are also a small number of LuxR homologues, such as EsaR, from Pantoea stewartii subsp. stewartii, that naturally act as repressors. The cells regulate the biosynthesis of exopolysaccharides by the EsaR/EsaI QS system, where EsaI encodes AHL-synthase in the same way as other LuxI-type family proteins. The transcription factor EsaR represses or activates transcription in the absence of AHL (at low cell densities), and derepresses or deactivates transcription when combined with AHL (at high cell densities) [15].
The Gram-positive bacteria Staphylococcus aureus QS is encoded by the Agr (accessory gene regulator) system, which plays a crucial role in regulating the syntheses of a wide range of S. aureus virulence factors and complex association with biofilm formation [6][16]. The Agr QS system is composed of the response regulator protein, AgrA, and the sensor histidine kinase, AgrC, which expresses a regulatory RNA, RNAIII, in response to autoinducing peptides [17].
The QS proteins of Gram-positive bacteria (e.g., Bacillus sp., Enterococcus faecalis) consisting of certain aspartyl phosphate phosphatases (Rap proteins), the regulator of the glucosyltransferase gene (Rgg), the neutral protease regulator (NprR), the phospholipase C regulator (PlcR), and the pheromone receptor (PrgX) form a protein family named RNPP, which is characterized by the presence of the tetratricopeptide repeat. There are only a few examples of the applications of these QS systems in biotechnological processes. For example, a significant regulatory role of RNPP-type QS systems in the solvent formation, cell motility and sporulation of Clostridium saccharoperbutylacetonicum N1-4 cells was reported [18].
The study of the effect of a number of AHL molecules (N-3-oxo-hexanoyl homoserine lactone (3OC6-HSL), N-hexanoyl homoserine lactone (C6-HSL), N-octanoyl homoserine lactone (C8-HSL), and N-dodecanoyl homoserine lactone (C12-HSL)) on metabolic processes in ANAMMOX consortia and on the ANAMMOX activity of granular sludge biomass has made it possible to establish that all studied AHL molecules are involved in the regulation of (i) bacterial activity in ANAMMOX consortia, (ii) the synthesis of amino acids (Ala, Val, Glu, Asp, and Leu), and (iii) the pathways of N-acetylmannosamine (ManNAc) and uridine-diphospho-N-acetylgalactosamine (UDP-GlcNAc), promoting the production of extracellular polymeric substances. C6-HSL and C8-HSL molecules significantly affected the metabolic activity of cells and the formation of a granular biocatalyst [19][20][21]. A significant effect of the substrate shock (excess concentration of the substrate) (total nitrogen) on the level of synthesis of AHL molecules in the granules of ANAMMOX bacteria and the correlation between the concentration of AHLs (C6-HSL and C8-HSL), excretion of extracellular polymeric substances, and the characteristics of self-forming granules of the biocatalyst has been shown [22]. The study of HdtS-type AHL synthases (JqsI-1 and JqsI-2) identified in the ANAMMOX bacteria Candidatus Jettenia caeni revealed a positive correlation between AHL concentration, AHL synthase gene expression, and the hydrazine synthase gene hzsA (genetic marker of ANAMMOX activity) [23].
Microbial synthesis through metabolic engineering is a widely used approach to increase the production of target products in various industries. However, this requires constant regulation of the concentration of the cell population to maintain their productivity. Recently, the majority of studies have focused on the development of various strategies for the use of the components of the QS, a mechanism of interaction between microorganisms induced by various quorum sensing molecules (QSMs), for the dynamic regulation of metabolic pathways for the synthesis of target products [24][25][26].
E. coli cells are most often used as host cells for the production of a number of products due to the presence of thoroughly studied metabolic pathways, engineering tools, and the ease of culturing highly concentrated populations [16][24][25][26][27][28][29][30][31]. In studies [24][26][27][29], synthetic microbial biocatalysts were developed and constructed using components of the Lux-type QS system in genetic circuits to regulate metabolic pathways for the biosynthesis of isopropanol, salicylic acid, 4-hydroxycoumarin, bisabolene, alginate lyase, and esterase. A modified version of the QS-MTS (metabolic toggle switch) system to stabilize the efficiency of QS signaling and regulation of metabolism was constructed in E. coli cells to increase the production of pyruvate and acetate [31].
Components of the Esa-type QS system have also been used in the development of genetic circuits and have been successfully applied for dynamic regulation of the biosynthetic pathways of 4-hydroxyphenylacetic acid (4HPAA) and 5-aminolevulinic acid (ALA) in E. coli cells [16][30].
New expression cassettes based on the switchable feedback promoters (SFP) regulatory motif, where small RNAs are used as transcription regulators to integrate additional levels of control over transcription outputs was developed in the research [25]. Lux- and Esa-type QS systems have been used to regulate these expression cassettes (riboregulated SFP, rSFP), which have been successfully applied for the regulation of the amorphadiene and oxygenated taxane metabolic pathways. The construction of two orthogonal, autonomous, and customizable genetic circuits based on the components of Lux- and Agr-type QS systems for dynamic modulation of the expression of two different sets of enzymes has made it possible to achieve a significant increase in the yield of medium-chain fatty acids, both in cultures in flasks and in bioreactors [28].
In addition to E. coli cells, a number of other microorganisms, in particular bacteria of the genus Bacillus, Gluconobacter, Pseudomonas, etc., are also actively used as host cells in metabolic engineering [13][32][33][34][35][36][37].
The integration of two modular QS systems, Phr60-Rap60-Spo0A and PhrQ-RapQ-DegU, for the dynamic control of metabolic pathways for the synthesis of menaquinone-7 (vitamin K2) or γ-polyglutamic acid (γ-PGA), respectively, in B. subtilis 168 cells resulted in a significant increase in the level of their production [32][33]. The study [34] proposed a completely autonomous system for controlling gene expression in B. subtilis cells based on the components of the Lux-type QS system from Gram-negative V. fischeri cells. The success of such QS modification was demonstrated in the production of vitamin B2.
A programmed cell-death module based on the Lux-type QS system was installed in G. oxydans cells to optimize the process and eliminate the occurrence of competition between G. oxydans and Ketogulonicigenium vulgare in a consortium of three species (G. oxydans—K. vulgare—Bacillus megaterium) for one-step production of 2-keto-L-gulonic acid (2-KGA) from sorbitol [37].
The study of the possibility of regulating denitrification by P. aeruginosa cells under aerobic conditions using two QS systems Las and Rhl has made it possible to establish that the Las and Rhl systems suppress the expression of target genes napA, nirS, norB, norC, and nosZ, thereby negatively affecting the activity of enzymes that catalyze denitrification, namely, nitrate reductase (NAP), nitrite reductase (NIR), nitric oxide reductase (NOR), and nitrous oxide reductase (NOS) [13].
Genetic engineering techniques can also be applied to control QS. It is known that surfactin correlates with the carbon metabolism in B. amyloliquefaciens cells which are relative of B. subtilis [35]. Mutation of srfA (ΔsrfA—a gene cluster for biosynthesizing surfactin) in B. subtilis led to enhanced production of acetate [36].
Thus, various approaches can be applied to stimulate QS in different bacterial cells by adding QSMs to the cell culture medium, metabolic engineering, and genetic modification. A natural or artificial increase in the concentration of the cells and their immobilization can also be used to improve the characteristics of biotechnological processes based on cellular BCs, and this will be discussed further.
3. QS in Biosynthesis of Polysaccharides by Bacterial BCs
The interest in the production of various polysaccharides (PSs) by biotechnological methods is enormous today [38]. At the same time, it was clearly shown that there is a relationship between QS, which depends on the population density of bacterial cells in the medium, and the level of exopolysaccharide synthesis [39]. Bacterial cells are the main source for obtaining active BCs that produce various PSs, since it is bacteria that have the natural ability to form biofilms that play a key role in their adhesion and protection from negative factors, including toxic and antimicrobial substances [40], as well as in the implementation of the survival strategy with a decrease in the concentration of available substrates. Various PSs (cellulose, levan and alginate) were found in the composition of bacterial biofilms [38][41].
In recent years, a growing number of studies have focused on studying the main mechanisms underlying the regulation of PS biosynthetic pathways. This is necessary for understanding the basic principles of functioning of processes and their shift towards the formation of the target product [42][43]. Most of the enzymatic steps in PS biosynthesis occur within the cells, while polymerization and secretion are localized in the cell wall. There are also examples of extracellular biosynthesis of PSs (dextran or levan). However, in both cases, the effect of QS on PS production is extremely interesting, since it can increase the efficiency of this process by activating or inhibiting key enzymes of the synthesis [42][43]. Thus, the biosynthesis of bacterial cellulose (BacC) depends on the presence of an allosteric regulator (cyclic guanosine monophosphate, c-GMP), which accumulates during the formation of biofilms, and can increase in its presence by almost 100 times. The mechanism of this regulation was revealed when the crystal structure of the cellulose synthase complex was studied and it was shown that the enzyme, being in an inactive state, interacts with c-GMP, which contributes to its activation [44].
The level of productivity of the main metabolites in the cells in biofilms is at a significantly higher level in comparison with free cells. It should be noted that poorly concentrated cell samples did not give improved levels of accumulated products even in immobilized form compared to the same free cells [45]. The influence of some carriers used for immobilization of cells in the QS-state and their low porosity created additional limitations for mass transfer to realize the advantages of QS; therefore, the yields of the products were comparable to those typical for free concentrated cells [46][47].
A significant increase in the biosynthesis [48][49][50][51][52][53][54][55][56][57][58] or the achievement of a concentration comparable to that accumulated by free cells in highly concentrated suspensions, which are generally formed by the end of the logarithmic growth phase [45][46][47], was shown for various PS producers as a result of their immobilization. In addition, the use of different methods of immobilization (adsorption on an insoluble carrier [46][51][52][53][55][56][57][58] or inclusion in a gel matrix [45][47][48][49][50][54]) led to the transition of bacterial cells into the QS state because the procedure resulted in a high-density cell culture and in keeping the cells in this state. The immobilization of PS producers in gel matrix has long been considered impractical due to diffusion difficulties and hampered mass transfer, which limits cellular metabolism [38]. However, the successful biosynthesis of such high molecular weight polymers as BacC, dextran, and pullulan has been proven by incorporating producer cells into PVA cryogel [48][49][50]. It was also noted that the accumulation of free cells in the medium during the cultivation of thus immobilized producers was six times less than in the case of suspension cultures, and an increase in the concentration of cells immobilized in a gel matrix led to a noticeable increase in PS synthesis [38][48][49][50].
Compared to a simple increase in the concentration of cells, the presence of the matrix intensified the biosynthesis even more, for example, of BacC by K. xylinum bacteria cells, which synthesized and easily pushed out the resulting BacC strands through the pores of the polymer carrier, which over time combined into a dense gel film, without covering the cells. The latter, therefore, were deprived of the ability to transform into a state of rest and even more actively carried out the synthesis of BacC [18]. A similar effect of stimulating the functioning of BacC in the QS state was demonstrated in G. kombuchae cells immobilized using a loofa sponge [51]. The level of BacC synthesis increased by 1.6–2.1 times with their participation, in comparison with highly concentrated suspensions of free cells.
The role of QS in BacC synthesis was confirmed when it was shown that the activation of proteins responsible for alginate synthesis depends on the presence of an allosteric regulator (cyclic dimeric guanosine monophosphate, c-di-GMP), which is responsible for the QS of cells. It turned out that the Alg8 enzyme, being in an inactive state, interacts with the c-di-GMP-binding domain PILZ of the Alg44 protein, which leads to its significant activation. Alginate biosynthesis is regulated at the transcriptional and post-translational levels [59].
It should be noted that in the case of alginate producers (A. vinelandii) immobilized in a composite agar layer/microporous membrane, additional stimulation of PS synthesis was shown to increase by 2.7 times per 1 g of consumed substrate (sucrose) in comparison with concentrated suspension-free cells, also in the state of QS [45]. During the synthesis of xanthan by X. campestris cells immobilized on polyurethane foam, an increase in its biosynthesis by 267–274% was also observed [52][53].
Such a significant increase in the yield of alginate when using immobilized BCs is explained by an even greater decrease in the consumption of consumed sucrose for bacterial growth in comparison with free cells when the cells in the carrier matrix are limited by oxygen. Interestingly, the natural self-immobilization of bacteria in biofilms also turned out to lead to “overproduction” of PSs, similar to what was obtained with artificially immobilized cultures. In this regard, the use of immobilized BCs based on PS-producing cells is of great interest for biotechnology.
However, it should be noted that despite many examples of the fact that higher yields of many target PSs can be obtained as a result of the use of immobilized cells compared to concentrated suspension cultures [48][49][50][51][52][53][54][55][56][57][58], there are examples of when such an effect is absent. In particular, this applies to PSs synthesized by lactic acid bacteria [46][47]. The production of PSs using six BCs in the form of lactic acid bacteria strains (Lactobacillus bulgaricus, L. acidophilus, L. plantarum, L. casei, Streptococcus thermophilus, and Lactococcus lactis) was investigated. The highest production of PSs was obtained in the case of L. plantarum cells (1513.1 mg/L). Immobilization of the cells of this strain in various carriers (Ca-alginate gel, K-carrageenan, agar, gelatin cross-linked with glutaraldehyde) made it possible to obtain only 1489.9 mg/L of PSs.
In this regard, it must be emphasized that QS for immobilized cells remains a poorly studied phenomenon. A deep understanding of all the mechanisms of possible regulation of the functioning of immobilized cells as very promising BCs will allow their large-scale use in various processes in practice to be reached faster.
4. Biocatalysts for Biotechnological Processes Based on Highly Concentrated Yeast Populations in Suspension and Immobilized Form
It is known that morphological transformations take place with yeast cells (formation of pseudohyphae) under the action of their QSMs when changing the density of their populations. At the same time, the cells form flocculi and sediment, forming hyphae forms that are more stable to the effects of various negative factors. It is established that farnesol, tyrosol and dodecyl alcohol are QSMs for Candida albicans cells. The secretion of phenylethyl alcohol and tryptophol is observed in the yeast Saccharomyces cerevisiae during nitrogen starvation, and these compounds regulate cell density and their morphological changes.
In wastewater treatment, various environmental stressors can cause changes in the morphology of yeast cells and lead to a decrease in treatment efficiency. The effect of phenylalanine on C. tropicalis cell density, cell flocculation, and metabolism in wastewater treatment has been investigated [60]. Phenylalanine has been used to simulate induction factors of filamentous form. It turned out that with an increase in cell density to 108 CFU/mL, the utilization of phenylalanine by cells decreased, the concentrations of tyrosine in extracellular metabolites increased, and the concentration of ethanol and organic (acetic, propionic, butyric, and valeric) acids decreased. Molecules of phenylethyl alcohol were found in metabolites as QSMs, and their concentration increased with the growth of the cell population, which proves that the change in cell morphology depends on QS [60][61]. The BCs generally functioned well, and the COD removal rate reached 95.3% [61].
It should be noted that in a large-scale biotechnological process, an increase of only a few percent in the yield of ethanol is of significant value for the alcohol industry. During the fermentation process, S. cerevisiae converts sugars into ethanol via glycolysis, producing secondary metabolites such as glycerol, acids, alcohols, and aldehydes. The synthesis of these by-products reduces the yield of ethanol. Another reason for the increase in ethanol yield (carbon substrate conversion efficiency) is a decrease in biomass growth during fermentation so that more substrate can be converted into the desired product. According to studies [60][61][62][63], QSMs allow the regulation of the yield of ethanol. Interestingly, the addition of phenylethanol in flow bioreactor experiments [62] reduces the rate of ethanol production and glucose consumption, indicating an effect of this compound on the rate of yeast catabolism.
From the scientific and practical point of view, it is extremely interesting to study the possibility of regulating ethanol fermentation with the participation of several different yeast cultures used in the QS state [64][65][66]. It has been shown that in this case the mechanisms of the regulation of processes under the action of QSMs become significantly more complicated, the dependences on the introduced concentrations of QSMs in such biotechnological media change, and the expected effects do not have the linearity that can be expected when using individual yeast cultures as a BC [64][65][66]. Thus, the effect of tryptophol or melatonin on alcoholic fermentation was investigated under the action of a BC in the form of a mixture of S. cerevisiae suspension cells and four species of yeast that do not belong to the genus Saccharomyces (T. delbrueckii, M. pulcherrima, H. uvarum, and S. bacillaris). It was found that the addition of 0.5 g/L tryptophol to the fermentation medium resulted in the inhibition of fermentation by S. cerevisiae cells, which continued throughout the entire process. However, this effect disappeared in the presence of other non-Saccharomyces strains [65].
It should be noted that changes in the morphological characteristics of yeast cells contribute to their high adhesive strength, which is caused by the presence of glycoproteins (adhesins) on the surface of yeast cell walls [67]. These adhesins are also involved in the interactions of yeast cells with each other and with cells of other microorganisms. In particular, it is these filamentous forms of cells capable of forming biofilms that are characterized by the highest adhesiveness in the yeast C. albicans [68][69][70]. It turned out that the aromatic alcohols tryptopol and phenylethyl alcohol activate the QS pathway in S. cerevisiae cells, regulating filamentation.
Hyperfilamentation is stimulated in the same cells in response to exposure to several short-chain alcohols, including isoamyl alcohol and 1-butanol. Alcohols stimulate the appearance of cyclic AMP in yeast cells, which indirectly promotes the transcriptional activation of adhesin, which is involved in cell adhesion to each other and different surfaces and enhances yeast cell filamentation [71].
The ability of emerging hyphae to secrete adhesins promotes the maturation of yeast biofilms, providing their architectural stability and the strength of emerging self-immobilizing yeast populations [72]. In this regard, the introduction of such alcohols into a medium with an increased concentration of yeast cells contributes to their transition to a productive and stable state. In support of this, the influence of the introduction of tryptophol and 2-phenylethanol into the fermentation medium on the efficiency of the process of obtaining ethanol from hydrolysates of Desmodesmus armatus algae was studied. Yeast S. cerevisiae cells immobilized in Ca-alginate gel were used as a BC. It was shown that the addition of 2-phenylethanol at a concentration of 0.2% to the medium leads to an increase in the yield of ethanol by 7.4% [73].
It was found that in addition to ethanol synthesis during alcoholic fermentation, highly concentrated yeast cells also synthesize a number of metabolites [74][75] that specifically inhibit the metabolic activity of possible competitors: other types of yeast and bacteria that can contaminate the reaction medium. That is, such BCs not only provide high yields of the target product, but actually also maintain the “sterility” of the fermentation medium. The triggering of protective substance production mechanisms depends on cell density and is also regulated by QS. Thus, it was shown that the death of yeasts other than Saccharomyces in ethanol fermentation with mixed cultures was caused only when the cultures reached a high cell density (about 107 cells/mL) [75].
It should be noted that in a number of yeast cells, more than 12 vol% ethanol accumulated in the medium leads to the complete suppression of QSMs synthesis. It has been established that with an increase in the concentration of ethanol, the rate of production of 2-phenylethanol, tryptophol, and tyrosol decreases, which is associated with a general disruption of numerous cellular processes caused by ethanol stress [76]. In light of that, regulation of the fermentation process by controlled introduction of QSMs that stimulate yeast cells to maintain high levels of ethanol production in reaction media with highly concentrated cell populations is of interest.
Therefore, the introduction of QSMs into biocatalytic systems based on yeast cells allows the formation of BCs with improved characteristics and ensures the biotechnological processes proceed effectively by increasing the cell tolerance to negative factors, including contamination of foreign microflora.
References
- Abbamondi, G.R.; Tommonaro, G. Research progress and hopeful strategies of application of quorum sensing in food, agriculture and nanomedicine. Microorganisms 2022, 10, 1192.
- Bettenworth, V.; Steinfeld, B.; Duin, H.; Petersen, K.; Streit, W.R.; Bischofs, I.; Becker, A. Phenotypic heterogeneity in bacterial quorum sensing systems. J. Mol. Biol. 2019, 431, 4530–4546.
- Westman, J.O.; Franzén, C.J. Current progress in high cell density yeast bioprocesses for bioethanol production. Biotechnol. J. 2015, 10, 1185–1195.
- Maddela, N.R.; Sheng, B.; Yuan, S.; Zhou, Z.; Villamar-Torres, R.; Meng, F. Roles of quorum sensing in biological wastewater treatment: A critical review. Chemosphere 2019, 221, 616–629.
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638.
- Eickhoff, M.J.; Bassler, B.L. Snapshot: Bacterial quorum sensing. Cell 2018, 174, 1328.e1.
- Dow, L. How do quorum-sensing signals mediate algae–bacteria interactions? Microorganisms 2021, 9, 1391.
- Majdura, J.; Jankiewicz, U.; Gałązka, A.; Orzechowski, S. The role of quorum sensing molecules in bacterial–plant interactions. Metabolites 2023, 13, 114.
- Efremenko, E.N. (Ed.) Immobilized Cells: Biocatalysts and Processes; Rior: Moscow, Russia, 2018; p. 524. ISBN 978-5-369-02004-3.
- Hauser, M. Synchronisation of glycolytic activity in yeast cells. Curr. Genet. 2022, 68, 69–81.
- Xinming, C.; Kaiqiang, Z.; Zhai, C. Analyze and control on the membrane ethanol fermentation process with periodic exogenous signals. Chem. Eng. Process. 2022, 181, 109174.
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588.
- Cui, X.; Ruan, X.; Yin, J.; Wang, M.; Li, N.; Shen, D. Regulation of las and rhl quorum sensing on aerobic denitrification in Pseudomonas aeruginosa PAO1. Curr. Microbiol. 2021, 78, 659–667.
- Kievit, T.R.; Kakai, Y.; Register, J.K.; Pesci, E.C.; Iglewski, B.H. Role of the Pseudomonas aeruginosa las and rhl quorum-sensing systems in rhlI regulation. FEMS Microb.Lett. 2002, 212, 101–106.
- Schu, D.J.; Scruggs, J.M.; Geissinger, J.S.; Michel, K.G.; Stevens, A.M. Acyl-homoserine lactone recognition and response hindering the quorum-sensing regulator EsaR. PLoS ONE 2014, 9, e107687.
- Shen, Y.P.; Fong, L.S.; Yan, Z.B.; Liu, J.Z. Combining directed evolution of pathway enzymes and dynamic pathway regulation using a quorum-sensing circuit to improve the production of 4-hydroxyphenylacetic acid in Escherichia coli. Biotechnol. Biofuels. 2019, 12, 94.
- Paulander, W.; Varming, A.N.; Bojer, M.S.; Friberg, C.; Bæk, K.; Ingmer, H. The agr quorum sensing system in Staphylococcus aureus cells mediates death of sub-population. BMC Res. Notes 2018, 11, 503.
- Feng, J.; Zong, W.; Wang, P.; Zhang, Z.T.; Gu, Y.; Dougherty, M.; Borovok, I.; Wang, Y. RRNPP-type quorum-sensing systems regulate solvent formation, sporulation and cell motility in Clostridium saccharoperbutylacetonicum. Biotechnol. Biofuels 2020, 13, 84.
- Zhao, R.; Zhang, H.; Zhang, F.; Yang, F. Fast start-up anammox process using acyl-homoserine lactones (AHLs) containing supernatant. J. Environ. Sci. 2018, 65, 127–132.
- Tang, X.; Guo, Y.; Wu, S.; Chen, L.; Tao, H.; Liu, S. Metabolomics uncovers the regulatory pathway of acyl-homoserine lactones based quorum sensing in anammox consortia. Environ. Sci. Technol. 2018, 52, 2206–2216.
- Zhang, J.; Li, J.; Zhao, B.H.; Zhang, Y.C.; Wang, X.J.; Chen, G.H. Long-term effects of N-acyl-homoserine lactone-based quorum sensing on the characteristics of ANAMMOX granules in high-loaded reactors. Chemosphere 2019, 218, 632–642.
- Zhang, J.; Zhang, Y.C.; Wang, X.J.; Li, J.; Zhou, R.X.; Wei, J.; Liang, D.B.; Zhang, K. Effects of substrate shock on release of AHL signals in ANAMMOX granules and properties of granules. Environ. Sci. Water Res. Technol. 2019, 5, 756–768.
- Tang, X.; Guo, Y.; Zhu, T.; Tao, H.; Liu, S. Identification of quorum sensing signal AHLs synthases in Candidatus Jettenia caeni and their roles in anammox activity. Chemosphere 2019, 225, 608–617.
- Honjo, H.; Iwasaki, K.; Soma, Y.; Tsuruno, K.; Hamada, H.; Hanai, T. Synthetic microbial consortium with specific roles designated by genetic circuits for cooperative chemical production. Met. Eng. 2019, 55, 268–275.
- Glasscock, C.J.; Biggs, B.W.; Lazar, J.T.; Arnold, J.H.; Burdette, L.A.; Valdes, A.; Kang, M.K.; Tullman-Ercek, D.; Tyo, K.E.J.; Lucks, J.B. Dynamic control of gene expression with riboregulated switchable feedback promoters. ACS Synth. Biol. 2021, 10, 1199–1213.
- Ge, C.; Yu, Z.; Sheng, H.; Shen, X.; Sun, X.; Zhang, Y.; Yan, Y.; Wang, J.; Yuan, Q. Redesigning regulatory components of quorum-sensing system for diverse metabolic control. Nat. Commun. 2022, 13, 2182.
- Kim, E.M.; Woo, H.M.; Tian, T.; Yilmaz, S.; Javidpour, P.; Keasling, J.D.; Lee, T.S. Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli. Metabol. Eng. 2017, 44, 325–336.
- Wu, J.; Bao, M.; Duan, X.; Zhou, P.; Chen, C.; Gao, J.; Cheng, S.; Zhuang, Q.; Zhao, Z. Developing a pathway-independent and full-autonomous global resource allocation strategy to dynamically switching phenotypic states. Nat. Commun. 2020, 11, 5521.
- Cao, Z.; Liu, Z.; Zhang, G.; Mao, X. PluxI mutants with different promoting period and their application for quorum sensing regulated protein expression. Food Sci. Hum. Wellness 2023, 12, 1841–1849.
- Gu, F.; Jiang, W.; Mu, Y.; Huang, H.; Su, T.; Luo, Y.; Liang, Q.; Qi, Q. Quorum sensing-based dual-function switch and its application in solving two key metabolic engineering problems. ACS Synth. Biol. 2020, 9, 209–217.
- Soma, Y.; Takahashi, M.; Fujiwara, Y.; Shinohara, T.; Izumi, Y.; Hanai, T.; Bamba, T. Design of synthetic quorum sensing achieving induction timing-independent signal stabilization for dynamic metabolic engineering of E. coli. ACS Synth. Biol. 2021, 10, 1384–1393.
- Cui, S.; Lv, X.; Wu, Y.; Li, J.; Du, G.; Ledesma-Amaro, R.; Liu, L. Engineering a bifunctional Phr60-Rap60-Spo0A quorum-sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis. ACS Synth. Biol. 2019, 8, 1826–1837.
- Hu, L.X.; Zhao, M.; Hu, W.S.; Zhou, M.J.; Huang, J.B.; Huang, X.L.; Gao, X.L.; Luo, Y.N.; Li, C.; Liu, K.; et al. Poly-γ-glutamic acid production by engineering a degU quorum-sensing circuit in Bacillus subtilis. ACS Synth. Biol. 2022, 11, 4156–4170.
- Corrêa, G.G.; Lins, M.R.D.C.R.; Silva, B.F.; de Paiva, G.B.; Zocca, V.F.B.; Ribeiro, N.V.; Picheli, F.P.; Mack, M.; Pedrolli, D.B. A modular autoinduction device for control of gene expression in Bacillus subtilis. Metabol. Eng. 2020, 61, 326–334.
- Chen, B.; Wen, J.; Zhao, X.; Ding, J.; Qi, G. Surfactin: A quorum-sensing signal molecule to relieve CCR in Bacillus amyloliquefaciens. Front. Microbiol. 2020, 11, 631.
- Wen, J.; Zhao, X.; Si, F.; Qi, G. Surfactin, a quorum sensing signal molecule, globally affects the carbon metabolism in Bacillus amyloliquefaciens. Metab. Eng. Commun. 2021, 12, e00174.
- Wang, E.X.; Liu, Y.; Ma, Q.; Dong, X.T.; Ding, M.Z.; Yuan, Y.J. Synthetic cell–cell communication in a three-species consortium for one-step vitamin C fermentation. Biotechnol. Lett. 2019, 41, 951–961.
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377.
- Sengupta, D.; Datta, S.; Biswas, D. Towards a better production of bacterial exopolysaccharides by controlling genetic as well as physico-chemical parameters. Appl. Microbiol. Biotechnol. 2018, 102, 1587–1598.
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468.
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140.
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583.
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490.
- Zimmer, J. Activation of bacterial cellulose biosynthesis by cyclic di-GMP. In Microbial Cyclic Di-Nucleotide Signaling; Chou, S.H., Guiliani, N., Lee, V., Römling, U., Eds.; Springer: Cham, Switzerland, 2020; pp. 211–221.
- Saude, N.; Junter, G.A. Production and molecular weight characteristics of alginate from free and immobilized-cell cultures of Azotobacter vinelandii. Process Biochem. 2002, 37, 895–900.
- Ishola, R.O.; Adebayo-Tayo, B.C. Mutagenesis and immobilization effect on exopolysaccharide production by Weissella confusa and Lactobacillus delbrueckii. J. Adv. Microbiol. 2018, 10, 1–10.
- Tao, J.; Huang, X.; Ling, F.; Yu, B.; Zhou, X.; Shen, Q.; Sagratini, G. Immobilization of Lactic acid bacteria for production of extracellular polysaccharides. Food Sci. Technol. 2022, 42, e99021.
- Stepanov, N.A.; Senko, O.V.; Efremenko, E.N. Biocatalytic production of extracellular exopolysaccharide dextran synthesized by cells of Leuconostoc mesenteroides. Catal. Ind. 2017, 9, 339–343.
- Senko, O.V.; Efremenko, E.N. Highly concentrated populations of Aureobasidium pullulans cells in biocatalytic pullulan production processes. Catal. Ind. 2017, 9, 344–348.
- Stepanov, N.; Efremenko, E. “Deceived” concentrated immobilized cells as biocatalyst for intensive bacterial cellulose production from various sources. Catalysts 2018, 8, 33.
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of bacterial cellulose using Gluconacetobacter kombuchae immobilized on Luffa aegyptiaca support. Sci. Rep. 2021, 11, 1–15.
- Mesquita, R.A.; Hassemer, G.; Marchiori, V.; Kiedis, J.; Valduga, E.; Junges, A.; Malvessi, E.; Cansian, R.L.; Zeni, J. Synthesis of xanthan gum from Xanthomonas campestris immobilized in polyurethane. Ind. Biotechnol. 2018, 14, 276–281.
- Nejadmansouri, M.; Shad, E.; Razmjooei, M.; Safdarianghomsheh, R.; Delvigne, F.; Khalesi, M. Production of xanthan gum using immobilized Xanthomonas campestris cells: Effects of support type. Biochem. Eng. J. 2020, 157, 107554.
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D. Production of xanthan gum by free and immobilized cells of Xanthomonas campestris and Xanthomonas pelargonii. Int. J. Biol. Macromol. 2016, 82, 751–756.
- Santos, V.A.Q.; Cruz, C.H.G. Zymomonas mobilis immobilized on loofa sponge and sugarcane bagasse for levan and ethanol production using repeated batch fermentation. Braz. J. Chem. Eng. 2017, 34, 407–418.
- Castro, C.C.; Nobre, C.; Duprez, M.E.; De Weireld, G.; Hantson, A.L. Screening and selection of potential carriers to immobilize Aureobasidium pullulans cells for fructo-oligosaccharides production. Biochem. Eng. J. 2017, 118, 82–90.
- Ortiz Martinez, C.; Pereira Ruiz, S.; Carvalho Fenelon, V.; Rodrigues de Morais, G.; Luciano Baesso, M.; Matioli, G. Characterization of curdlan produced by Agrobacterium sp. IFO 13140 cells immobilized in a loofa sponge matrix, and application of this biopolymer in the development of functional yogurt. J. Sci. Food Agric. 2016, 96, 2410–2417.
- Ruiz, S.P.; Martinez, C.O.; Noce, A.S.; Sampaio, A.R.; Baesso, M.L.; Matioli, G. Biosynthesis of succinoglycan by Agrobacterium radiobacter NBRC 12665 immobilized on loofa sponge and cultivated in sugar cane molasses. Structural and rheological characterization of biopolymer. J. Mol. Catal. B Enzym. 2015, 122, 15–28.
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Alginate biosynthesis and biotechnological production. In Alginates and Their Biomedical Applications; Rehm, B., Moradali, M., Eds.; Springer: Singapore, 2018; pp. 1–25.
- Sun, J.; Sun, H.; Lv, W.; Zhang, Q.; Wan, P.; Jiang, L.; Zhong, Y. Quorum sensing mediates yeast cell morphology to improve settle ability: Implication for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105817.
- Zhang, Q.; Xiang, J.; Lv, W.; Liu, Y.; Sun, J.; Wan, P.; Jiang, L. Quorum sensing molecules in yeast wastewater treatment and their regulation of yeast cell morphology. J. Water Process Eng. 2022, 49, 103191.
- Sooklim, C.; Samakkarn, W.; Thongmee, A.; Duangphakdee, O.; Soontorngun, N. Enhanced aroma and flavour profile of fermented Tetragonula pagdeni Schwarz honey by a novel yeast T. delbrueckii GT-ROSE1 with superior fermentability. Food Biosci. 2022, 50, 102001.
- Tian, J.; Lin, Y.; Su, X.; Tan, H.; Gan, C.; Ragauskas, A.J. Effects of Saccharomyces cerevisiae quorum sensing signal molecules on ethanol production in bioethanol fermentation process. Microbial. Res. 2023, 271, 127367.
- Nath, B.J.; Mishra, A.K.; Sarma, H.K. Assessment of quorum sensing effects of tyrosol on fermentative performance by chief ethnic fermentative yeasts from northeast India. J. Appl. Microbiol. 2021, 131, 728–742.
- Valera, M.J.; Morcillo-Parra, M.Á.; Zagórska, I.; Mas, A.; Beltran, G.; Torija, M.J. Effects of melatonin and tryptophol addition on fermentations carried out by Saccharomyces cerevisiae and non-Saccharomyces yeast species under different nitrogen conditions. Int. J. Food Microbiol. 2019, 289, 174–181.
- Nath, B.J.; Das, K.K.; Talukdar, R.; Sarma, H.K. Tyrosols retrieved from traditionally brewed yeasts assist in tolerance against heavy metals and promote the growth of cells. FEMS Microbiol. Lett. 2021, 368, fnab152.
- Willaert, R.G. Adhesins of yeasts: Protein structure and interactions. J. Fungi 2018, 4, 119.
- Rosiana, S.; Zhang, L.; Kim, G.H.; Revtovich, A.V.; Uthayakumar, D.; Sukumaran, A.; Gedde-McAlister, J.; Kirienko, N.V.; Shapiro, R.S. Comprehensive genetic analysis of adhesin proteins and their role in virulence of Candida albicans. Genetics 2021, 217, iyab003.
- Jung, P.; Mischo, C.E.; Gunaratnam, G.; Spengler, C.; Becker, S.L.; Hube, B.; Jacobs, K.; Bischoff, M. Candida albicans adhesion to central venous catheters: Impact of blood plasma-driven germ tube formation and pathogen-derived adhesins. Virulence 2020, 11, 1453–1465.
- Kumar, A. The complex genetic basis and multilayered regulatory control of yeast pseudohyphal growth. Annu. Rev. Genet. 2021, 55, 1–21.
- Tian, X.; Ding, H.; Ke, W.; Wang, L. Quorum sensing in fungal species. Annu. Rev. Microbiol. 2021, 75, 449–469.
- Gaálová-Radochová, B.; Kendra, S.; Jordao, L.; Kursawe, L.; Kikhney, J.; Moter, A.; Bujdáková, H. Effect of quorum sensing molecule farnesol on mixed biofilms of Candida albicans and Staphylococcus aureus. Antibiotics 2023, 12, 441.
- Huang, X.F.; Reardon, K.F. Strategies to achieve high productivity, high conversion, and high yield in yeast fermentation of algal biomass hydrolysate. Eng. LifeSci. 2022, 22, 119–131.
- Ndubuisi, I.A.; Qin, Q.; Liao, G.; Wang, B.; Moneke, A.N.; Ogbonna, J.C.; Jin, C.; Fang, W. Effects of various inhibitory substances and immobilization on ethanol production efficiency of a thermotolerant Pichia kudriavzevii. Biotechnol. Biofuels 2020, 13, 91.
- Avbelj, M.; Zupan, J.; Raspor, P. Quorum-sensing in yeast and its potential in wine making. Appl. Microbiol. Biotechnol. 2016, 100, 7841–7852.
- Prakash, J.; Kalia, V.C. Application of quorum sensing systems in production of green fuels. In Quorum Sensing and Its Biotechnological Applications; Kalia, V., Ed.; Springer: Singapore, 2018; pp. 155–166.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
01 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




