Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Phan Anh Duong | -- | 2761 | 2023-05-29 10:16:52 | | | |
| 2 | Sirius Huang | + 1 word(s) | 2762 | 2023-05-30 03:17:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Duong, P.A.; Ryu, B.R.; Song, M.K.; Nguyen, H.V.; Nam, D.; Kang, H. Hazards of the Ammonia Bunkering Process. Encyclopedia. Available online: https://encyclopedia.pub/entry/44953 (accessed on 09 March 2026).
Duong PA, Ryu BR, Song MK, Nguyen HV, Nam D, Kang H. Hazards of the Ammonia Bunkering Process. Encyclopedia. Available at: https://encyclopedia.pub/entry/44953. Accessed March 09, 2026.
Duong, Phan Anh, Bo Rim Ryu, Mi Kyoung Song, Hong Van Nguyen, Dong Nam, Hokeun Kang. "Hazards of the Ammonia Bunkering Process" Encyclopedia, https://encyclopedia.pub/entry/44953 (accessed March 09, 2026).
Duong, P.A., Ryu, B.R., Song, M.K., Nguyen, H.V., Nam, D., & Kang, H. (2023, May 29). Hazards of the Ammonia Bunkering Process. In Encyclopedia. https://encyclopedia.pub/entry/44953
Duong, Phan Anh, et al. "Hazards of the Ammonia Bunkering Process." Encyclopedia. Web. 29 May, 2023.
Copy Citation
Ammonia is thought to be a potential alternative for hydrogen storage in the future, allowing for CO2-free energy systems. Ammonia’s beneficial characteristics with regard to hydrogen storage include its high volumetric hydrogen density, low storage pressure, and long-term stability. However, ammonia is characterized by toxicity, flammability, and corrosiveness, making safety a challenge compared to other alternative fuels. In specific circumstances, leakage from ammonia bunkering can cause risks, dispersion, and unsafe areas due to its flammability and toxicity. To avoid dispersion, fire, and explosion hazards on ships, it is crucial to conduct thorough risk analyses.
ammonia
marine vessels
risk assessment
1. General Information and Physical Properties of Ammonia
Ammonia is composed of one nitrogen and three hydrogen atoms (NH3). The calorific value of ammonia is 22.5 MJ/kg [1]. Ammonia is a colorless gas with a pungent odor [2] that consists of 17.6% hydrogen by weight [3]. The average unit price of ammonia is about USD 250–300 [4]. The contribution of the ammonia production process to the total GHG emissions of the world has been estimated as about 1% [5]. Ammonia is known to have a variety of advantageous properties as fuel, making it appealing as a possible medium for hydrogen storage. The volumetric hydrogen density of ammonia is 45% greater than that of liquid hydrogen. This suggests that there is more hydrogen in liquid ammonia than there is in an equivalent volume of liquid hydrogen [6]. Compared to ethanol, methanol, liquid hydrogen, and gasoline, ammonia is a hydrogen carrier with a higher volumetric hydrogen density [7]. The storage of ammonia is simpler than the storage of hydrogen, the other carbon-free fuel. The storage of ammonia takes place either at room temperature at 10 bars or at 33 °C at 1 bar [2]. The basic properties of ammonia are presented in Table 1.
| Properties | Unit | Value |
|---|---|---|
| Energy density | MJ/L | 12.7 |
| Density at standard pressure and temperature | Kg/m3 | 0.769 |
| Latent heat of vaporization | MJ/kg | 188 |
| Vapor pressure at 20 °C | kPa | 858 |
| Heat of vaporization | kJ/kg | 1371 |
| Autoignition temperature | °C | 651 |
| Heat capacity at constant pressure | kJ/mol.°C | 0.037 |
| Minimum ignition energy | mJ | 680 |
| Heat capacity at constant volume | kJ/mol.°C | 0.028 |
| Liquid density | kg/m3 | 600 |
| Adiabatic flame temperature at 1 bar | °C | 1800 |
| Molar mass | g/mol | 17.031 |
| Melting point | °C | −77.7 |
| Condensation pressure at 25 °C | MPa | 0.99 |
| Boiling point at 1 bar | °C | −33.6 |
| Critical temperature | °C | 132.25 |
| Critical pressure | bar | 113 |
| Adiabatic flame temperature | °C | 1800 |
| Flammable range in dry air | % | 15.15 to 27.35 |
| Max. laminar burning | m/s | 0.07 |
| Cetane number | 0 | |
| Octane number | ~130 |
Special safety precautions are necessary for the storage of ammonia given its toxic and corrosive nature. Compared to commonly used fuels such as methanol and diesel, the hazard level of ammonia is over three times higher [11]. The event of an ammonia leak into water can be harmful to aquatic life, but its natural degradation process and the nitrogen cycle can facilitate the regeneration of aquatic life. It should be noted that ammonia has a very low odor threshold (0.037 to 1.0 ppm), making it detectable by most individuals even in small amounts that do not pose a health risk.
Gaseous ammonia has a lower density than air (1.225 kg/m3 compared to 0.769 kg/m3 at STP), and under normal atmospheric conditions, it can quickly dissipate into the atmosphere, lowering the risk of explosion or fire in the event of a leak. In addition, compared to hydrogen, which has an auto-ignition temperature of 520 °C, ammonia’s higher auto-ignition temperature (650 °C) means a lower risk of fire. Liquid ammonia is highly toxic and has a vapor pressure relative to toxicity at atmospheric temperature that is roughly three orders of magnitude higher than those of gasoline and methanol.
2. Ammonia Bunkering Methods
Bunkering is a vital operation that supplies fuel to power the machinery of a ship. Ammonia bunkering, similar to that of alternative fuels such as LNG, LPG, and hydrogen, can be categorized into four main types: ship-to-ship (STS), terminal-to-ship (TTS), truck-to-ship (T-TS), and ammonia portable tank (APT). The suitable ammonia bunkering method is selected after considering the amount of ammonia bunkering required, operational circumstances, and time constraints. Figure 1 illustrates the three most common ammonia bunkering methods.
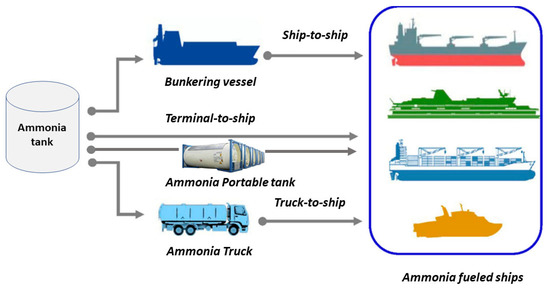
Figure 1. Ammonia bunkering methods.
A comparison of the advantages and disadvantages of each ammonia bunkering method is shown below in Table 2.
Table 2. Comparison of ammonia bunkering methods.
| Bunkering Methods | Advantages | Disadvantages |
|---|---|---|
| STS | Suitable for medium and large ammonia vessels. Quick, saving bunkering time. |
High degree of investment required. Bunkering procedure affected by weather and sea conditions. |
| TTS | Quick ammonia bunkering. Available for all kinds of ammonia vessels, especially large-scale vessels. |
Investment and construction cost of ammonia bunkering terminal is high. Bunkering only available for designated ports. |
| T-TS | Low cost of construction and operation. Suitable for small-scale ammonia bunkering. |
Speed of ammonia bunkering is slow. Limited for large ammonia vessels. |
| APT | Quick ammonia bunkering speed. Flexible bunkering location. Simple process. |
High costs for tank operation and maintenance. Suitable for small-scale ships only. |
3. Hazards of the Ammonia Bunkering Process
The dangers of ammonia can be grouped under three characteristics: (i) toxicity, (ii) corrosiveness, and (iii) flammability.
3.1. Toxic Effects of Ammonia on Humans
Ammonia is a gas that is colorless and poisonous, and it has a strong pungent smell at concentrations between 5 and 30 ppm. This gas has a lower density than air, and liquid ammonia can cause severe burns and injuries if it comes into contact with skin. There is a high risk of death when large quantities of ammonia are released, as it can form a toxic cloud that can be inhaled far away from the release site. Fatalities usually occur when people are exposed to high concentrations of the gas, or if they are trapped without an escape route, due to the strong odor of ammonia, which is intolerable at concentrations well below those that are harmful. Ammonia rapidly absorbs water and can cause dehydration upon contact with skin, while anhydrous ammonia can cause a loss of water from body tissues and chemical burns via the production of ammonium hydroxide. Additionally, frostbite can occur when liquid ammonia vaporizes, causing the removal of heat from body tissues within seconds. The effects of ammonia on human health are presented in Table 3.
Table 3. Effects of ammonia on humans [12].
| Ammonia Content (ppm) |
Impacts to Humans |
|---|---|
| 5–10 | Odor-based detection |
| 20–50 | Mild discomfort |
| 50–100 ppm for 2 h | Annoyance and inconvenience |
| 140 ppm for 2 h | Some irritation |
| 200–300 | Effects on throat and eyes |
| 300–500 | Bearable for 20–60 min |
| 2500–5000 | Rapidly fatal (life-threatening around 30 min) |
| 5000–10,000 | Promptly lethal |
The probability of human death due to the effects of an ammonia vapor cloud formed following a leak can be estimated by [13].
where Pd_toxic represents the probability of human death by exposure to toxic ammonia and Pd represents the probability of human death.
Here, t represents exposure time, C represents the cloud dispersion concentration (mg/m3), and erf(x) represents the Gaussian error.
It is important to note that the effects of ammonia toxicity can be cumulative, meaning that repeated exposure even to low concentrations of ammonia over a prolonged period can have significant health consequences. Therefore, it is essential to take appropriate precautions to prevent exposure to ammonia, such as wearing appropriate personal protective equipment (PPE) and working in well-ventilated areas.
3.2. Toxic Effects of Ammonia on the Environment
When ammonia leaks occur in seawater during bunkering, the absorbed ammonia can have a severe impact on aquatic life, as lethal levels are easily surpassed, causing death to most species in close proximity. Due to its exothermic reaction with water, ammonia quickly evaporates. As ammonia gas is lighter than air, it will rise to the top of the atmosphere in the form of a cloud. However, this ammonia gas cloud can pose a significant danger to creatures in its immediate vicinity, as it can expose them to deadly amounts of ammonia. This cloud remains a threat until it is completely diluted through the processes of cloud evaporation and continuous air mixing.
Overall, it is important to minimize the release of ammonia into the environment by taking appropriate precautions during storage, handling, and use. This includes proper ventilation, safe disposal methods, and the careful use of fertilizers and other ammonia-based products.
3.3. Flammability of Ammonia
The auto-ignition temperature of ammonia under atmospheric conditions is 651 °C, with a flammable range of 15.15–27.35%. Compared to other fuels, the likelihood of ammonia auto-igniting is extremely low due to its high minimum ignition energy of 680 mJ, which is 2000 times greater than that of CH4. However, if ammonia spills from a high-pressure storage container, it can cause severe harm since it is lighter than air and diffuses more quickly. Moreover, due to the increasing use of hydrogen as an alternative for fuel storage, refrigeration, and the post-treatment of combustion exhaust gas in industrial settings, it is important to implement fire protection measures.
Similar to other hydrocarbons, condensed ammonia does not burn continuously. The reason for this is that the heat emitted from the flames is not sufficient to reach the pool. If an external heat source such as the ground or water is present, enough ammonia can vaporize to keep the fire burning.
As presented in Figure 2, the primary dangers related to ammonia bunkering are fires and explosions, which may occur due to leaks and spills around ignition sources. Without ignition, ammonia dissipates by vaporization and forms a vapor cloud that disperses in the air. However, in the event of ignition, there are four potential risk scenarios for ammonia, including vapor cloud flash fire, jet fire, pool fire, and vapor cloud explosion. Additionally, the consequences of ammonia fires and explosions are dependent on factors such as the initial temperature and composition of the ammonia and the diameter of the pool fire. Compared to LNG and LPG, ammonia has a lower risk of fire due to its lower burning rate.
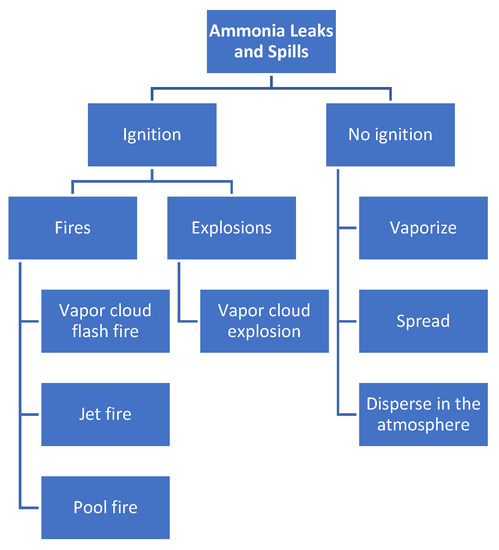
Figure 2. Main hazards of the ammonia bunkering process.
The use of water spray, fog, or foam can be effective in extinguishing large ammonia flames, while dry chemicals or CO2 are more appropriate for small ammonia fires. However, it is important to avoid directing a water jet directly towards a leak or liquid ammonia source as this may cause a hazardous reaction. Responders must always wear protective equipment with an oxygen supply, even if the ammonia concentration is as low as 25 ppm.
3.4. Corrosiveness
Ammonia is a substance that can cause corrosion and harm to various materials, such as metals, plastics, and rubber. The corrosive effect of ammonia is due to its ability to react with water and form ammonium hydroxide, which is strongly alkaline in solution. The following are some of the ways in which ammonia can cause corrosion:
Metals: Ammonia can cause the corrosion of metals, particularly those that are not resistant to alkaline substances. When ammonia comes into contact with metal surfaces, it can cause the metal to become pitted, corroded, or even discolored. This can lead to weakened structural integrity and the potential failure of metal components.
Plastics: Ammonia can also cause damage to certain types of plastics, particularly those that are not resistant to alkaline substances. When ammonia comes into contact with plastic surfaces, it can cause the plastic to become brittle, cracked, or even discolored. This can lead to the potential failure of plastic components, particularly those that are subjected to stress or pressure.
Rubber: Ammonia can cause damage to rubber materials, particularly those that are not resistant to alkaline substances. When ammonia comes into contact with rubber surfaces, it can cause the rubber to become soft, swollen, or even disintegrate. This can lead to the potential failure of rubber components, particularly those that are subjected to pressure or friction.
Overall, it is important to handle ammonia carefully and take appropriate precautions to prevent its exposure to materials that are vulnerable to its corrosive effects. This includes proper storage and handling procedures, as well as the use of protective coatings and materials that are resistant to alkaline substances.
3.5. Other Hazards
Ammonia is highly susceptible to hydraulic shock due to its high boiling point and expansion rate. Hydraulic shock is the result of a sudden change in liquid flow velocity, causing a localized pressure surge that may lead to severe damage to equipment, valves, and piping. When a refrigerated system is exposed to a defrosted system, this may cause hydraulic shock.
Additionally, the release of pressurized ammonia into the environment poses another risk. About 8–9% of the ammonia in a container will vaporize and expand rapidly once released, continuing to do so even after the pressure is brought down. Ammonia can expand up to 710 times from its liquid state to a vapor state, and it can continue to evaporate even after it rains out. A catastrophic tank failure at room temperature could result in a significant release of ammonia.
A BLEVE (boiling liquid expanding vapor explosion) is a type of physical explosion that can occur when a pressurized liquid boils rapidly due to a loss in pressure. For a BLEVE to occur, the temperature of the liquid at the time of pressure loss must be higher than its superheating level (TSL). The critical temperature for ammonia is 89.8 °C, which is significantly higher than its recommended ambient and storage temperatures. Therefore, it can be assumed that the risk of a BLEVE is low.
3.6. Accidents in Ammonia Bunkering
Ammonia is denser than air and can cause harm to humans when it is released into the environment, especially to the eyes, nose, and respiratory system. At a temperature of −33.33 °C, liquid ammonia is less dense than seawater with a density of 0.696 g/cm3, which makes it float in seawater [14]. It is difficult to ignite ammonia due to its high auto-ignition temperature of approximately 651 °C and narrow flammable range between 15.75% and 27.35%. In the case of a leak, ammonia has the potential to corrode various metals and compounds, including copper and zinc. Hence, it is vital to have a venting mechanism in place that can safely release ammonia during an emergency. Table 1 provides additional information on the properties of ammonia.
The widespread application of ammonia in the industrial sector has led to significant consequences for society, property, the environment, and facilities, particularly during unexpected incidents. According to statistics, from 1985 to 2019, there were approximately 71 accidents involving anhydrous ammonia. The primary causes of deaths and injuries were identified as inhalation of the gas or fires [15]. Chemical-based hazards have a high percentage of injuries, fatalities, and evacuations, which is in line with the alarming number of serious incidents caused by ammonia leaks. Accidents involving ammonia explosions and dispersion can be caused by various factors, such as mechanical failures, operational difficulties, and human error.
There have been numerous incidents where ammonia leaked and negatively impacted the ecosystem. Typical accidents caused by released ammonia are described in Table 4.
Table 4. Records of ammonia incidents.
| Reference | Time | Place | Accident Description | Results |
|---|---|---|---|---|
| Junior et al. [16] | 2007 | Brazil | Ammonia was released from a fish cooperative in Brazil (around 40 kg of AA released). | There were 2 deaths and 18 persons presenting symptoms. |
| Ojha et al. [17] | 1973 | South Africa | An ammonia tank rupture resulted in 38 tons of ammonia leaked. | This accident caused 18 deaths. The ammonia cloud formed by dispersion had a diameter of about 150 m and a depth of 20 m. |
| U.S. CSB [18] | 23 August 2010 | Theodore, Alabama | The catastrophic rupture of a 12-inch pipe caused around 32,000 pounds of ammonia to be released. | The dispersion cloud of ammonia reached about 0.25 miles in size. About 800 contractors at a nearby shipyard were affected. Inside the facility, the ammonia concentration was recorded as about 500 to 600 ppm. |
| Reuters [19] | 31 August 2013 | Shanghai, China | Liquid ammonia leaked from a refrigeration plant. The reason was reported as pipe/valve failure. | There were about 15 deaths and 25 injured. The ammonia dispersion affected residents 15 km downwind from the leakage point. |
| Jain et al. [15] | 7 July 2017 | Elk Grove Village, Illinois | Ammonia leaked from a room (80 × 40 × 20 feet) under a pressure of 150 psi. | About 100 employees were affected. The building was damaged by the explosion. |
Based on the analysis of typical accidents in ammonia plants and operations, researchers determined that ammonia can be released due to various reasons, such as human error, operational mistakes, and maintenance and inspection failures during storage tank operations, the bunkering process, and pipeline operations. A release of ammonia may pose a threat to nearby areas, and its level of toxicity depends on the extent of diffusion, which can be estimated based on the release grade.
The key method to prevent unforeseen incidents and reduce the negative impact of ammonia release on people and facilities is to conduct a risk and consequence analysis for potential ammonia leakage. Therefore, it is essential to create a safety zone that prohibits unauthorized individuals and vehicles from operating within the ammonia bunkering area.
References
- Al-Enazi, A.; Okonkwo, E.C.; Bicer, Y.; Al-Ansari, T. A review of cleaner alternative fuels for maritime transportation. Energy Rep. 2021, 7, 1962–1985.
- Ayvali, T.; Edman Tsang, S.C.; Van Vrijaldenhoven, T. The position of ammonia in decarbonising maritime industry: An overview and perspectives: Part I. Johns. Matthey Technol. Rev. 2021, 65, 275–290.
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108.
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310.
- Bicer, Y.; Dincer, I.; Zamfirescu, C.; Vezina, G.; Raso, F. Comparative life cycle assessment of various ammonia production methods. J. Clean. Prod. 2016, 135, 1379–1395.
- Le Fevre, C. A Review of Demand Prospects for LNG as a Marine Transport Fuel; Oxford Institute for Energy Studies: Oxford, UK, 2018.
- Inal, O.B.; Dere, C.; Deniz, C. Onboard hydrogen storage for ships: An overview. In Proceedings of the Fifth International Hydrogen Technologies Congress, Online, 26–28 May 2021.
- National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia (accessed on 1 March 2023).
- Jeong, S.Y.; Jang, D.; Lee, M.C. Property-based quantitative risk assessment of hydrogen, ammonia, methane, and propane considering explosion, combustion, toxicity, and environmental impacts. J. Energy Storage 2022, 54, 105344.
- Li, M.; Zhu, D.; He, X.; Moshammer, K.; Fernandes, R.; Shu, B. Experimental and kinetic modeling study on auto-ignition properties of ammonia/ethanol blends at intermediate temperatures and high pressures. Proc. Combust. Inst. 2022; in press.
- Mckinlay, C.J.; Turnock, S.R.; Hudson, D.A. A Comparison of Hydrogen and Ammonia for Future Long Distance Shipping Fuels. In Proceedings of the LNG/LPG and Alternative Fuels, London, UK, 29–30 January 2020.
- Class NK. Part C ‘Guidelines for the Safety of Ships Using Ammonia as Fuel’ of Guidelines for Ships Using Alternative Fuels; Class NK: Tokyo, Japan, 2018; pp. 63–73.
- Declerck, L. Quantitative risk assessment. Top. Model. Clust. Data 2002, 6, 157–172.
- Dharmavaram, S.; Tilton, J.; Gardner, R. Fate and transport of ammonia spilled from a barge. J. Hazard. Mater. 1994, 37, 475–487.
- Jain, P. What Has the Industry Experience Been with Ammonia Manufacturing Plants? What Is Their Track Record for Having Serious Process Safety Incidents? What Root Causes Have Typically Led to Them? 2019. Available online: https://engineering.purdue.edu/P2SAC/presentations/documents/Industry-Experience-With-Ammonia-Manufacturing-Plants-Fall-2019.pdf (accessed on 20 March 2023).
- Junior, M.M.; e Santos, M.S.; Vidal, M.; Carvalho, P. Overcoming the blame game to learn from major accidents: A systemic analysis of an Anhydrous Ammonia leakage accident. J. Loss Prev. Process. Ind. 2012, 25, 33–39.
- Ojha, M.; Dhiman, A. Problem, Failure and Safety Analysis of Ammonia Plant: A Review. Int. Rev. Chem. Eng. 2010, 2, 631–646.
- U.S. Chemical Safety and Hazard Investigation Board. Key Lessons for Preventing Hydraulic Shock in Industrial Refrigeration Systems Anhydrous Ammonia Release at Millard; U.S. Chemical Safety and Hazard Investigation Board: Washington, DC, USA, 2015; pp. 1–15.
- Reuters. 2013. Available online: www.reuters.com (accessed on 20 March 2023).
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
30 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





