Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sizhou Feng | -- | 2378 | 2023-05-29 16:55:31 | | | |
| 2 | Rita Xu | Meta information modification | 2378 | 2023-05-30 03:51:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, N.; Zhang, L.; Feng, S. Invasive Mucormycosis in Patients with Hematological Malignancies. Encyclopedia. Available online: https://encyclopedia.pub/entry/44975 (accessed on 07 February 2026).
Yang N, Zhang L, Feng S. Invasive Mucormycosis in Patients with Hematological Malignancies. Encyclopedia. Available at: https://encyclopedia.pub/entry/44975. Accessed February 07, 2026.
Yang, Nuobing, Lining Zhang, Sizhou Feng. "Invasive Mucormycosis in Patients with Hematological Malignancies" Encyclopedia, https://encyclopedia.pub/entry/44975 (accessed February 07, 2026).
Yang, N., Zhang, L., & Feng, S. (2023, May 29). Invasive Mucormycosis in Patients with Hematological Malignancies. In Encyclopedia. https://encyclopedia.pub/entry/44975
Yang, Nuobing, et al. "Invasive Mucormycosis in Patients with Hematological Malignancies." Encyclopedia. Web. 29 May, 2023.
Copy Citation
The incidence rate of invasive mucormycosis (IM) in patients with hematological malignancies (HMs) is increasing year by year, ranging from 0.07% to 4.29%, and the mortality rate is mostly higher than 50%. With the ongoing pandemic of COVID-19, COVID-19-associated mucormycosis (CAM) also became a global health threat.
invasive mucormycosis
hematological malignancies
high risk factors
1. Introduction
In recent years, the incidence rate of invasive mucormycosis (IM) in patients with hematological malignancies (HMs) increased, making it the most common disease among non-Aspergillus invasive mold infections (NAIMIs) [1]. Acute leukemia, allogeneic hematopoietic stem cell transplantation (allo-HSCT), chronic severe neutropenia, use of immunosuppressants, complicated with diabetes mellitus, iron overload, and use of deferoxamine to reduce serum iron are all high risk factors for HMs patients to be infected by Mucorales. Although IM is relatively rare in patients with HMs, its mortality rate is over 50% [2][3], which deserves clinical attention. In addition, the ongoing COVID-19 pandemic leads to immune dysregulation and increased use of steroids, thus making COVID-19 patients more susceptible to Mucorales and contributing to the surge of COVID-19-associated mucormycosis (CAM) cases.
Epidemiology and Risk Factors
The incidence rate of IM in patients with HMs is increasing year by year. An autopsy-based study showed that the prevalence of IM in patients with HMs increased from 0.006 cases per 100 autopsies in 1989–1993 to 0.018 cases per 100 autopsies in 2004–2008 (p = 0.04) [4]. Another study collected data from autopsy reports published during 2008–2013, and found that the incidence rate of invasive fungal infections in patients with HMs was 25% (711/2804), of which IM accounted for 6%, as the third commonest invasive fungal infection after invasive aspergillosis (IA) (55.5%) and invasive candidiasis (IC) (28.5%) [5]. A Spanish study reported that the prevalence of IM increased from 1.2 per 100,000 inpatients between 1988 and 2006 to 3.3 per 100,000 inpatients between 2007 and 2015, of which 52.6% had HMs [6]. From 2001 to 2006, the Transplant-Associated Infection Surveillance Network (TRANSNET) in America reported 44 IM (0.3%) of 15,820 HSCT patients [7]. A multicenter, retrospective study conducted in America found that 1133 of 962,428 HMs patients suffered from IM (0.12%) between 2007 and 2019 [8]. From 2007 to 2017, a survey of the Children’s Cancer Hospital in Egypt found 45 cases of proven IM among 13,735 hospitalized children (0.33%) who suffered from tumors [9].
There are apparent differences in the risk factors of IM among countries with geographical and economic differences. A prospective study in India between 2016 and 2017 on 465 patients diagnosed with proven IM showed that diabetes mellitus was the major risk factor associated with IM (n = 342, 73.5%), whereas epidemiological studies on patients with IM in Japan and North America showed that patients suffered from HMs accounted for 56.39% (75/133) and 61.2% (74/121) of the total number of patients with proven or probable IM, respectively, indicating that HMs were the most common underlying diseases in these countries [10][11][12]. Typically, those suffering from HMs have poorer prognosis than patients with other underlying diseases. An epidemiological study in France between 2005 and 2007 included 101 patients diagnosed with proven or probable IM, among whom 50 (50%) were complicated with HMs, 23 with diabetes (23%), and 18 with trauma (18%) [13]. The result showed that there was a significant difference in the mortality rate of these three types of patients infected with IM (60% vs. 32% vs. 11%, p = 0.008), and the mortality was higher for patients with HMs compared with patients with diabetes mellitus or with trauma.
Acute leukemia, neutropenia, steroid therapy, allo-HSCT, and graft-versus-host disease (GvHD) are factors contributing to high susceptibility to IM in patients with HMs. Table 1 collects data of risk factors for IM in patients with HMs or those undergoing HSCT from 9 studies. In a retrospective study of 32,815 patients with HMs and 1765 patients undergoing HSCT, the incidence rate of IM was highest in allo-HSCT recipients (1.19%), followed by acute lymphoblastic leukemia (0.75%) patients and acute myeloid leukemia (0.45%) patients and IM was associated with the shortest median survival time compared with IA and IC (3 months vs. 7 months vs. 7 months) [14]. Riches et al. found 6.01 cases of IM per 1000 patients who received allo-HSCT, and the high-risk factors for IM included history of Aspergillus infection (relative risk (RR) 4.91, p = 0.0007), preceding acute GVHD (RR 1.78, p = 0.027), and age > 50 years (RR 2.28, p = 0.0006) [15].
Table 1. Risk factors for IM in patients with HMs or HSCT.
| Characteristics of Studies | Risk Factors/Underlying Diseases, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Time Period |
Countries of Origin of Cases |
Total Number of Patients |
AL | Hyperglycemia | Neutropenia | Steroids | HSCT | GvHD | Voriconazole |
| Park et al., 2011 [7] | 2001–2006 | America | 105 | 28 (26.7%) | 46 (43.8%) | 39 (37.1%) | 59 (56.2%) | 76 (72.4%) | 61 (58.1%) | 47 (44.8%) |
| Kontoyiannis et al., 2000 [16] | 1989–1998 | America | 24 | 9 (37.5%) | 6 (25.0%) | 22 (91.7%) | 20 (83.3%) | 10 (41.7%) | 2 (8.3%) | NA |
| Kontoyiannis et al., 2014 [10] | 2004–2008 | North America | 74 | NA | 24 (32.4%) | 45 (60.8%) | 54 (73.0%) | 32 (43.2%) | 10 (13.5%) | 40 (54.1%) |
| Lanternier et al., 2012 [13] | 2005–2007 | France | 50 | 27 (54.0%) | 9 (18.0%) | 41 (82.0%) | 13 (26.0%) | 12 (24.0%) | 5 (10.0%) | NA |
| Xhaard et al., 2012 [17] | 2003–2008 | France | 29 | 12 (41.4%) | 14 (48.3%), | 6 (20.7%) | 26 (89.7%) | 29 (100%) | 22 (75.9%) | 12 (41.3%) |
| Pagano et al., 1997 [2] | 1987–1995 | Italy | 37 | 32 (86.5%) | NA | 33 (89.2%) | 37 (100.0%) | NA | NA | NA |
| Muggeo et al., 2019 [18] | 2009–2016 | Italy | 15 | 11 (73.3%) | 5 (33.3%) | 10 (66.6%) | 13 (86.6%) | 5 (33.3%) | NA | 3 (20.0%) |
| Pagano et al., 2004 [19] | 1987–2001 | Multi-center | 59 | 46 (78.0%) | 10 (16.9%) | 47 (79.7%) | 59 (100.0%) | 5 (8.5%) | NA | NA |
| Madney et al., 2019 [9] | 2007–2017 | Egypt | 45 | 39 (86.7%) | NA | 41 (91.1%) | 16 (35.6%) | 1 (2.2%) | 1 (2.2%) | 13 (28.9%) |
Breakthrough mucormycosis (BT-MCR) usually occurs in patients who are treated with antifungals without anti-Mucorales activity, mainly voriconazole and echinocandins. However, many studies found that HMs patients with high-risk factors such as active HMs, recurrent/refractory leukemia, prolonged neutropenia, and so on, will still have BT-MCR even under the prophylaxis or treatment of Mucorales-active antifungals, and such patients often have low survival rate and poor prognosis. By retrospectively analyzing the clinical data of 24 cases of breakthrough invasive mold infections that occurred during posaconazole (n = 8) or voriconazole (n = 16) prophylaxis among patients with HMs or undergoing transplantation (HSCT or lung transplantation) during 2009–2013 at Duke University, Lamoth et al. found that BT-MCR was one of the most common breakthrough invasive mold infections (9/24, 37.5%), of which seven cases received voriconazole and two cases received posaconazole for prophylaxis [20]. Another retrospective study of 145 HMs patients and HSCT recipients who received isavuconazole prophylaxis between 2016 and 2018 found that 12 patients (8.3%) had breakthrough invasive fungal infections including five cases of Aspergillus fumigatus, two cases of other Aspergillus species, two cases of Mucorales, two cases of Fusarium species, and one case of Candida glabrata, and all 12 patients had a median duration of neutropenia of 25.5 days and relapsed/refractory acute leukemia [21]. Between 2000 and 2020, 103 patients experienced BT-MCR in a single center, among whom 16 patients developed BT-MCR while on Mucorales-active antifungals (nine cases of isavuconazole, six cases of posaconazole, one case of AmB) and the other 87 patients developed BT-MCR while on antifungals without anti-Mucorales activity such as voriconazole, echinocandins, and itraconazole [22]. The 42-day mortality of patients developing BT-MCR while on Mucorales-active antifungals was higher than that of the remaining patients (63% vs. 25%, p = 0.006), and exposure to Mucorales-active antifungals was an independent predictor of death in patients with BT-MCR (hazard ratio (HR) 4.64, p < 0.001). Table 2 summarized characteristics of patients with BT-MCR.
Table 2. Characteristics of patients with BT-MCR.
| Reference | Number of Patients | Underlying Disease | BT-MCR Patients | Prophylactic Drugs | Characteristics of BT-MCR Patients |
|---|---|---|---|---|---|
| Rothe et al. (2021) [23] | 15 | AML, ALL, MDS, MM | 6 | Posaconazole (n = 5), isavuconazole (n = 1). | All patients required invasive mechanical ventilation and were treated with broad-spectrum antibiotics. |
| Lerolle et al. (2014) [24] | 270 | AML, GvHD | 2 | Posaconazole oral suspension. | Patients received broad spectrum antibiotics the month before BT-MCR onset, and were neutropenic at the time of BT-MCR onset. |
| Fontana et al. (2020) [21] | 145 | AML, MDS, HSCT | 2 | Isavuconazole. | Patients had a median duration of neutropenia of 25.5 days and relapsed/refractory acute leukemia. |
| Rausch et al. (2018) [25] | 100 | AML, ALL | 4 | Isavuconazole. | Patients were with prolonged neutropenia and relapsed/refractory leukemia at the time of BT-MCR. |
| Axell-House et al. (2021) [22] | 103 | Leukemia, MDS | 103 | Mucorales-active antifungals (9 cases of isavuconazole, 6 cases of posaconazole, 1 case of AmB); antifungals without anti-Mucorales activity (52 voriconazole, 22 echinocandins, 8 itraconazole, 5 echinocandin + voriconazole). | Patients developing BT-MCR while on Mucorales-active antifungals had a higher 42-day mortality (63% vs. 25%, p = 0.006). |
The recent surge in cases of CAM accompanied with the pandemic of the coronavirus disease 2019 (COVID-19) made it a global health threat. As of 7 June 2021, India recorded 28,252 cases of IM, among whom 24,370 cases with a history of COVID-19 [26]. Moreover, CAM cases were also reported worldwide including in Turkey, Egypt, China, America, Iran, Spain, and so on [27][28]. Most of these patients were complicated with poorly controlled blood glucose and were treated with heavy steroids and broad-spectrum antibiotics for SARS-CoV-2 infection, which resulted in weakened immune system and highly susceptibility to Mucorales [28][29]. Arora et al. conducted a case–control study comparing cases diagnosed with CAM with controls who recovered from COVID-19 without developing CAM [30]. A total of 152 patients of CAM (cases), including 120 proven and 32 probable, and 200 controls were included in the study. The result showed that diabetes (92.1% vs. 28%, p < 0.001), poor glycemic control (90.6% vs. 51.5%, p < 0.001), severe COVID-19 (21% vs. 9.9%, p < 0.001), systemic use of steroids (65.8% vs. 48%, p = 0.001) were more frequently observed in cases than controls.
A study in Australia on 74 patients diagnosed with proven or probable IM, among whom 36 (48.6%) were complicated with HMs, and found that Rhizopus spp. (20/36, 55.6%) was the most common genus of Mucorales organisms, followed by Mucor spp. (6/36, 16.7%) and Rhizomucor spp. (4/36, 11.1%) [31]. Another meta-analysis reported 851 patients with proven or probable IM, of which 275 (33%) were complicated with HMs, and it documented that Rhizopus spp. was the major pathogen of IM, followed by Mucor spp. (14%) and Lichtheimia spp. (13%) [32]. Other pathogenic genera included Apophysomyces spp., Cunninghamella spp., Rhizomucor spp., Saksenaea spp., and Synchephalastrum spp., but they were relatively rare. Of note, the mortality associated with Cunninghamella infections was remarkably higher than that caused by other genera of Mucorales organisms (71% vs. 44%, p < 0.001). The author of this research summarized the clinical data of 1568 patients with IM (whose underlying diseases included HMs, diabetes mellitus, trauma, etc.) from nine studies (which included more than 50 cases) [7][10][11][13][31][32][33][34][35], and found that the three major pathogenic genera were Rhizopus spp. (n = 778, 53.8%), Mucor spp. (n = 199, 13.8%), and Lichtheimia spp. (n = 152, 10.5%). Figure 1 illustrates the general distribution of common pathogenic genera of Mucorales organisms.
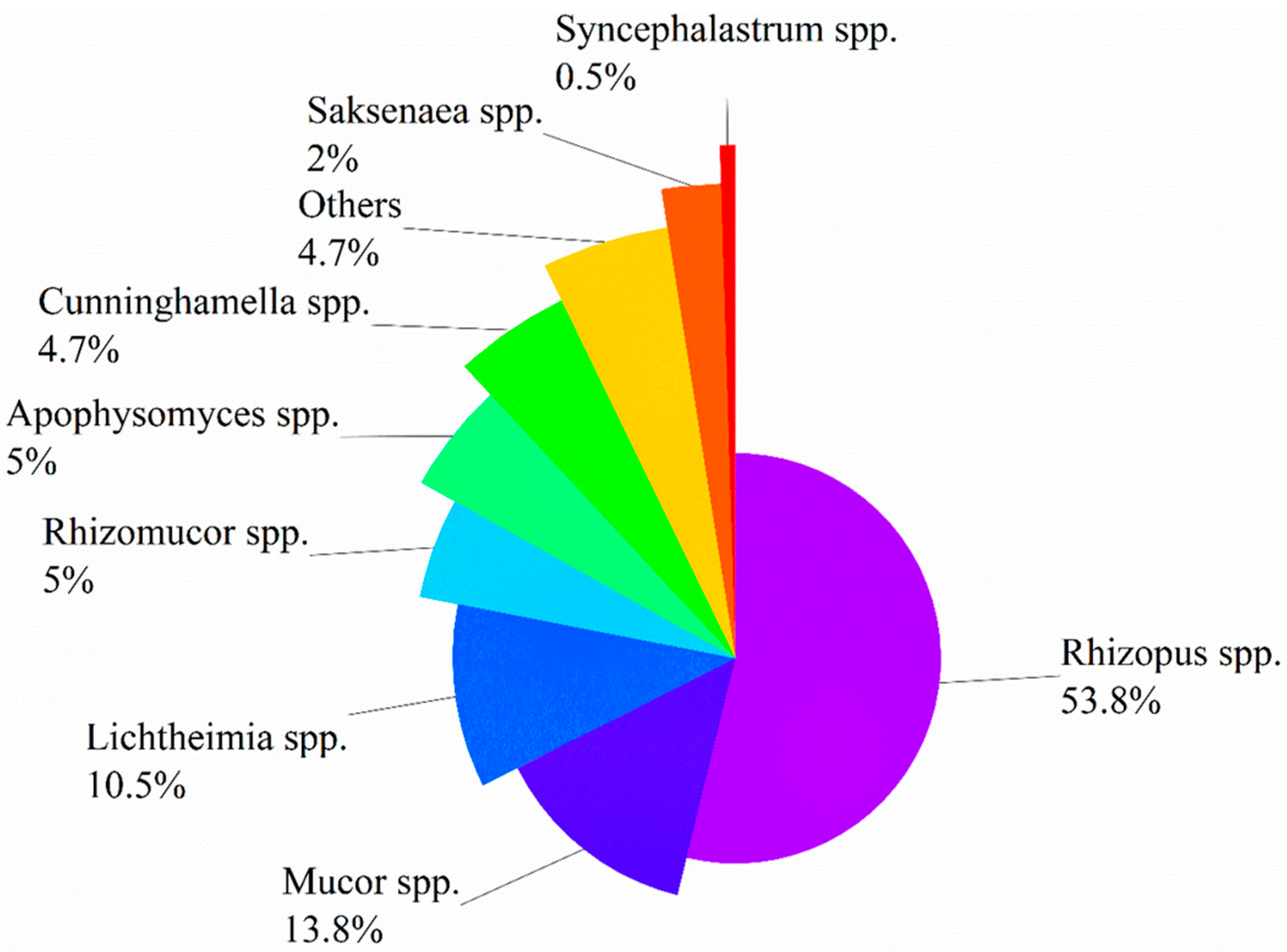
Figure 1. Distribution of Mucorales organisms causing infection.
To sum up, HMs are the most common risk factors associated with IM in developed countries, contrasting to diabetes mellitus in developing countries. Factors such as acute leukemia, neutropenia, allo-HSCT, and steroid therapy make patients with HMs highly susceptible to IM. BT-MCR still occurs in patients with active HMs, recurrent/refractory leukemia, and prolonged neutropenia under the prophylaxis of Mucorales-active antifungals, and such patients often have relatively high mortality and poor prognosis. Rhizopus spp. is the most common pathogen of IM, followed by Mucor spp. and Lichtheimia spp.
2. Clinical Manifestations
According to anatomic localizations and clinical manifestations, IM is divided into the following six clinical types: pulmonary mucormycosis (PM), rhino-orbital-cerebral mucormycosis (ROCM), disseminated mucormycosis, cutaneous/soft tissue mucormycosis, gastrointestinal mucormycosis and other rare forms, such as renal infection, endocarditis, osteomyelitis, peritonitis, and so on. CAM and IM in patients with diabetes mellitus usually manifest as ROCM. In contrast, most scholars believe that PM and disseminated mucormycosis occurs most often in patients with HMs. By analyzing cases of proven or probable IM in pediatric (≤19 years) patients, Pana et al. found that among 29 patients with PM, 22 had HMs, which were independently correlated with PM (odds ratio (OR) 4.4, p = 0.01) [36]. However, Slavin et al. analyzed 162 patients with NAIMIs (145 proven and 17 probable), of whom 74 (45.7%) were IM, and found HMs independently predicted disseminated infections (OR 2.7, p = 0.03) [35]. A study in Europe between 2005 and 2007 on 102 patients with HMs who were complicated with proven or probable IM indicated that the major sites of IM in these patients were pulmonary (n = 35, 34%) and disseminated (n = 28, 27%) [33]. On the other hand, in a retrospective study that included 20 patients with HMs who were diagnosed with proven IM, the most frequently involved site of IM was paranasal sinuses (n = 19, 95%) and PM only occurred in one patient (5%) [37]. Researchers collected and analyzed infection sites of 604 cases of IM in HMs patients and HSCT patients from 11 studies [2][3][7][9][10][11][13][17][19][33][36] which included more than 25 cases and had detailed records of involved sites of IM (some patients have two or more infection sites). The result showed that the most common form of IM in patients with HMs or patients undergoing HSCT is PM (44.4%), followed by ROCM (27.6%) and disseminated mucormycosis (16%), which is shown in Figure 2 and Figure 3.
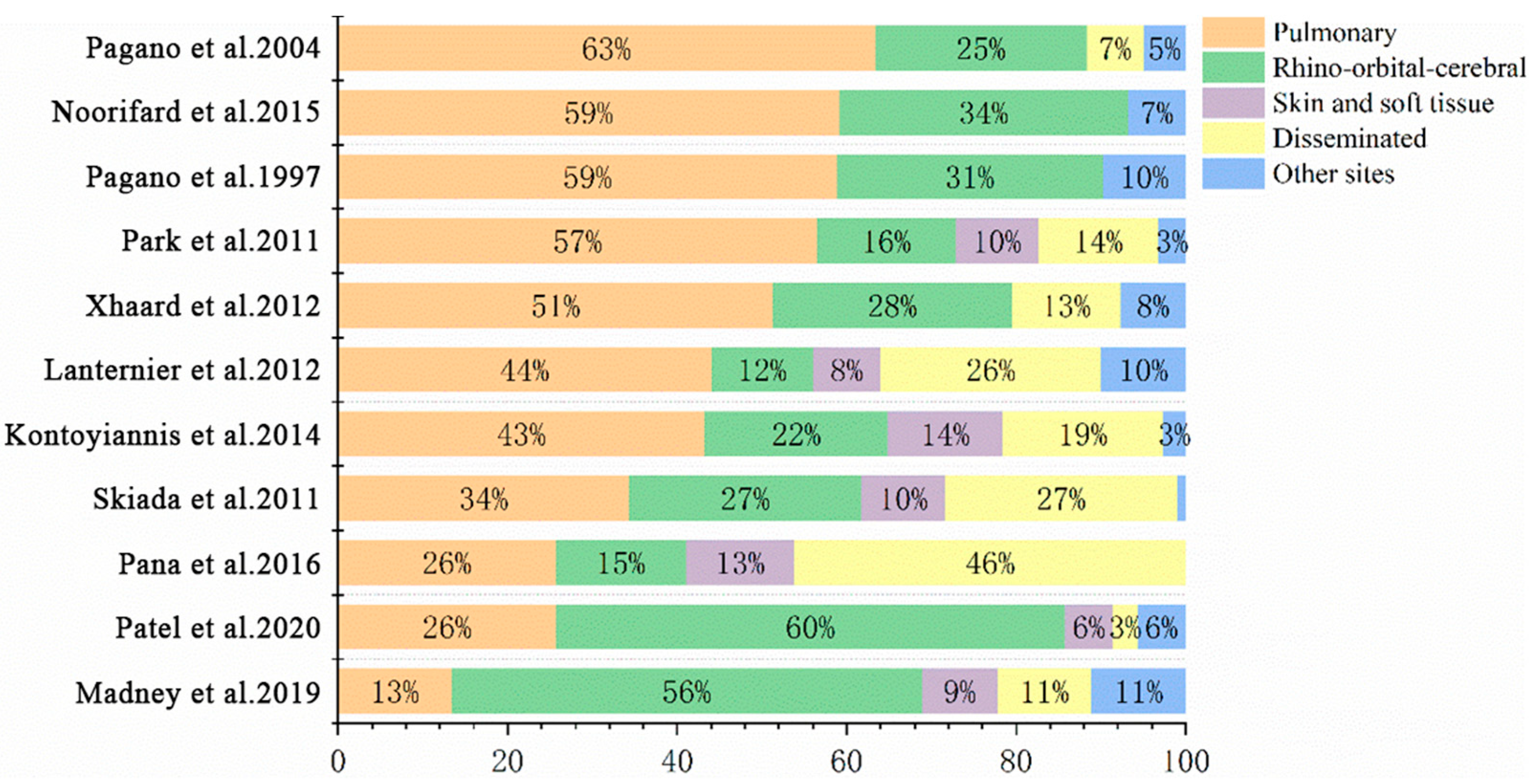
Figure 2. Sites of IM in patients with HMs.
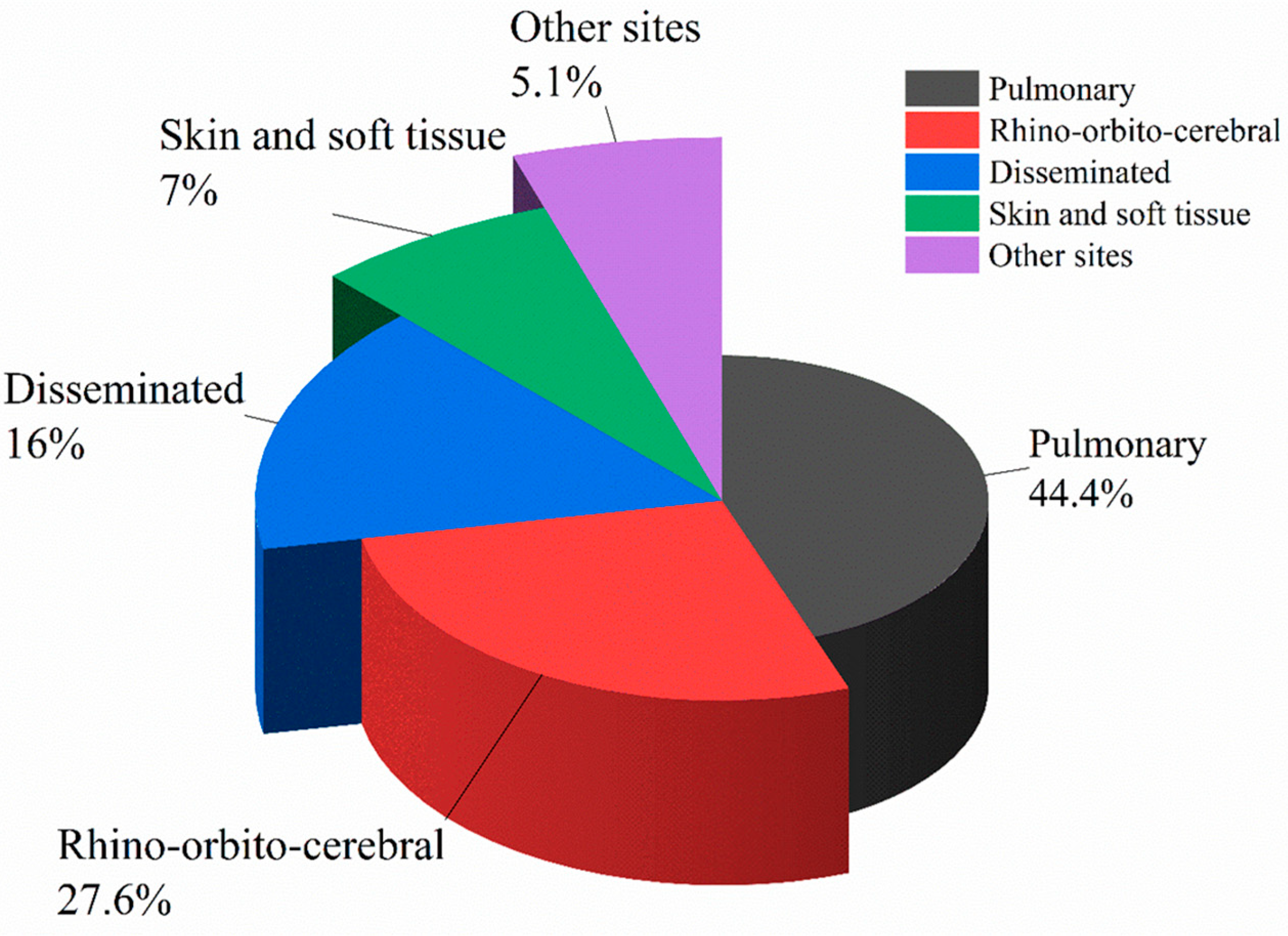
Figure 3. Proportion of various types of IM differentiated by sites in patients with HMs.
Fever is the most common clinical manifestation of IM, and almost all patients with IM have varying degrees of fever. Table 3 summarized clinical and imaging manifestations of PM, rhino-orbital mucormycosis, and central nervous system (CNS) mucormycosis. The triad of “cough, dyspnea, and chest pain” is a relatively specific sign of PM. Imaging manifestations of PM include exudation, cavity, ground-glass lesion, consolidation, pleural effusion, atelectasis, halo sign, reverse halo sign, air-crescent sign, etc. A retrospective study on HMs patients with IM or IA, including 59 proven IM patients and 541 proven IA patients showed that, compared with patients with IA, patients with IM had a significantly higher frequency of local pain syndrome (53% vs. 5%, p = 0.0001), hemoptysis (32% vs. 6%, p = 0.001), pleural effusion (53% vs. 7%, p = 0.003), destructive lesions (38% vs. 8%, p = 0.0001), and “reverse halo” sign (17% vs. 3%) [38]. In addition, although neutropenia and lymphocytopenia represented the major risk factors in both groups, patients with IM had a longer duration of severe neutropenia (30 vs. 14 days, p = 0.0001) and lymphocytopenia (25 vs. 14 days, p = 0.001) [38]. Both Jung et al. and Chamilos et al. compared the CT findings of PM and IA in patients with HMs, and they found the frequency of “reverse halo” sign (54% vs. 6% p < 0.001), multiple (≥10) nodules (64% vs. 18%, p = 0.02) and pleural effusion (63% vs. 33%, p = 0.1) in patients with PM were significantly higher than those in patients with IA [39][40]. The CT findings of patients with rhino-orbital mucormycosis include thickened oedematous mucosa, opacification or obliteration of paranasal sinuses and bony destruction, while MRI shows non-enhancing mucosal tissue within the involved sinuses and turbinates, also known as “black turbinate sign” [41][42]. CNS mucormycosis can be isolated, but it can also occur due to contiguous spread from the paranasal sinuses and orbits or hematogenous spread [43]. Compared with CT, MRI can identify intracranial infections, such as intraventricular “fungus balls”, thrombosis of intracranial arteries, the inflammatory alterations of the cavernous sinus and the involvement of adjacent structures (such as meninges), more sensitively and accurately [44].
Table 3. Clinical and imaging characteristics of IM.
| Clinical Manifestations | Imaging Manifestations | |
|---|---|---|
| Pulmonary mucormycosis | The triad of “cough, dyspnea, chest pain”, hemoptysis. | Exudation, cavity, ground-glass lesion, consolidation, pleural effusion, atelectasis, halo sign, reverse halo sign, air-crescent sign. |
| Rhino-orbital mucormycosis | Facial edema, pain, nasal congestion, rhinorrhea, eye pain, chemosis, proptosis, epiphora, and palatal ulcer destruction. | Thickened oedematous mucosa, opacification or obliteration of paranasal sinuses and bony destruction in CT, “black turbinate sign” in MRI. |
| Central nervous system mucormycosis | Headache, facial nerve palsy, ptosis, diplopia, hemiplegia, epilepsy. | Intraventricular “fungus balls”, thrombosis of intracranial arteries, the inflammatory alterations of the cavernous sinus and the involvement of adjacent structures (such as meninges). |
In conclusion, the most common form of IM in patients with HMs is PM, followed by ROCM and disseminated mucormycosis. “Reverse halo” sign, multiple (≥10) nodules, and pleural effusion are relatively specific imaging manifestations of patients with PM, while the imaging manifestations of ROCM patients are not very specific. MRI can better reflect the intracranial infections than CT.
References
- Valentine, J.C.; Morrissey, C.O.; Tacey, M.A.; Liew, D.; Patil, S.; Ananda-Rajah, M. A population-based analysis of attributable hospitalisation costs of invasive fungal diseases in haematological malignancy patients using data linkage of state-wide registry and costing databases: 2009–2015. Mycoses 2020, 63, 162–171.
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.; Cudillo, L.; Montillo, M.; Cenacchi, A.; Pacilli, L.; Fabbiano, F.; Del Favero, A. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto). Br. J. Haematol. 1997, 99, 331–336.
- Noorifard, M.; Sekhavati, E.; Khoo, H.J.; Hazraty, I.; Tabrizi, R. Epidemiology and clinical manifestation of fungal infection related to Mucormycosis in hematologic malignancies. J. Med. Life 2015, 8, 32–37.
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645.
- Dignani, M.C. Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000Prime Rep. 2014, 6, 81.
- Guinea, J.; Escribano, P.; Vena, A.; Munoz, P.; Martinez-Jimenez, M.D.C.; Padilla, B.; Bouza, E. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE 2017, 12, e0179136.
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864.
- Zhang, Y.; Sung, A.H.; Rubinstein, E.; Benigno, M.; Chambers, R.; Patino, N.; Aram, J.A. Characterizing patients with rare mucormycosis infections using real-world data. BMC Infect. Dis. 2022, 22, 154.
- Madney, Y.; Khedr, R.; Ahmed, N.; El-Mahallawy, H.; Youssef, A.; Taha, H.; Hassanain, O.; Ahmed, G.; Hafez, H. Overview and outcome of mucormycosis among children with cancer: Report from the Children’s Cancer Hospital Egypt. Mycoses 2019, 62, 984–989.
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance((R)): Focus on mucormycosis. Mycoses 2014, 57, 240–246.
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.S.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15.
- Ueno, R.; Nishimura, S.; Fujimoto, G.; Ainiwaer, D. The disease burden of mucormycosis in Japan: Results from a systematic literature review and retrospective database study. Curr. Med. Res. Opin. 2021, 37, 253–260.
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study, G. A global analysis of mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54 (Suppl. S1), S35–S43.
- Valentine, J.C.; Morrissey, C.O.; Tacey, M.A.; Liew, D.; Patil, S.; Peleg, A.Y.; Ananda-Rajah, M.R. A population-based analysis of invasive fungal disease in haematology-oncology patients using data linkage of state-wide registries and administrative databases: 2005–2016. BMC Infect. Dis. 2019, 19, 274.
- Riches, M.L.; Trifilio, S.; Chen, M.; Ahn, K.W.; Langston, A.; Lazarus, H.M.; Marks, D.I.; Martino, R.; Maziarz, R.T.; Papanicolou, G.A.; et al. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: A CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant. 2016, 51, 277–282.
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 2000, 30, 851–856.
- Xhaard, A.; Lanternier, F.; Porcher, R.; Dannaoui, E.; Bergeron, A.; Clement, L.; Lacroix, C.; Herbrecht, R.; Legrand, F.; Mohty, M.; et al. Mucormycosis after allogeneic haematopoietic stem cell transplantation: A French Multicentre Cohort Study (2003–2008). Clin. Microbiol. Infect. 2012, 18, E396–E400.
- Muggeo, P.; Calore, E.; Decembrino, N.; Frenos, S.; De Leonardis, F.; Colombini, A.; Petruzziello, F.; Perruccio, K.; Berger, M.; Burnelli, R.; et al. Invasive mucormycosis in children with cancer: A retrospective study from the Infection Working Group of Italian Pediatric Hematology Oncology Association. Mycoses 2019, 62, 165–170.
- Pagano, L.; Offidani, M.; Fianchi, L.; Nosari, A.; Candoni, A.; Picardi, M.; Corvatta, L.; D’Antonio, D.; Girmenia, C.; Martino, P.; et al. Mucormycosis in hematologic patients. Haematologica 2004, 89, 207–214.
- Lamoth, F.; Chung, S.J.; Damonti, L.; Alexander, B.D. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621.
- Fontana, L.; Perlin, D.S.; Zhao, Y.; Noble, B.N.; Lewis, J.S.; Strasfeld, L.; Hakki, M. Isavuconazole Prophylaxis in Patients With Hematologic Malignancies and Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2020, 70, 723–730.
- Axell-House, D.B.; Wurster, S.; Jiang, Y.; Kyvernitakis, A.; Lewis, R.E.; Tarrand, J.J.; Raad, I.I.; Kontoyiannis, D.P. Breakthrough Mucormycosis Developing on Mucorales-Active Antifungals Portrays a Poor Prognosis in Patients with Hematologic Cancer. J. Fungi 2021, 7, 217.
- Rothe, K.; Braitsch, K.; Okrojek, R.; Heim, M.; Rasch, S.; Verbeek, M.; Schmid, R.M.; Busch, D.H.; Lahmer, T. Clinical and microbiological features and outcomes of mucormycosis in critically ill patients. Int. J. Infect. Dis. 2021, 109, 142–147.
- Lerolle, N.; Raffoux, E.; Socie, G.; Touratier, S.; Sauvageon, H.; Porcher, R.; Bretagne, S.; Bergeron, A.; Azoulay, E.; Molina, J.M.; et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: A 4-year study. Clin. Microbiol. Infect. 2014, 20, O952–O959.
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough Fungal Infections in Patients With Leukemia Receiving Isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613.
- Mahalaxmi, I.; Jayaramayya, K.; Venkatesan, D.; Subramaniam, M.D.; Renu, K.; Vijayakumar, P.; Narayanasamy, A.; Gopalakrishnan, A.V.; Kumar, N.S.; Sivaprakash, P.; et al. Mucormycosis: An opportunistic pathogen during COVID-19. Environ. Res. 2021, 201, 111643.
- Dilek, A.; Ozaras, R.; Ozkaya, S.; Sunbul, M.; Sen, E.I.; Leblebicioglu, H. COVID-19-associated mucormycosis: Case report and systematic review. Travel Med. Infect. Dis. 2021, 44, 102148.
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021, 64, 1452–1459.
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260.
- Arora, U.; Priyadarshi, M.; Katiyar, V.; Soneja, M.; Garg, P.; Gupta, I.; Bharadiya, V.; Berry, P.; Ghosh, T.; Patel, L.; et al. Risk factors for Coronavirus disease-associated mucormycosis. J. Infect. 2022, 84, 383–390.
- Kennedy, K.J.; Daveson, K.; Slavin, M.A.; van Hal, S.J.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Chapman, B.; Halliday, C.L.; Hajkowicz, K.; et al. Mucormycosis in Australia: Contemporary epidemiology and outcomes. Clin. Microbiol. Infect. 2016, 22, 775–781.
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34.
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867.
- Otto, W.R.; Pahud, B.A.; Yin, D.E. Pediatric Mucormycosis: A 10-Year Systematic Review of Reported Cases and Review of the Literature. J. Pediatr. Infect. Dis. Soc. 2019, 8, 342–350.
- Slavin, M.; van Hal, S.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Daveson, K.; Kennedy, K.; Hajkowicz, K.; Halliday, C.; Athan, E.; et al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin. Microbiol. Infect. 2015, 21, 490.e1–490.e10.
- Pana, Z.D.; Seidel, D.; Skiada, A.; Groll, A.H.; Petrikkos, G.; Cornely, O.A.; Roilides, E.; Collaborators of Zygomyco.net and/or FungiScope™ Registries. Invasive mucormycosis in children: An epidemiologic study in European and non-European countries based on two registries. BMC Infect. Dis. 2016, 16, 667.
- Kara, I.O.; Tasova, Y.; Uguz, A.; Sahin, B. Mucormycosis-associated fungal infections in patients with haematologic malignancies. Int. J. Clin. Pract. 2009, 63, 134–139.
- Klimko, N.; Khostelidi, S.; Shadrivova, O.; Volkova, A.; Popova, M.; Uspenskaya, O.; Shneyder, T.; Bogomolova, T.; Ignatyeva, S.; Zubarovskaya, L.; et al. Contrasts between mucormycosis and aspergillosis in oncohematological patients. Med. Mycol. 2019, 57 (Suppl. S2), S138–S144.
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18.
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin. Infect. Dis. 2005, 41, 60–66.
- Han, Q.; Escott, E.J. The Black Turbinate Sign, A Potential Diagnostic Pitfall: Evaluation of the Normal Enhancement Patterns of the Nasal Turbinates. AJNR Am. J. Neuroradiol. 2019, 40, 855–861.
- Kim, J.H.; Kang, B.C.; Lee, J.H.; Jang, Y.J.; Lee, B.J.; Chung, Y.S. The prognostic value of gadolinium-enhanced magnetic resonance imaging in acute invasive fungal rhinosinusitis. J. Infect. 2015, 70, 88–95.
- Lersy, F.; Royer-Leblond, J.; Lhermitte, B.; Chammas, A.; Schneider, F.; Hansmann, Y.; Lefebvre, N.; Denis, J.; Sabou, M.; Lafitte, F.; et al. Cerebral mucormycosis: Neuroimaging findings and histopathological correlation. J. Neurol. 2021, 269, 1386–1395.
- Andreani, G.; Fadda, G.; Gned, D.; Dragani, M.; Cavallo, G.; Monticone, V.; Morotti, A.; De Gobbi, M.; Guerrasio, A.; Barbui, A.M.; et al. Rhino-Orbital-Cerebral Mucormycosis after Allogeneic Hematopoietic Stem Cell Transplantation and Isavuconazole Therapeutic Drug Monitoring during Intestinal Graft versus Host Disease. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019061.
More
Information
Subjects:
Infectious Diseases; Hematology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
534
Revisions:
2 times
(View History)
Update Date:
30 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




