Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nuha Mashaan | -- | 2457 | 2023-05-30 09:45:22 | | | |
| 2 | Catherine Yang | Meta information modification | 2457 | 2023-05-30 09:54:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jwaida, Z.; Dulaimi, A.; Mashaan, N.; Othuman Mydin, M.A. Geopolymers for Engineering Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/44995 (accessed on 06 March 2026).
Jwaida Z, Dulaimi A, Mashaan N, Othuman Mydin MA. Geopolymers for Engineering Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/44995. Accessed March 06, 2026.
Jwaida, Zahraa, Anmar Dulaimi, Nuha Mashaan, Md Azree Othuman Mydin. "Geopolymers for Engineering Applications" Encyclopedia, https://encyclopedia.pub/entry/44995 (accessed March 06, 2026).
Jwaida, Z., Dulaimi, A., Mashaan, N., & Othuman Mydin, M.A. (2023, May 30). Geopolymers for Engineering Applications. In Encyclopedia. https://encyclopedia.pub/entry/44995
Jwaida, Zahraa, et al. "Geopolymers for Engineering Applications." Encyclopedia. Web. 30 May, 2023.
Copy Citation
Geopolymers have the impact in global warming and climate change resulting from greenhouse gas emissions. Geopolymers are an effective cementitious binder substitute for cement in various applications.
geopolymer
waste materials
review
sustainability
1. Introduction
For the development of a country, the construction industry has a significant role, accounting for more than 6% of the gross domestic product globally [1]. The development of infrastructure and urbanization require producing considerable amounts of concrete. Cement represents a main constituent of concrete which binds filler and aggregate materials.
Cement mainly consists of silicon, calcium, and aluminum and minerals such as iron ore, chalk, clay, and limestone [2], which are mined or quarried from natural resources. Moreover, during the manufacturing of cement, the required fossil fuels such as coal and petroleum are also considered natural resources. The energy required for the manufacturing also includes that needed for the grinding and heating of raw materials to more than 1450 °C. Additionally, the thermal decomposition of limestone emits greenhouse gases directly into the atmosphere, including carbon dioxide (CO2) [3][4], with small quantities of other toxic gases such as sulfur dioxide (SO2) and nitrogen dioxide (NO2) [4].
The production of cement is considered a significant contributor to the emissions of carbon dioxide (CO2). From 2015 to 2020, each ton of cement produced increased CO2 emissions by 1.8% annually. It was also estimated that about a 3% reduction of CO2 is needed to achieve net zero emissions by 2050 [5]. It is estimated that the process of cement production causes 4–10% of CO2 emissions globally [6][7]. During the use of electric power, combustion of fuel, and the process of calcination, each ton of cement produced emits about 0.66–1.5 tons of CO2 depending on the production system and technique [8][9]. Each ton of cement produced requires 3.2–6.3 GJ of energy and 2.65–2.8 tons of raw materials [10][11]. In addition, dust is generated during cement production which contributes to industrial air pollution [12]. Thus, reducing the manufacturing of cement reduces the amount of CO2 emission. With the increasing need for concrete for infrastructure and housing, cement production is increasing daily. It was estimated that about 4100 million metric tons of cement were produced worldwide in 2018 [13]. It was reported by Schneider et al. [14] that the production of cement will increase in 2050 to 4.38 billion tons due to the increasing global demand. In addition, Müller and Harnisch [15] expected a cement production of 5 billion tons in 2030. Such predictions are alarming as the production of cement requires a substantial quantity of energy and raw materials, reducing the available non-renewable fossil fuel and natural resources [12][16].
On the other hand, significant amounts of solid waste are produced by municipalities, agriculture, and industry due to the increasing global population. The amount of discharged waste was estimated at 10.4 billion tons in 2010 and will rise to 148 billion tons by 2025 [17][18]. Although solid wastes can be dealt with by various methods such as decomposition, crushing, incineration, and gasification [19], residues result which cause secondary pollution [17]. Thus, effective reusing or recycling of the residues can minimize the burdens on the environment [20]. With the consideration of the adverse effect of utilizing and producing cement, various researchers have attempted to provide solutions for sustainable technologies. Some of the suggested approaches are to use supplementary cementitious materials (SCMs) to partially replace cement and chemical additives for reducing the consumption of water, usage of alternative fuels, an effective system of cement production, and use of water and recycled materials and concrete [21][22][23][24].
Geopolymer binders use by-products such as ground granulated blast furnace slag (GGBS) and fly ash with minimal emission of greenhouse gases from the production of alkali activators. By-product materials have been used in concrete only as a partial replacement since they need to react with the cement hydration product to form binding materials. Thus, there has been substantial interest in developing alternatives that can reduce the dependence on cement while providing approximately similar engineering properties to cement. Geopolymer represents a new binding material that can be produced from the activation of aluminosilicate material with alkaline solutions [4][25]. Thus, geopolymer binders were proposed as an effective solution to fully or partially replace the use of cement. It is estimated by Davidovits [26] that only 0.15 to 0.20 tons of cement are produced from each ton of geopolymer, significantly lower than when using cement alone. This shows the effective potential of replacing cement with geopolymers as an environmentally friendly alternative. Geopolymers have been considered a sustainable solution and eco-friendly disposal of industrial by-product materials such as ground granulated blast furnace slag (GGBS) [27][28][29][30], fly ash [28][31][32][33][34], metakaolin [34][35][36], bottom ash [27][35][37], and rice husk ash [27][33][38].
2. Geopolymers
In 1976, this alkali-activated material was defined by Joseph Davidovits [39]. Alkali-activated material (AAM) represents an effective alternative to cement due to its better performance and lower environmental impacts. Although the concept of alkali-activated material and geopolymer technology has been widely explored over recent decades, no consensus has been reached for distinguishing between the terminologies [24]. However, according to Provis and Bernal [40], AAM is the widest classification, referring to any system of an aluminosilicate-rich precursor with either no or high calcium content and activated by alkaline activators, whereas geopolymers are a subset of AAM, referring to a system of high aluminosilicate and low calcium content with the presence of alkaline activators [41][42].
Geopolymer is a term for a binder produced from the chemical reactions of a precursor containing silica with alumina material by the presence of alkaline activators. The main components are the source materials which should be rich in major constituents, such as silicon (Si) and aluminum (Al), from either natural or waste and by-product materials, such as clays, kaolinite, etc. or red mud, fly ashes, slags, etc. The liquid is usually based on sodium (hydroxide or silicate) or potassium (hydroxide or silicate) [41][42]
Initially, geopolymer binders were produced as amorphous and inorganic material for their non-combustible, inflammable, and heat-resistant properties for reducing the fire risks of the use of plastic materials in the 1970s in Europe. Then, the use of geopolymer binders was shifted to structural engineering since promising results were obtained by different studies in terms of their behavior and potential of replacing cement [12][43]. Alternative names for geopolymers were also introduced, including geocement, alkali-bonded ceramics and hydroceramics, aluminosilicate inorganic polymers (AIPs), inorganic polymers (IPs), inorganic phosphate cement (IPC), and alkali-activated materials (AAMs), based on the composition and formulation [12][42].
Geopolymer binders are innovative cement-based materials that can substitute OPC composites while emitting less CO2 than OPC [44]. The desired goal of studies about geopolymer binders in industry and academia has been to obtain an effective alternative to cement. Thus, based on traditional cement-related techniques and science, development and synthesis processes were aligned and evaluated. However, the synthesis and chemistry processes of geopolymers and cement are different due to the different natures of materials and constituents. The binders of cement are synthesized depending on the hydration reactions of silicon dioxide and calcium oxide to produce calcium silicate hydrates (C-S-H) [45][46].
On the other hand, the structure of geopolymer binders as inorganic polymers consists of chained repetitive elements called monomers, controlling the characteristics of geopolymers. The structure is made of sialate (of silico-oxo-aluminate) or alkali aluminosilicate gel with alumina (Al) or silica (Si) ions, presenting alumina and silica bonding by a bridge of oxygen. Geopolymer has an amorphous microstructure similar to a crystalline structure, which allows the reaction with other materials to form binders, while the composition is similar to natural zeolite [45][47]. Van Jaarsveld et al. [48] found that the crystalline and amorphous degree in fly-ash-based geopolymers is a key parameter impacting the mechanical and physical characteristics.
According to Davidovits [26], the term polysialate is used to chemically designate aluminosilicate-based geopolymers, while sialate is the abbreviation for silico-oxo-aluminate (Si-O-Al-O) [49]. The process of geopolymerization involves the chemical reaction of aluminosilicate oxide minerals with a high-alkaline solution. The process is a very fast reaction and, during the process, the geopolymer blocks are created by polymeric bonds and three-dimensional sialate (Si-O-Al-O) chained structures [50][51]. The quantity of alumina and silica in the structure impacts the degree of reaction and bonding and thereby the characteristics of geopolymers. The following formula represents the created polysialate chained structure:
where M is a cation or alkaline element (e.g., potassium, sodium, etc.), z is a value of 1–3 or higher depending on the reaction chemistry, n is the polymerization degree, w is the hydration degree [39]. The polysialate formation and its chemical structure are illustrated in Figure 1 as depicted by Davidovits [26]. The polysialate structure depends on the spatial arrangement and organization of alternately linked SiO4 and AlO4 with all shared oxygen ions, which can form several repeating units (monomers).
Mn [-(SiO2)z-AlO2)]n.wH2O

Figure 1. The polysialate formation of geopolymerization process [26].
However, the geopolymerization mechanism is not thoroughly understood due to the existence of influential parameters in the process. Various studies indicated that the process consists of three main stages: dissolution and destruction of alumina and silica ions from precursors by hydrolysis of alkaline activators, the re-orientation and transportation of cation ions into monomers, and polycondensation of the monomers into polymeric structures. The stages can overlap with each other and therefore are difficult to study or isolate [12][52][53][54].
In the 1950s, alkaline-activated mechanisms were first explored by Glukhovsky, who divided the process into three phases: destruction–coagulation; coagulation–condensation; condensation–crystallization [42]. Later on, researchers such as Shi et al. [51], Fernandez-Jimenez et al. [55], and Duxson et al. [56] reviewed the Glukhovsky model and proposed a new model for the geopolymerization process, as shown in Figure 2. Figure 3 illustrates the chemical reactions of geopolymerization [57][58][59]. Alkaline activators dissolve aluminosilicate materials into silicate and aluminum ions (AlO4 and SiO4 tetrahedral units) with a pH greater than 10. Aluminosilicate gel is formed by the shift of the ions to an equilibrium state and then interaction. A small degree of structural order is observed, which favors a stable Al-rich compound. Silicon and aluminum tetrahedrons join, producing rings with four secondary tetrahedral units [55]. The high concentration of aluminum in the precipitation of gel 1 (SiAl = 1) results from the significant concentration of Al + 3 ions in the alkaline medium at the beginning of the reaction [40][60]. Firstly, the gel consists of alumina since it dissolves faster than alumina, then following the dissolution of silicate, the gel is said to be a zeolite precursor gel. This gel is more stable than aluminum gel due to stronger SiO bonds than Al-O bonds [61]. More Si–O bonds are broken from the precursors as the reaction progresses, which dissolve and thereby increase the concentration of silicon in the solution, reaching the gel 2 precipitation model (SiAl = 2). This rise in the ratio of Si/Al ratio improves the mechanical characteristics of the formed aluminosilicate gels [62]. Finally, the formation of an amorphous to semi-crystalline aluminosilicate network is developed with the desired physical characteristics. The final products are based on the characteristics of alkaline activators and source materials and the contents of calcium, aluminosilicate, or magnesium. Such products can be sodium aluminosilicate hydrate (N-A-S-H) (Na + Al + Si), polymers or zeolite from alumina or silica, or C-S-H gel [61][63][64]. The existence of alumina, silica, and calcium species can produce aluminosilicate hydrate (C-A-S-H) gel products, which can often be seen in GGBS geopolymer binders. In terms of the secondary products, in addition to the formation of the C-S-H gel inside the material, they include portlandite, ettringite, and calcium monosulfoaluminate [41][65].
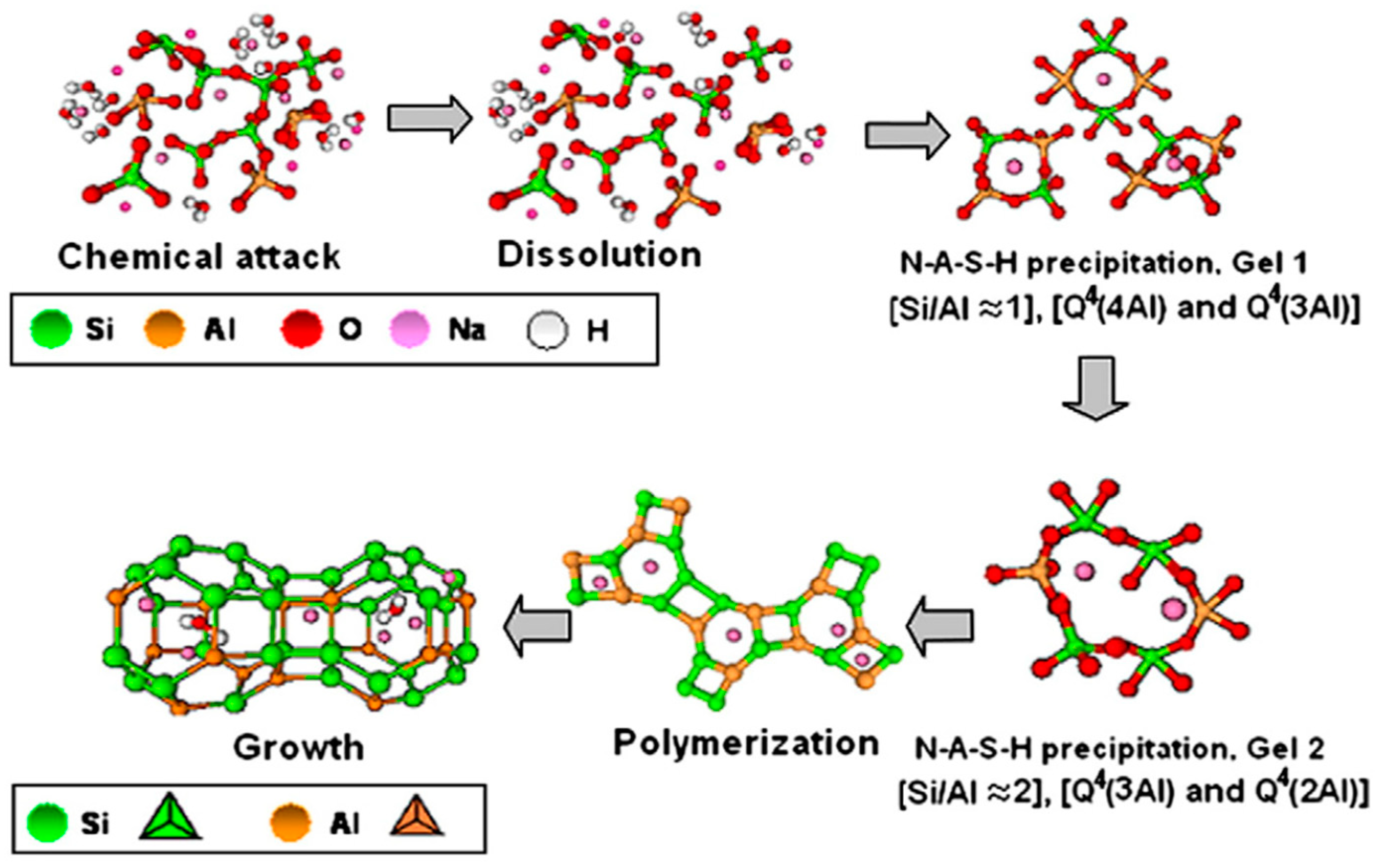
Figure 2. Stages of geopolymerization process [54].
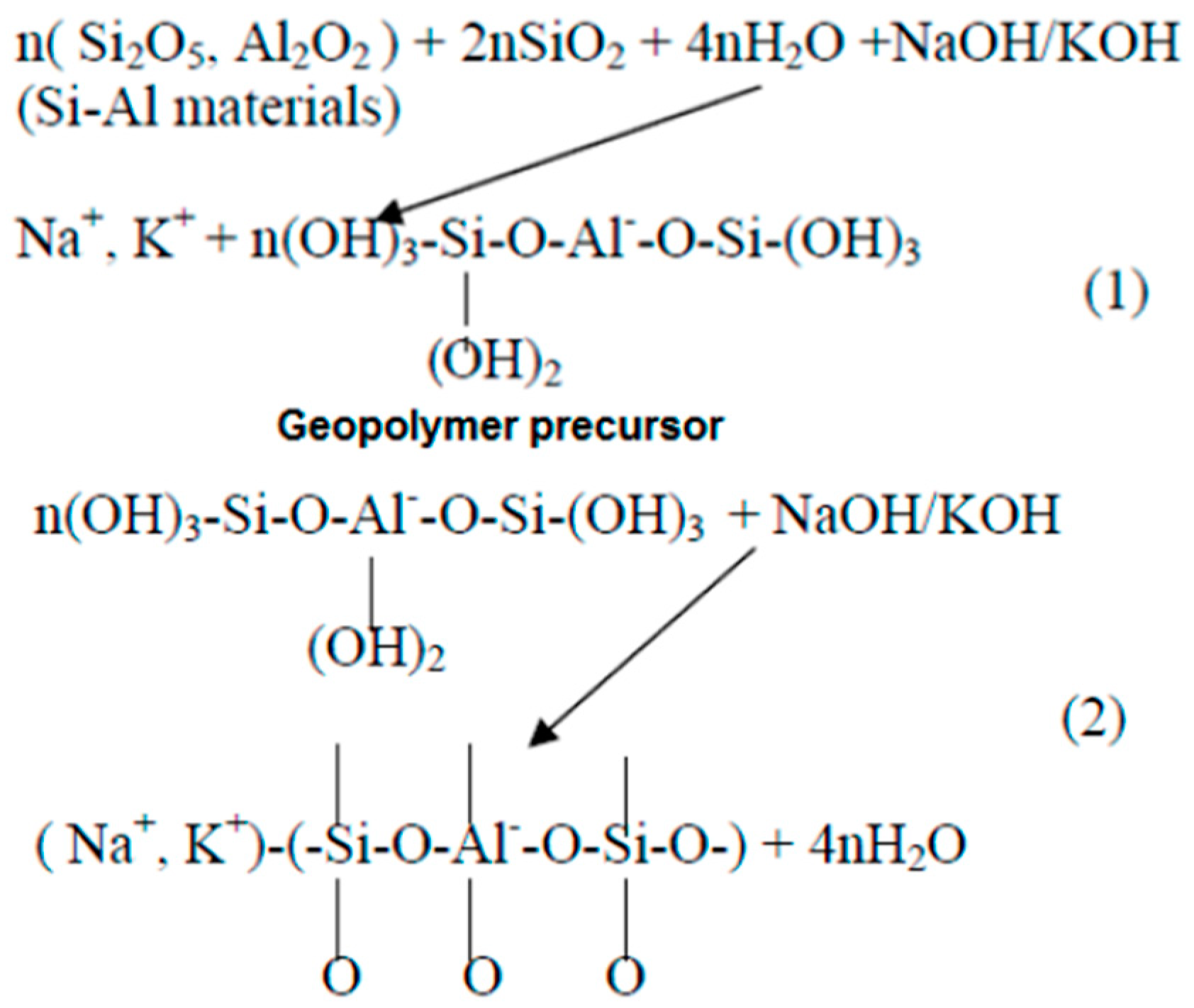
Figure 3. Reactions during geopolymerization [59]. (1) Dissolution of Si and Al atoms from the source material through the action of hydroxide ions, (2) Transportation or orientation or condensation of precursor ions into monomers followed by Setting or polycondensation/polymerisation of monomers into polymeric structures.
Additionally, water (H2O) is a product of the process indicated by the proposed model, chemical reactions, and the formulation of the polysialate chain structure. Hardjito and Rangan [47] mentioned that chemically free water resulted during the chemical reactions of the process from the matrix of the geopolymer and was removed in the drying and curing steps. However, the role of water is negligible in comparison with the hydration process of cement, though it impacts the workability and produces micro- and nanovoids in the matrix [66]. Since water lacks involvement in the process, the properties and microstructure binders are influenced by the ingress water resistance, elevated temperatures, chemical attacks, and alkali-aggregate reactivity [67].
When the amount of activator is lower than required, some of the aluminosilicate precursors do not undergo a reaction due to the lack of reaction medium. Low dissolution of aluminosilicate does not cause a change in the setting time. In addition, various researchers [68][69] reported that the geopolymer structure is similar to zeolites, though the geopolymer has denser mesoporous and semi-crystalline or amorphous structures in comparison with the crystalline structure of zeolites [70][71]. This could result from the vast dissolution of the glassy constituents during the mixing of the alkali activators with the precursors. In this case, space and time are not adequate for the growth of the gel into a well-crystallized structure, forming semi-crystalline, amorphous, or microcrystalline structures [72].
Studies have indicated that the content of active calcium in the raw materials determines the nanostructures of geopolymers. A highly crosslinked zeolite-like N-A-S-H structure is formed with a low amount of active calcium, with Q4 (4Al) as the main units of the structure [73][74]. With the presence of humidity, oligomeric aluminum-rich colloids are formed by the rapid combination of aluminum–oxygen and silicon–oxygen tetrahedrons. In contrast, the polymerization degree of aluminum-rich colloids is low with low levels of humidity. With a high content of calcium, C-A-S-H gel is formed, similar to tobermorite in structure. N-A-S-H and C-A-S-H can both occur in the system, though at high pH levels of more than 12, the N-A-S-H is slowly transformed into C-A-S-H [75].
3. Potential of Geopolymer Concrete Applications in Construction
Geopolymer technology has been considered as a promising technique to reduce or replace the use of conventional concrete by solving the emission and disposal problems from inductors such as rice milling, thermal plants, steel industries, etc. However, challenges are present in achieving comparable or better properties than conventional concrete. These include the requirement of high dosages of alkaline activators and curing temperatures [76]. Another significant challenge is the properties of raw materials such as GGBS, FA, RHA, POFA, MK, etc. It has been reported that the sources of these materials impact their properties, thereby requiring different chemical activator dosages, curing regimes, etc. Thus, mixing proportion criteria are yet to be obtained. This research revealed various parameters for various waste- and by-product-based geopolymers to bridge the gap between laboratories and actual industrial applications. Some advanced curing regimes can be developed regarding the emitted gas from industries by adjusting them to the required temperature inside the chamber where the curing is carried out. Some advanced methods such as nuclear magnetic resonance can be used under XRD that can elucidate the amorphous products formed by mixing various source materials. Furthermore, the pH of the reaction products can be studied to check their acidity or usage in conjunction with steel reinforcement or whether it is too basic to cause other deteriorations.
References
- Prospects, M. Global Construction Trends. 2022. Available online: https://www.marketprospects.com/articles/global-construction-industry-trends (accessed on 1 January 2023).
- PCA How Cement Is Made. 2019. Available online: https://www.cement.org/cement-concrete/how-cement-is-made (accessed on 26 April 2023).
- Hendriks, C.A.; Worrell, E.; De Jager, D.; Blok KRiemer, P. Emission reduction of greenhouse gases from the cement industry. In Proceedings of the Fourth International Conference on Greenhouse Gas Control Technologies, Interlaken, Switzerland, 30 August–2 September 1998; IEA GHG R&D Programme: Interlaken, Austria, 1998.
- Neupane, K. Evaluation of environmental sustainability of one-part geopolymer binder concrete. Clean. Mater. 2022, 6, 100138.
- Direct CO2 Intensity of Cement Production in the Net Zero Scenario, 2015–2030. 2021. Available online: https://www.iea.org/reports/cement (accessed on 21 December 2022).
- Van Oss, H.G.; Padovani, A.C. Cement manufacture and the environment part II: Environmental challenges and opportunities. J. Ind. Ecol. 2003, 7, 93–126.
- Farooq, F.; Jin, X.; Faisal Javed, M.; Akbar, A.; Izhar Shah, M.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762.
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485.
- Carreño-Gallardo, C.; Tejeda-Ochoa, A.; Perez-Ordonez, O.I.; Ledezma-Sillas, J.E.; Lardizabal-Gutierrez, D.; Prieto-Gomez, C.; Valenzuela-Grado, J.A.; Hernandez, F.R.; Herrera-Ramirez, J.M. In the CO2 emission remediation by means of alternative geopolymers as substitutes for cements. J. Environ. Chem. Eng. 2018, 6, 4878–4884.
- Mehta, K.P. Reducing the environmental impact of concrete. Concr. Int. 2001, 23, 61–66.
- Suhendro, B. Toward green concrete for better sustainable environment. Procedia Eng. 2014, 95, 305–320.
- Negahban, E. Investigation of Geopolymer Concrete for Pavement Applications; Swinburne University of Technology: Melbourne, Australia, 2022.
- ICR. Global Cement Report, 13th ed.; CemNet: Dorking, UK; International Cement Review: Dorking, UK, 2019.
- Schneider, M.; Romer, M.; Tschudin MBolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650.
- Müller, N.a.J.H. A Blueprint for a Climate-Friendly Cement Industry: How to Turn around the Trend of Cement Related Emissions in the Developing World; World Wide Fund for Nature: Gland, Switzerland, 2008; p. 101.
- Berry, M.; Cross, D.; Stephens, J. Changing the Environment: An Alternative “ Green ” Concrete Produced without Portland Cement. In Proceedings of the 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 May 2009.
- Alam, O.; Qiao, X. An in-depth review on municipal solid waste management, treatment and disposal in Bangladesh. Sustain. Cities Soc. 2020, 52, 101775.
- Patwa, A.; Parde, D.; Dohare, D.; Vijay, R.; Kumar, R. Solid waste characterization and treatment technologies in rural areas: An Indian and international review. Environ. Technol. Innov. 2020, 20, 101066.
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211.
- Harbec, D.; Zidol, A.; Tagnit-Hamou, A.; Gitzhofer, F. Mechanical and durability properties of high performance glass fume concrete and mortars. Constr. Build. Mater. 2017, 134, 142–156.
- Pacewska, B.; Wilińska, I. Usage of supplementary cementitious materials: Advantages and limitations. J. Therm. Anal. Calorim. 2020, 142, 371–393.
- Fantilli, A.P.; Jóźwiak-Niedźwiedzka, D. Special Issue: Supplementary Cementitious Materials in Concrete, Part I. Materials 2021, 14, 2291.
- Amin, A. Application of Supplementary Cementitious Materials in Precast Concrete Industry. In Sustainability of Concrete with Synthetic and Recycled Aggregates; Hosam, M.S., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 8.
- Jiang, X.; Zhang, Y.; Zhang, Y.; Ma, J.; Xiao, R.; Guo, F.; Bai, Y.; Huang, B. Influence of size effect on the properties of slag and waste glass-based geopolymer paste. J. Clean. Prod. 2023, 383, 135428.
- Xiao, R.; Huang, B.; Zhou, H.; Ma, Y.; Jiang, X. A state-of-the-art review of crushed urban waste glass used in OPC and AAMs (geopolymer): Progress and challenges. Clean. Mater. 2022, 4, 100083.
- Davidovits, J. 30 Years of Successes and Failures in Geopolymer Applications. Market Trends and Potential Breakthroughs. In Proceedings of the Geopolymer 2002 Conference, Melbourne, Australia, 28–29 October 2002.
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300.
- Kurtoglu, A.E.; Alzeebaree, R.; Aljumaili, O.; Nis, A.; Gulsan, M.E.; Humur, G.; Cevik, A. Mechanical and durability properties of fly ash and slag based geopolymer concrete. Adv. Concr. Constr. 2018, 6, 345.
- Mehta, A.; Siddique, R. Sustainable geopolymer concrete using ground granulated blast furnace slag and rice husk ash: Strength and permeability properties. J. Clean. Prod. 2018, 205, 49–57.
- Venkatesan, R.P.; Pazhani, K.C. Strength and durability properties of geopolymer concrete made with Ground Granulated Blast Furnace Slag and Black Rice Husk Ash. KSCE J. Civ. Eng. 2016, 20, 2384–2391.
- Adak, D.; Sarkar, M.; Mandal, S. Structural performance of nano-silica modified fly-ash based geopolymer concrete. Constr. Build. Mater. 2017, 135, 430–439.
- Jiang, X.; Xiao, R.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A laboratory investigation of steel to fly ash-based geopolymer paste bonding behavior after exposure to elevated temperatures. Constr. Build. Mater. 2020, 254, 119267.
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143.
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Properties of metakaolin-high calcium fly ash geopolymer concrete containing recycled aggregate from crushed concrete specimens. Constr. Build. Mater. 2018, 161, 365–373.
- Pouhet, R.; Cyr, M. Formulation and performance of flash metakaolin geopolymer concretes. Constr. Build. Mater. 2016, 120, 150–160.
- Zhang, H.Y.; Qiu, G.H.; Kodur, V.; Yuan, Z.S. Spalling behavior of metakaolin-fly ash based geopolymer concrete under elevated temperature exposure. Cem. Concr. Compos. 2020, 106, 103483.
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958.
- Zabihi, S.M.; Tavakoli, H.; Mohseni, E. Engineering and microstructural properties of fiber-reinforced rice husk–ash based geopolymer concrete. J. Mater. Civ. Eng. 2018, 30, 04018183.
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441.
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327.
- de Oliveira, L.B.; de Azevedo, A.R.G.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of geopolymers with industrial waste. Case Stud. Constr. Mater. 2022, 16, e00839.
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933.
- Provis, J.L.; Van Deventer, J.S. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: New York, NY, USA, 2009.
- Unis Ahmed, H.; Mahmood, L.J.; Muhammad, M.A.; Faraj, R.H.; Qaidi, S.M.A.; Hamah Sor, N.; Mohammed, A.S.; Mohammed, A.A. Geopolymer concrete as a cleaner construction material: An overview on materials and structural performances. Clean. Mater. 2022, 5, 100111.
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455.
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 19–47.
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Resaerch report; Curtin University of Technology Perth: Bentley, Australia, 2005.
- van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280.
- Fu, C.; Ye, H.; Zhu, K.; Fang, D.; Zhou, J. Alkali cation effects on chloride binding of alkali-activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2020, 114, 103721.
- Ambily, P.S.; Ravisankar, K.; Umarani, C.; Dattatreya, J.K.; Iyer, N.R. Development of ultra-high-performance geopolymer concrete. Mag. Concr. Res. 2014, 66, 82–89.
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763.
- Petermann, J.C.; Saeed, A.; Hammons, M.I. Alkali-Activated Geopolymers: A Literature Review; Applied Research Associates Inc.: Panama City, FL, USA, 2010.
- Rangan, B.V. 11—Engineering Properties of Geopolymer Concrete in Geopolymers; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 211–226.
- Cheah, C.B.; Samsudin, M.H.; Ramli, M.; Part, W.K.; Tan, L.E. The use of high calcium wood ash in the preparation of Ground Granulated Blast Furnace Slag and Pulverized Fly Ash geopolymers: A complete microstructural and mechanical characterization. J. Clean. Prod. 2017, 156, 114–123.
- Fernández-Jiménez, A.; Vallepu, R.; Terai, T.; Palomo, A.; Ikeda, K. Synthesis and thermal behavior of different aluminosilicate gels. J. Non-Cryst. Solids 2006, 352, 2061–2066.
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597.
- Zhang, D.-W.; Wang, D.-m.; Liu, Z.; Xie, F.-z. Rheology, agglomerate structure, and particle shape of fresh geopolymer pastes with different NaOH activators content. Constr. Build. Mater. 2018, 187, 674–680.
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314.
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299.
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085.
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729.
- Zhao, M.; Zhang, G.; Htet, K.W.; Kwon, M.; Liu, C.; Xu, Y.; Tao, M. Freeze-thaw durability of red mud slurry-class F fly ash-based geopolymer: Effect of curing conditions. Constr. Build. Mater. 2019, 215, 381–390.
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697.
- van Overmeir, A.L.; Figueiredo, S.C.; Šavija, B.; Bos, F.P.; Schlangen, E. Design and analyses of printable strain hardening cementitious composites with optimized particle size distribution. Constr. Build. Mater. 2022, 324, 126411.
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2014.
- Nath, P. Study of fly ash based geopolymer concrete cured in ambient condition. In School of Civil and Mechanical Engineering; Curtin University: Perth, Australia, 2014.
- Kadhim, A.; Sadique, M.; Al-Mufti, R.; Hashim, K. Developing one-part alkali-activated metakaolin/natural pozzolan binders using lime waste. Adv. Cem. Res. 2021, 33, 342–356.
- Granizo, N.; Palomo, A.; Fernandez-Jiménez, A. Effect of temperature and alkaline concentration on metakaolin leaching kinetics. Ceram. Int. 2014, 40, 8975–8985.
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533.
- Barbosa, T.R.; Foletto, E.L.; Dotto, G.L.; Jahn, S.L. Preparation of mesoporous geopolymer using metakaolin and rice husk ash as synthesis precursors and its use as potential adsorbent to remove organic dye from aqueous solutions. Ceram. Int. 2018, 44, 416–423.
- Lee, N.; Khalid, H.R.; Lee, H.-K. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater. 2016, 229, 22–30.
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746.
- Wongsa, A.; Siriwattanakarn, A.; Nuaklong, P.; Sata, V.; Sukontasukkul, P.; Chindaprasirt, P. Use of recycled aggregates in pressed fly ash geopolymer concrete. Environ. Prog. Sustain. Energy 2020, 39, e13327.
- Provis, J.L.; Yong, S.L.; Duxson, P. 5—Nanostructure/Microstructure of Metakaolin Geopolymers in Geopolymers; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 72–88.
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between, N-A-S-H and C-A-S-H gels. Study in the ternary diagram, Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931.
- Ahmari, S.; Zhang, L.; Zhang, J. Effects of activator type/concentration and curing temperature on alkali-activated binder based on copper mine tailings. J. Mater. Sci. 2012, 47, 5933–5945.
More
Information
Subjects:
Engineering, Civil
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
30 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





