
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Moon Dong-Oh | -- | 1232 | 2023-05-30 04:06:10 | | | |
| 2 | Fanny Huang | Meta information modification | 1232 | 2023-05-30 05:44:55 | | |
Video Upload Options
Calcium is an essential intracellular messenger that plays a vital role in controlling a broad range of cellular processes, including apoptosis. Cytosolic calcium levels are tightly regulated, and an imbalance in calcium concentrations can trigger apoptosis through various mechanisms involving cytosolic proteins.
1. Introduction
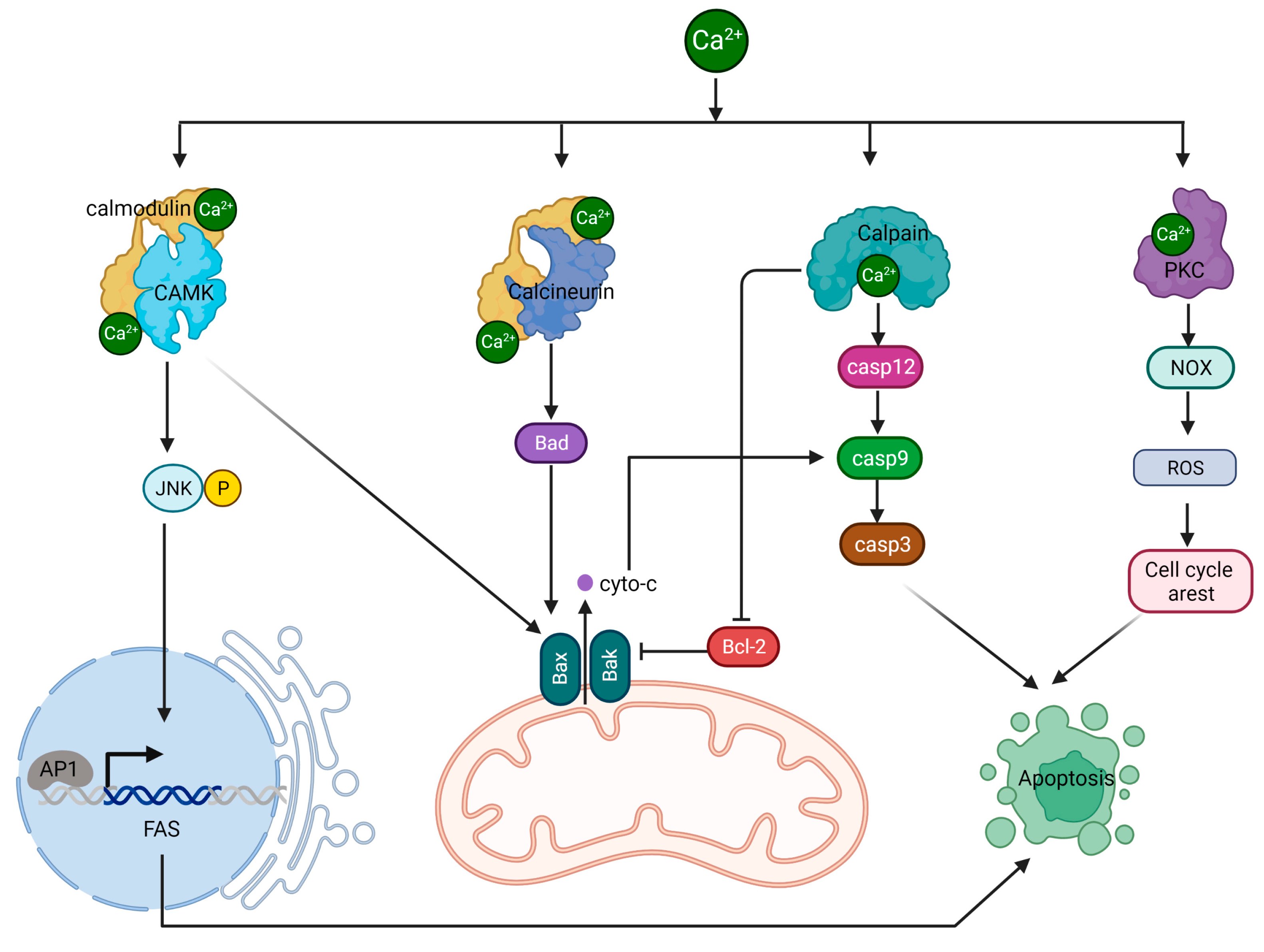
Calcium, an essential second messenger in cells, controls numerous cellular functions, including cell death [1]. The concentration of calcium varies in different cell compartments, with the cytoplasm maintaining relatively low levels to ensure effective cellular signaling and prevent cytotoxic effects. This balance is achieved through various mechanisms, such as calcium pumps, sodium–calcium exchangers, calcium-binding proteins, and the mitochondrial calcium uniporter (MCU), that help regulate cytoplasmic calcium levels [2]. Cancer cells are characterized by modifications in calcium channel, pump, and binding protein function, leading to calcium concentrations that surpass the usual limits seen in healthy cells. This calcium surplus fosters cell growth and malignancy. Elevated intracellular calcium levels in many cancer types can be attributed to heightened activity or irregular control of specific calcium channels and pumps. Examples include the overactivity of certain members of the transient receptor potential (TRP) channel family, like TRPM3, TRPC1, TRPC6, TRPV4, and TRPV6, seen across a variety of tumors [3]. In addition, channels like TRPA1 are notably more active in cancers such as those of the breast and lung [4]. Regulated Cell Death (RCD), which includes apoptosis, autophagy, necrosis, etc., is vital for maintaining homeostasis in living organisms, and calcium signaling plays a pivotal role in controlling it [5]. Calcium’s central role in apoptosis is particularly significant due to its influence on the key molecular events and signaling pathways determining cell fate. Understanding calcium’s function in cell death can potentially uncover new therapeutic targets for illnesses related to dysregulated cell death.
2. The Role of Calcium in Cytosolic Protein-Mediated Apoptosis
2.1. Calcium/CAMK II/JNK/Fas Pathway
2.2. Calcium/Calcineurin/Bcl-2 Pathway
2.3. Calpain/Caspases Pathway
2.4. PKC/PDK/ERK Pathway
References
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003, 4, 517–529.
- Berridge, M.J. Calcium hypothesis of Alzheimer’s disease. Pflug. Arch. 2010, 459, 441–449.
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2007, 1772, 937–946.
- Gautier, M.; Dhennin-Duthille, I.; Ay, A.S.; Rybarczyk, P.; Korichneva, I.; Ouadid-Ahidouch, H. New insights into pharmacological tools to TR(i)P cancer up. Br. J. Pharmacol. 2014, 171, 2582–2592.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541.
- Jiang, S.J.; Wang, W. Research progress on the role of CaMKII in heart disease. Am. J. Transl. Res. 2020, 12, 7625–7639.
- Chi, M.; Evans, H.; Gilchrist, J.; Mayhew, J.; Hoffman, A.; Pearsall, E.A.; Jankowski, H.; Brzozowski, J.S.; Skelding, K.A. Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells. Sci. Rep. 2016, 6, 33132.
- Peshes-Yaloz, N.; Rosen, D.; Sondel, P.M.; Krammer, P.H.; Berke, G. Up-regulation of Fas (CD95) expression in tumour cells in vivo. Immunology 2007, 120, 502–511.
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Investig. 2009, 119, 2925–2941.
- Creamer, T.P. Calcineurin. Cell Commun. Signal. 2020, 18, 137.
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521.
- Saraf, J.; Bhattacharya, P.; Kalia, K.; Borah, A.; Sarmah, D.; Kaur, H.; Dave, K.R.; Yavagal, D.R. A Friend or Foe: Calcineurin across the Gamut of Neurological Disorders. ACS Cent. Sci. 2018, 4, 805–819.
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug. Discov. 2016, 15, 854–876.
- Ahn, Y.J.; Kim, M.S.; Chung, S.K. Calpain and Caspase-12 Expression in Lens Epithelial Cells of Diabetic Cataracts. Am. J. Ophthalmol. 2016, 167, 31–37.
- Mandic, A.; Viktorsson, K.; Strandberg, L.; Heiden, T.; Hansson, J.; Linder, S.; Shoshan, M.C. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: Two separate pathways in cisplatin-induced apoptosis. Mol. Cell. Biol. 2002, 22, 3003–3013.
- Chan, S.L.; Mattson, M.P. Caspase and calpain substrates: Roles in synaptic plasticity and cell death. J. Neurosci. Res. 1999, 58, 167–190.
- Isakov, N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin. Cancer Biol. 2018, 48, 36–52.
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 2012, 13, 10697–10721.





