
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jialong Shen | -- | 3376 | 2023-05-26 16:58:49 | | | |
| 2 | Rita Xu | Meta information modification | 3376 | 2023-05-29 03:01:59 | | | | |
| 3 | Rita Xu | Meta information modification | 3376 | 2023-05-29 09:03:12 | | |
Video Upload Options
Fibrous membranes offer broad opportunities to deploy immobilized enzymes in new reactor and application designs, including multiphase continuous flow-through reactions. Enzyme immobilization is a technology strategy that simplifies the separation of otherwise soluble catalytic proteins from liquid reaction media and imparts stabilization and performance enhancement. Flexible immobilization matrices made from fibers have versatile physical attributes, such as high surface area, light weight, and controllable porosity, which give them membrane-like characteristics, while simultaneously providing good mechanical properties for creating functional filters, sensors, scaffolds, and other interface-active biocatalytic materials.
1. Introduction
| Class | Name | Catalyzed Reaction |
|---|---|---|
| 1 | Oxidoreductases | AH2 + B = A + BH2 or AH2 + B+ = A + BH + H+ |
| 2 | Transferases | AX + B = A + BX |
| 3 | Hydrolases | A-B + H2O = AH + BOH |
| 4 | Lyases | A = 1 B + X-Y = X-A-B-Y |
| 5 | Isomerases | A = B |
| 6 | Ligases | A + B + NTP = A-B + NDP + P or A + B + NTP = A-B + NMP + PP |
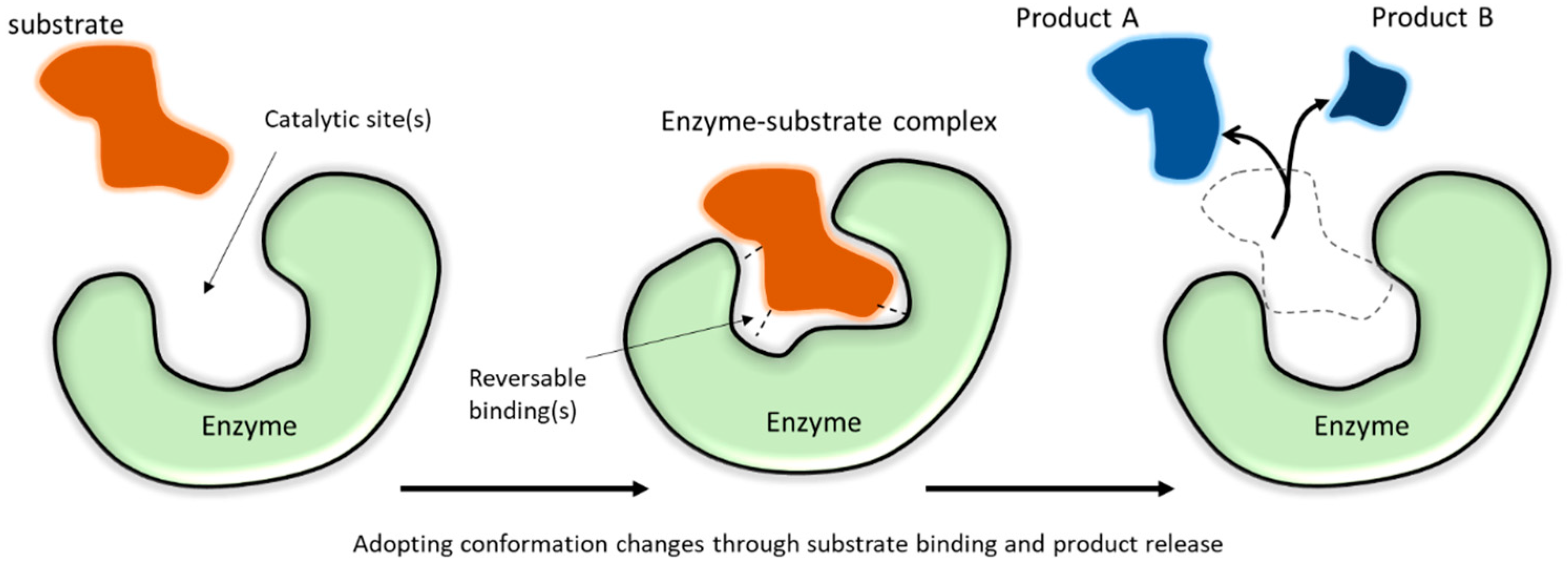
2. Functional Attributes of Enzymes
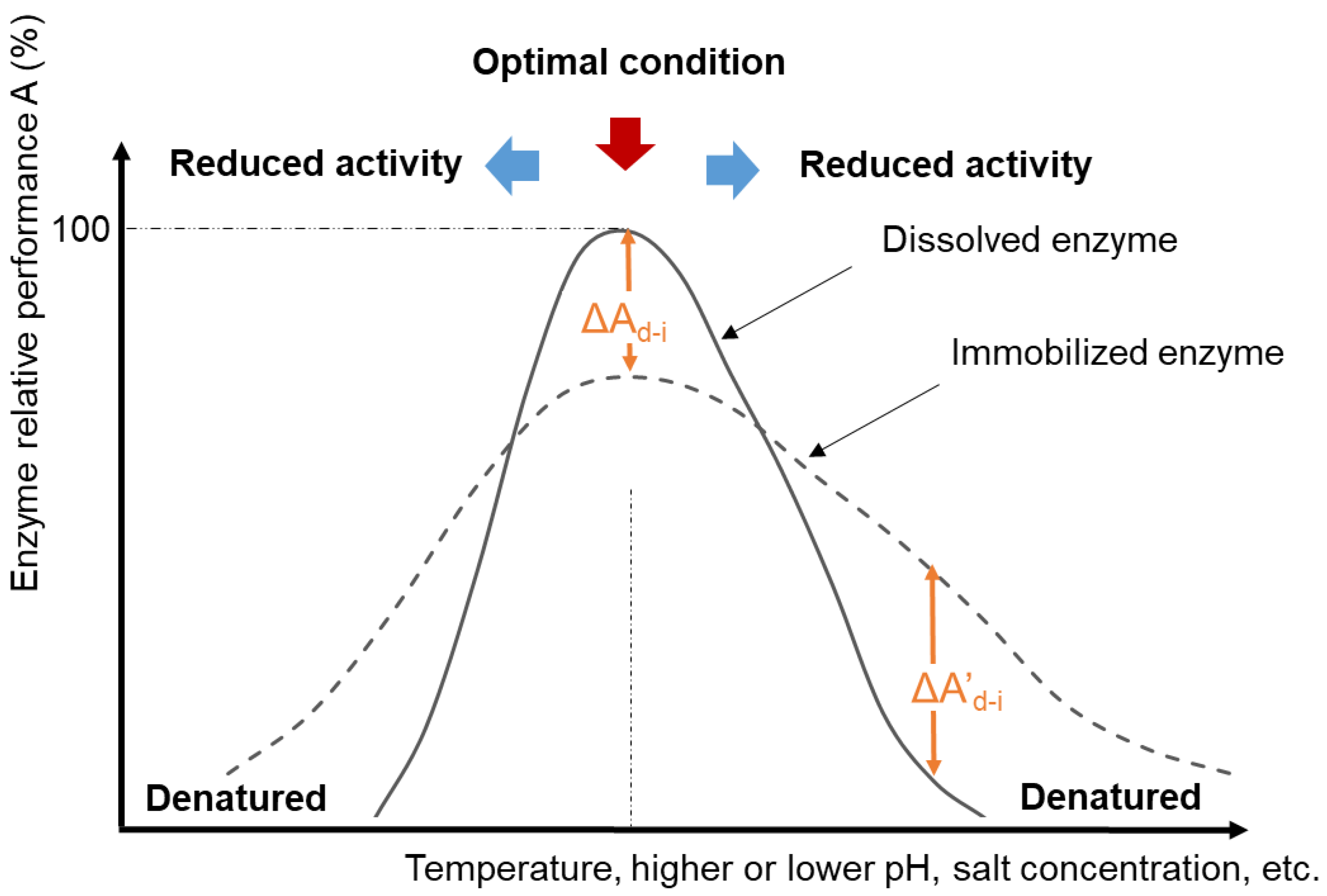
3. Quantifying Immobilized Enzyme Performance
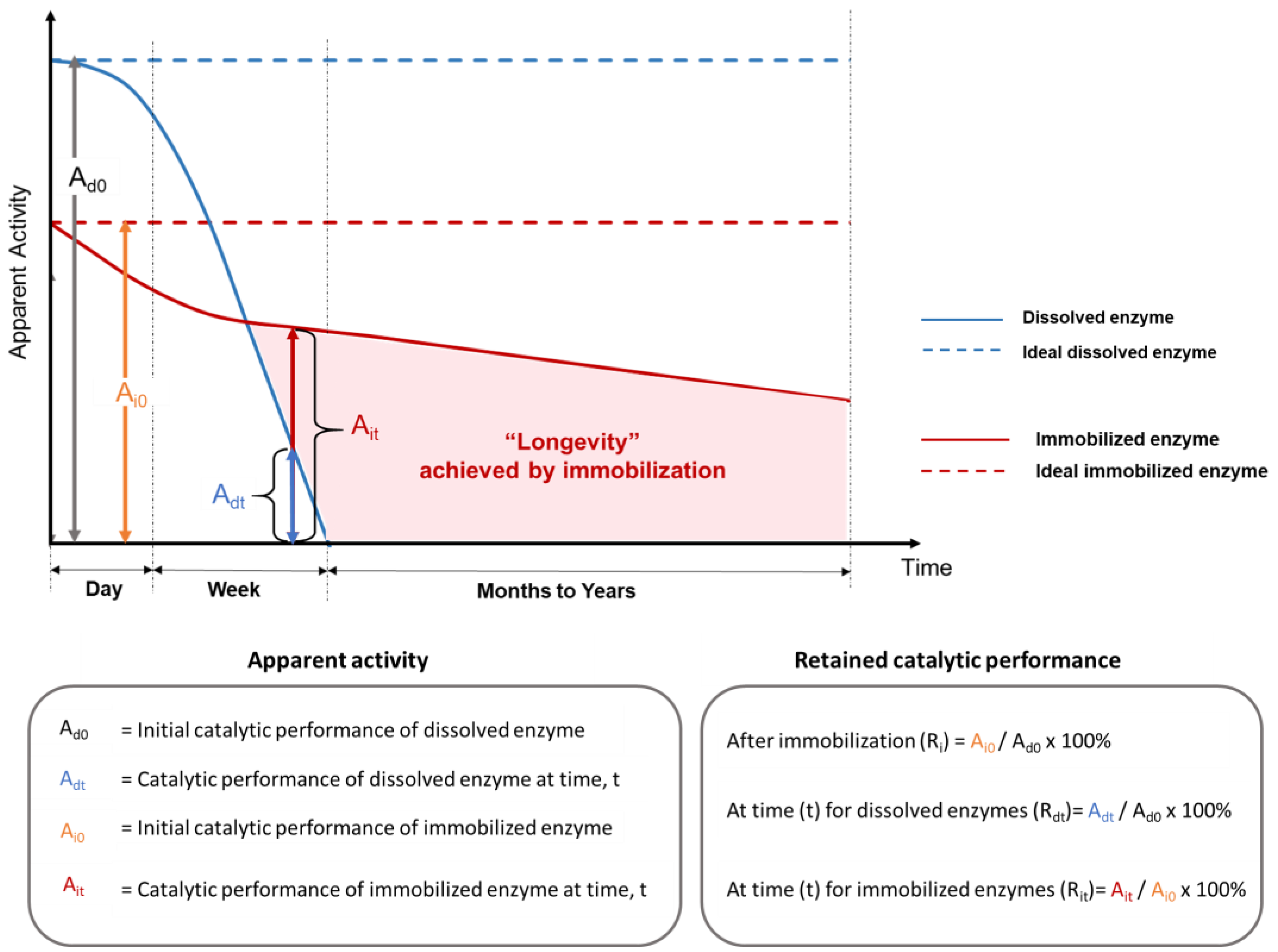
3.1. Retained Activity after Immobilization
3.2. Enzyme Activity over Extended Periods
3.3. Mass Transfer and Surface Property Considerations
References
- Buchholz, K.; Kasche, V.; Bornscheuer, U.T. Biocatalysts and Enzyme Technology, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; ISBN 9783527672004.
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 9783527312320.
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised Enzymes: Carrier-Bound or Carrier-Free? Curr. Opin. Biotechnol. 2003, 14, 387–394.
- Sheldon, R.A. Cross-Linked Enzyme Aggregates (CLEA®s): Stable and Recyclable Biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587.
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-Linked Enzyme Aggregates (CLEA) in Enzyme Improvement—A review. Biocatalysis 2016, 1, 166–177.
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307.
- Pialis, P.; Saville, B.A. Production of l-DOPA from Tyrosinase Immobilized on Nylon 6,6: Enzyme Stability and Scaleup. Enzym. Microb. Technol. 1998, 22, 261–268.
- Gupta, R.; Chaudhury, N. Entrapment of Biomolecules in Sol–Gel Matrix for Applications in Biosensors: Problems and Future Prospects. Biosens. Bioelectron. 2007, 22, 2387–2399.
- Liu, Y.; Yu, J. Oriented Immobilization of Proteins on Solid Supports for Use in Biosensors and Biochips: A Review. Microchim. Acta 2016, 183, 1–19.
- Asakura, T.; Kitaguchi, M.; Demura, M.; Sakai, H.; Komatsu, K. Immobilization of Glucose Oxidase on Nonwoven Fabrics with Bombyx Mori Silk Fibroin Gel. J. Appl. Polym. Sci. 1992, 46, 49–53.
- Baliyan, A.; Sital, S.; Tiwari, U.; Gupta, R.; Sharma, E.K. Long Period Fiber Grating Based Sensor for the Detection of Triacylglycerides. Biosens. Bioelectron. 2016, 79, 693–700.
- Kimmel, J.D.; Arazawa, D.T.; Ye, S.-H.; Shankarraman, V.; Wagner, W.; Federspiel, W.J. Carbonic Anhydrase Immobilized on Hollow Fiber Membranes Using Glutaraldehyde Activated Chitosan for Artificial Lung Applications. J. Mater. Sci. Mater. Med. 2013, 24, 2611–2621.
- Arazawa, D.T.; Oh, H.-I.; Ye, S.-H.; Johnson, C.A.; Woolley, J.R.; Wagner, W.; Federspiel, W.J. Immobilized Carbonic Anhydrase on Hollow Fiber Membranes Accelerates CO2 Removal from Blood. J. Membr. Sci. 2012, 403–404, 25–31.
- Babadi, A.A.; Bagheri, S.; Hamid, S.B.A. Progress on Implantable Biofuel Cell: Nano-Carbon Functionalization for Enzyme Immobilization Enhancement. Biosens. Bioelectron. 2016, 79, 850–860.
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in Carbon Dioxide Separation and Capture: A Review. J. Environ. Sci. 2008, 20, 14–27.
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.; Silva, C.G. Enhanced Biocatalytic Sustainability of Laccase by Immobilization on Functionalized Carbon Nanotubes/Polysulfone Membranes. Chem. Eng. J. 2018, 355, 974–985.
- Braham, S.A.; Hussain, F.; Morellon-Sterling, R.; Kamal, S.; Kornecki, J.F.; Barbosa, O.; Kati, D.E.; Fernandez-Lafuente, R. Cooperativity of Covalent Attachment and Ion Exchange on Alcalase Immobilization Using Glutaraldehyde Chemistry: Enzyme Stabilization and Improved Proteolytic Activity. Biotechnol. Prog. 2018, 35, e2768.
- Bagheri, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Rogers, R.D. Ionic Liquid-Based Preparation of Cellulose−Dendrimer Films as Solid Supports for Enzyme Immobilization. Biomacromolecules 2007, 9, 381–387.
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What Macromolecular Crowding Can Do to a Protein. Int. J. Mol. Sci. 2014, 15, 23090–23140.
- McDonald, A.G.; Tipton, K.F. Fifty-Five Years of Enzyme Classification: Advances and Difficulties. FEBS J. 2013, 281, 583–592.
- Asad, A.; Sameoto, D.; Sadrzadeh, M. Overview of Membrane Technology. In Nanocomposite Membranes for Water and Gas Separation; Sadrzadeh, M., Mohammadi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–28. ISBN 978-0-12-816710-6.
- Bayne, L.; Ulijn, R.V.; Halling, P.J. Effect of Pore Size on the Performance of Immobilised Enzymes. Chem. Soc. Rev. 2013, 42, 9000–9010.
- Engel, P. Enzymes: A Very Short Introduction; Oxford University Press: Oxford, UK, 2020; ISBN 9780198824985.
- Wolfenden, R.; Snider, M.J. The Depth of Chemical Time and the Power of Enzymes as Catalysts. Accounts Chem. Res. 2001, 34, 938–945.
- Roux, P.; Delepierre, M.; Goldberg, M.E.; Chaffotte, A.-F. Kinetics of Secondary Structure Recovery during the Refolding of Reduced Hen Egg White Lysozyme. J. Biol. Chem. 1997, 272, 24843–24849.
- Grisham, D.R.; Nanda, V. Hydrodynamic Radius Coincides with the Slip Plane Position in the Electrokinetic Behavior of Lysozyme. Proteins: Struct. Funct. Bioinform. 2018, 86, 515–523.
- Janin, J.; Miller, S.; Chothia, C. Surface, Subunit Interfaces and Interior of Oligomeric Proteins. J. Mol. Biol. 1988, 204, 155–164.
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51.
- Richards, F.M. Areas, volumes, packing, and protein structure. Annu. Rev. Biophys. Bioeng. 1977, 6, 151–176.
- Miller, S.; Janin, J.; Lesk, A.; Chothia, C. Interior and Surface of Monomeric Proteins. J. Mol. Biol. 1987, 196, 641–656.
- Rubinson, K.A. Why Proteins are Big: Length Scale Effects on Equilibria and Kinetics. Protein J. 2019, 38, 95–119.
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein−Ligand Binding. Chem. Rev. 2008, 108, 946–1051.
- Kirkman, H.N.; Gaetani, G.F. Mammalian Catalase: A Venerable Enzyme with New Mysteries. Trends Biochem. Sci. 2007, 32, 44–50.
- Siddiqui, K.S. Defying the Activity-Stability Trade-off in Enzymes: Taking Advantage of Entropy to Enhance Activity and Thermostability. Crit. Rev. Biotechnol. 2016, 37, 309–322.
- Wang, S.-C.; Lee, J.C.T. Enhanced Enzymatic Activity through Photoreversible Conformational Changes. Biochemistry 2007, 46, 14557–14566.
- Hosseinkhani, S.; Nemat-Gorgani, M. Partial Unfolding of Carbonic Anhydrase Provides a Method for its Immobilization on Hydrophobic Adsorbents and Protects it Against Irreversible Thermoinactivation. Enzym. Microb. Technol. 2003, 33, 179–184.
- Madhu, A.; Chakraborty, J. Developments in Application of Enzymes for Textile Processing. J. Clean. Prod. 2017, 145, 114–133.
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in Aqueous Solution, Reaction with Proteins, and Application to Enzyme Crosslinking. Biotechniques 2004, 37, 790–802.
- Asaduzzaman, F.; Salmon, S. Enzyme Immobilization: Polymer–Solvent–Enzyme Compatibility. Mol. Syst. Des. Eng. 2022, 7, 1385–1414.
- Liszka, M.J.; Clark, M.E.; Schneider, E.; Clark, D.S. Nature Versus Nurture: Developing Enzymes That Function Under Extreme Conditions. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 77–102.
- Bao, X.; Huang, X.; Lu, X.; Li, J.-J. Improvement of Hydrogen Peroxide Stability of Pleurotus Eryngii Versatile Ligninolytic Peroxidase by Rational Protein Engineering. Enzym. Microb. Technol. 2014, 54, 51–58.
- Liu, J.-Z.; Wang, T.-L.; Huang, M.-T.; Song, H.-Y.; Weng, L.-P.; Ji, L.-N. Increased Thermal and Organic Solvent Tolerance of Modified Horseradish Peroxidase. Protein Eng. Des. Sel. 2006, 19, 169–173.
- Pour, R.R.; Ehibhatiomhan, A.; Huang, Y.; Ashley, B.; Rashid, G.M.; Williams, S.; Bugg, T.D. Protein Engineering of Pseudomonas Fluorescens Peroxidase Dyp1B for Oxidation of Phenolic and Polymeric Lignin Substrates. Enzym. Microb. Technol. 2019, 123, 21–29.
- Yu, X.-W.; Tan, N.-J.; Xiao, R.; Xu, Y. Engineering a Disulfide Bond in the Lid Hinge Region of Rhizopus chinensis Lipase: Increased Thermostability and Altered Acyl Chain Length Specificity. PLoS ONE 2012, 7, e46388.
- Wang, Y.; Fu, Z.; Huang, H.; Zhang, H.; Yao, B.; Xiong, H.; Turunen, O. Improved Thermal Performance of Thermomyces Lanuginosus GH11 Xylanase by Engineering of an N-Terminal Disulfide Bridge. Bioresour. Technol. 2012, 112, 275–279.
- Mansfeld, J.; Vriend, G.; Dijkstra, B.W.; Veltman, O.R.; Burg, B.V.D.; Venema, G.; Ulbrich-Hofmann, R.; Eijsink, V.G. Extreme Stabilization of a Thermolysin-Like Protease by an Engineered Disulfide Bond. J. Biol. Chem. 1997, 272, 11152–11156.
- Razzaghi, M.; Homaei, A.; Vianello, F.; Azad, T.; Sharma, T.; Nadda, A.K.; Stevanato, R.; Bilal, M.; Iqbal, H.M.N. Industrial Applications of Immobilized Nano-Biocatalysts. Bioprocess Biosyst. Eng. 2021, 45, 237–256.
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process. Res. Dev. 2011, 15, 266–274.
- Huang, H.; Hu, N.; Zeng, Y.; Zhou, G. Electrochemistry and Electrocatalysis with Heme Proteins in Chitosan Biopolymer Films. Anal. Biochem. 2002, 308, 141–151.
- Altinkaynak, C.; Tavlasoglu, S.; Ÿzdemir, N.; Ocsoy, I. A New Generation Approach in Enzyme Immobilization: Organic-Inorganic Hybrid Nanoflowers with Enhanced Catalytic Activity and Stability. Enzym. Microb. Technol. 2016, 93–94, 105–112.
- Akgöl, S.; Kaçar, Y.; Özkara, S.; Yavuz, H.; Denizli, A.; Arica, M. Immobilization of Catalase via Adsorption onto L-Histidine Grafted Functional pHEMA Based Membrane. J. Mol. Catal. B: Enzym. 2001, 15, 197–206.
- Arola, S.; Tammelin, T.; Setälä, H.; Tullila, A.; Linder, M.B. Immobilization–Stabilization of Proteins on Nanofibrillated Cellulose Derivatives and Their Bioactive Film Formation. Biomacromolecules 2012, 13, 594–603.
- Hwang, S.; Lee, K.; Park, J.-W.; Min, B.-R.; Haam, S.; Ahn, I.-S.; Jung, J.-K. Stability Analysis of Bacillus Stearothermophilus L1 Lipase Immobilized on Surface-Modified Silica Gels. Biochem. Eng. J. 2004, 17, 85–90.
- Abdelrahim, M.Y.M.; Martins, C.F.; Neves, L.; Capasso, C.; Supuran, C.T.; Coelhoso, I.M.; Crespo, J.G.; Barboiu, M. Supported Ionic Liquid Membranes Immobilized with Carbonic Anhydrases for CO2 Transport at High Temperatures. J. Membr. Sci. 2017, 528, 225–230.
- Bankeeree, W.; Prasongsuk, S.; Lotrakul, P.; Punnapayak, H.; Imai, T. A Novel Xylan-Polyvinyl Alcohol Hydrogel Bead with Laccase Entrapment for Decolorization of Reactive Black 5. Bioresources 2016, 11.
- Qi, G.; Liu, K.; House, A.; Salmon, S.; Ambedkar, B.; Frimpong, R.A.; Remias, J.E.; Liu, K. Laboratory to Bench-Scale Evaluation of an Integrated CO2 Capture System Using a Thermostable Carbonic Anhydrase Promoted K2CO3 Solvent with Low Temperature Vacuum Stripping. Appl. Energy 2018, 209, 180–189.
- Messing, R.A.; Filbert, A.M. An Immobilized Glucose Isomerase for the Continuous Conversion ofGlucose to Fructose. J. Agric. Food Chem. 1975, 23, 920–923.
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported Ionic Liquid Phase (SILP) Facilitated Gas-Phase Enzyme Catalysis—CALB Catalyzed Transesterification of Vinyl Propionate. Catal. Sci. Technol. 2018, 8, 2460–2466.
- Shen, J.; Yuan, Y.; Salmon, S. Durable and Versatile Immobilized Carbonic Anhydrase on Textile Structured Packing for CO2 Capture. Catalysts 2022, 12, 1108.
- Al-Qodah, Z.; Al-Shannag, M.; Al-Busoul, M.; Penchev, I.; Orfali, W. Immobilized Enzymes Bioreactors Utilizing a Magnetic Field: A review. Biochem. Eng. J. 2017, 121, 94–106.
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. A Review of Mass Transfer Controlling the Reaction Rate in Heterogeneous Catalytic Systems. In Mass Transfer—Advanced Aspects; Nakajima, H., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 29.
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236–6249.
- Liu, J.; Iranshahi, A.; Lou, Y.; Lipscomb, G. Static Mixing Spacers for Spiral Wound Modules. J. Membr. Sci. 2013, 442, 140–148.
- Goldstein, L. Kinetic Behavior of Immobilized Enzyme Systems. Methods Enzymol. 1976, 44, 397–443.
- Illanes, A.; Wilson, L. Parameters for the Evaluation of Immobilized Enzymes Under Process Conditions. In Immobilization of Enzymes and Cells: Fourth Edition; Humana Press: Totowa, NJ, USA, 2020; pp. 65–81. ISBN 9781071602140.
- Rovito, B.J.; Kittrell, J.R. Film and pore diffusion studies with immobilized glucose oxidase. Biotechnol. Bioeng. 1973, 15, 143–161.
- Murzin, D.Y.; Salmi, T. Mass Transfer and Catalytic Reactions. In Catalytic Kinetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 589–664. ISBN 9780444637536.
- Rivero, J.R.; Panagakos, G.; Lieber, A.; Hornbostel, K. Hollow Fiber Membrane Contactors for Post-Combustion Carbon Capture: A Review of Modeling Approaches. Membranes 2020, 10, 382.
- Valencia, P.; Ibañez, F. Estimation of the Effectiveness Factor for Immobilized Enzyme Catalysts through a Simple Conversion Assay. Catalysts 2019, 9, 930.
- Marrazzo, W.N.; Merson, R.L.; McCoy, B.J. Enzyme Immobilized in a Packed-Bed Reactor: Kinetic Parameters and Mass Transfer Effects. Biotechnol. Bioeng. 1975, 17, 1515–1528.
- Regan, D.L.; Lilly, M.D.; Dunnill, P. Influence of Intraparticle Diffuisional Limitation on the Observed Kinetics of Immobilized Enzymes and on Catalyst Design. Biotechnol. Bioeng. 1974, 16, 1081–1093.
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.; Della Negra, M.; Kaiser, A.; Pinelo, M. Enzyme Immobilization on Inorganic Surfaces for Membrane Reactor Applications: Mass Transfer Challenges, Enzyme Leakage and Reuse of Materials. Adv. Synth. Catal. 2018, 360, 2578–2607.
- Fritzmann, C.; Wiese, M.; Melin, T.; Wessling, M. Helically Microstructured Spacers Improve Mass Transfer and Fractionation Selectivity in Ultrafiltration. J. Membr. Sci. 2014, 463, 41–48.
- Diamanti, E.; Santiago-Arcos, J.; Grajales-Hernández, D.; Czarnievicz, N.; Comino, N.; Llarena, I.; Di Silvio, D.; Cortajarena, A.L.; López-Gallego, F. Intraparticle Kinetics Unveil Crowding and Enzyme Distribution Effects on the Performance of Cofactor-Dependent Heterogeneous Biocatalysts. ACS Catal. 2021, 11, 15051–15067.
- Dittmeyer, R.; Emig, G. Simultaneous Heat and Mass Transfer and Chemical Reaction. Handb. Heterog. Catal. 2008, 1727–1784.
- Al-Muftah, A.E.; Abu-Reesh, I.M. Effects of Internal Mass Transfer and Product Inhibition on a Simulated Immobilized Enzyme-Catalyzed Reactor for Lactose Hydrolysis. Biochem. Eng. J. 2005, 23, 139–153.
- Godjevargova, T.; Gabrovska, K. Influence of Matrix on External Mass Transfer Resistance in Immobilized Urease Membranes. Enzym. Microb. Technol. 2006, 38, 338–342.
- Comite, A.; Bottino, A.; Capannelli, G.; Costa, C.; Di Felice, R. Multi-Phase Catalytic Membrane Reactors. In Woodhead Publishing Series in Energy; Basile, A., Ed.; Woodhead Publishing Limited: Soston, UK, 2013; Volume 2, ISBN 9780857097347.





