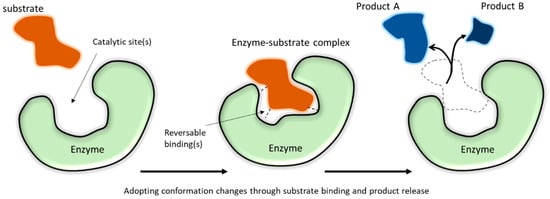
2. Functional Attributes of Enzymes
Enzymes are compact globular proteins with catalytic active sites that lower the transition state energy for specific chemical reactions to occur [
33], that in some cases would take hundreds or thousands of years to occur if not catalyzed [
34]. Enzymes range in physical size from small monomers (with single protein chain domains), such as hen egg white lysozyme that has a molecular weight of 14.3 kDa [
35] and a spherical shape with a diameter of around 3 nm [
36], to large multimers (having multiple protein chain domains that form a complex), such as tetrameric beef liver catalase that has a molecular weight of ~232 kDa [
37] and a diameter of at least 8 nm [
38,
39]. As is characteristic of proteins in general, enzymes exhibit self-assembly phenomena, have a density near 1.37 g cm
−1 [
38], tend to have more charged amino acid residues at their surface than in their interior [
40], and, when solvated, are intimately surrounded by 3 to 4 layers of hydrating water (with a thickness around 0.7–1 nm) [
41]. Often, but not always, the substrate size is small compared to the enzyme molecule, allowing substrates to diffuse into the enzyme active site. For example, bovine α-carbonic anhydrase has a molecular weight of ~30 kDa, while the substrate CO
2 is about 44 Da, with a weight ratio of about 680:1 [
42], and catalase is more than 6000 times larger than its substrate, hydrogen peroxide (34 Da) [
43]. The catalytic active sites in enzymes are formed by precise spatial relationships of chemically reactive amino acid side chains through correct folding of the protein polymer [
33].
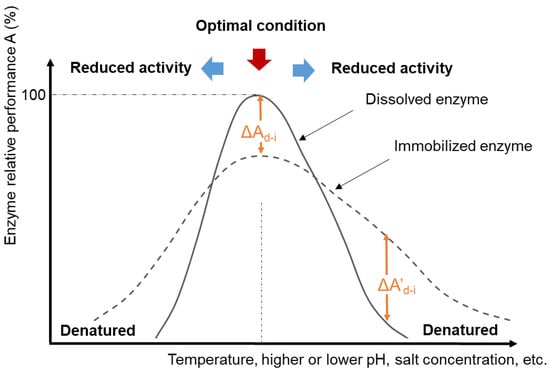
Enzymes exhibit the fastest catalytic effects at certain “optimal” conditions of temperature, pH, ionic strength, and other factors (
Figure 2). These conditions vary for specific isozymes and between enzyme classes. The optimal pH may be associated with the ionization state of functional groups within the active site when this is important for the catalytic mechanism. The optimal temperature often corresponds to the temperature just below the enzyme denaturation temperature because reaction rates generally increase as temperature increases. When enzymes denature, the protein structure unfolds to an extent that the three-dimensional orientation of the active site is disrupted, leading to a loss of catalytic activity. The optimal activity conditions may or may not correspond to the conditions at which the enzyme structure is most stable [
44]. For example, a partially denatured (unfolded) enzyme might exhibit higher than usual catalytic activity (provided that the active site is still intact) because the active site may be more exposed to its substrate [
45] and (especially if a higher temperature is a factor in the partial unfolding) the reaction kinetics will be faster [
46]. Outside of the optimal activity zone, enzymes may still catalyze the reaction, but at reduced rates. Compared to dissolved enzymes, research shows that immobilized enzymes often demonstrate a higher tolerance toward more extreme process conditions [
1,
2,
4,
47,
48,
49] (dashed line in
Figure 2). However, there can be trade-offs between enzyme stability (extended activity even under stressed conditions) and enzyme catalytic activity (the rate at which substrates are converted to products), which must be taken into consideration when comparing their overall catalytic efficiencies [
44].
Figure 2. Schematic diagram of normalized enzyme activity and/or structural stability and process conditions for catalytic reactions.
Protein engineering can create enzymes that are more robust than wild-type (those found in nature) isozymes through recombinant DNA techniques [
50,
51,
52,
53,
54,
55,
56]. Another strategy for preserving enzyme activity is to hold enzymes (by immobilization) at relatively mild conditions to produce more stable biocatalyst products for industrial applications [
57]. Immobilization also converts soluble enzymes to an insoluble form, making it readily separable from the process liquids during or after the catalytic cycle for recycling and to prevent enzyme contamination in products. In some cases, this separation prevents biocatalysts from being exposed to subsequent (harsher) process steps, thereby eliminating the risks of denaturation. Immobilization also makes it possible to install biocatalysts in continuous process flow reactions (such as packed-bed reactors), where extending enzyme performance longevity reduces enzyme consumption, resulting in higher overall productivity.
3. Quantifying Immobilized Enzyme Performance
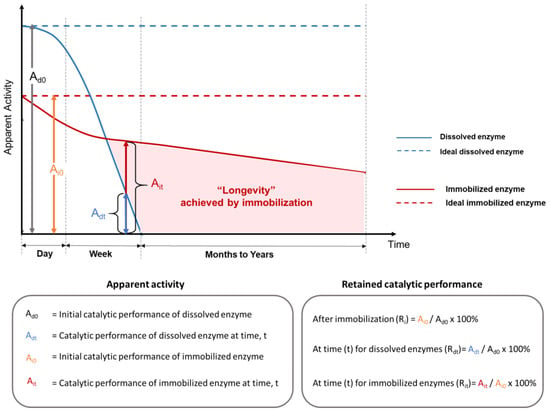
Measuring immobilized enzyme performance varies depending on which parameters are known and the purpose of the evaluation. Underlying these variabilities are the facts that different enzymes catalyze different chemical reactions at different rates with different optimal conditions, and immobilization techniques that work well for one enzyme type do not always translate well to others. Nevertheless, a number of metrics have become ‘expected’; however, these are not uniformly applied, causing comparison difficulties among published studies. The most important analytical parameter is enzyme activity, which is the enzyme-catalyzed reaction rate, often expressed in units of micromoles of substrate converted (or product generated) per minute. When the amount of enzyme protein is known, this value can be expressed as “specific activity”. However, the activity depends on many factors. As illustrated in Figure 3, even if reaction conditions are held constant, time and the impact of the immobilization itself influence the apparent enzyme activity. Other ways of quantifying immobilized enzyme performance are concerned with the catalyst consumption and conversion efficiency of the reaction as a basis for cost calculations. Productivity is an all-encompassing performance metric that is especially relevant for continuous reaction processes that are intended to operate for long periods of time (“longevity”).
Figure 3. Relative enzyme performance versus process time for free and immobilized enzymes as justification for immobilizing enzymes to fabricate biocatalytic materials. Ri is the retained catalytic performance after immobilization. Rdt is the retained catalytic performance at a specified time for the dissolved enzyme. Rit is the retained catalytic performance at a specified time for the immobilized enzyme.
3.1. Retained Activity after Immobilization
When enzymes are immobilized, their detectable level of activity usually changes, resulting in an apparent (or relative) enzyme activity (abbreviated here as “A”, vertical axis in
Figure 3). After immobilization, the measured activity is not only a consequence of specific enzyme activity and reaction conditions but also includes other factors, such as the mass fraction of immobilized enzymes in the support, the chemical or physical properties of the support materials, and the accessibility of enzymes in the immobilization matrix. The retained enzyme activity after immobilization can be quantified as A
i0/A
d0 (%), where A
i0 and A
d0 are the initial catalytic performance of immobilized and dissolved enzymes, respectively. For commercial processes, it is often essential that immobilized enzymes should have improved productivity (total amount of substrate converted per amount of enzyme protein) to generate sufficient cost savings to offset the extra cost of immobilization production and motivate adoption of the technology [
60]. There are some exceptions to this, such as the production of high-value products that cannot be made by other methods and the use of enzymes for certain kinds of sensor design, in which the redox potential of the metal bound at the enzyme active site is more important than the chemical catalytic function of the correctly folded enzyme molecule [
61]. Nevertheless, in most cases, extended enzyme catalytic longevity is indispensable, and the retention of enzyme structural stability is a prerequisite for enzyme activity.
It is not uncommon for immobilized enzymes, especially ones in retrievable solid matrices, such as membranes, to demonstrate lower activities at optimal conditions compared to that of the dissolved enzyme (A
i0/A
d0 < 100%). This lower activity is attributed to increased mass transfer barriers between substrates/products and enzymes in the presence of the immobilization matrix [
18,
62]. Conversely, many immobilized enzymes demonstrate better catalytic performance at broader pH or temperature ranges in comparison with dissolved enzymes, corresponding to a negative
A’
d-i (
Figure 2) at conditions outside the optimal conditions for the soluble dissolved enzyme [
10,
63,
64,
65,
66,
67]. The exact reasons for improved thermal and pH stability through immobilization are not always clear, but the most common explanation is that the presence of the support provides confined environments to restrict the unfolding of enzymes when conditions become unfavorable. Studies also found that the presence of macromolecule crowding around the protein can tighten the protein structure and assist the protein folding [
19].
3.2. Enzyme Activity over Extended Periods
Although a drop in the instantaneous enzyme performance between dissolved (A
d0 in
Figure 3) and immobilized enzymes with physical support (A
i0 in
Figure 3) is often observed at optimal reaction conditions for the soluble enzyme, the true benefit of using an immobilized enzyme emerges when the real application exposes the unprotected soluble enzymes to intolerable conditions, but spares the protected immobilized enzymes. A comparison of enzyme longevity for dissolved (A
d0 and A
dt) and immobilized (A
i0 and A
it) enzymes in terms of the apparent enzyme activity versus time is illustrated in
Figure 3. A
dt and A
it are the catalytic performance of dissolved and immobilized enzymes at time t, respectively. The depicted rapid activity loss of soluble enzyme is often observed experimentally, either due to enzyme denaturation or to the difficulties of recovering and reusing dissolved enzyme proteins. This type of significant performance drop is indicated by a low A
dt over short periods (hours to days) in real applications (solid blue line in
Figure 3), if no supplemental enzymes are added [
68]. In comparison, the retained activity of enzymes that are bound to a physical support over very long time periods (months to years) is a hallmark of the improved enzyme longevity that can be obtained via immobilization (shaded area in
Figure 3).
Over time, immobilized enzymes will eventually exhibit decreases (Ait < Ai0) in relative performance (red curve) compared with an ideal scenario (Ai0, red horizontal dashed line, Figure 3). Commonly, a first abrupt performance decrease is observed over a relatively short time period (e.g., hours to days), which is usually attributed to enzyme leaching from the support matrix. A slower biocatalyst inactivation or slower decrease in relative enzyme performance is then observed over a much longer period (e.g., weeks to years). A number of factors can contribute to this slower inactivation of immobilized enzymes, including gradual enzyme leaching, damage to immobilized enzymes, erosion or degradation of the physical support matrix, and accumulation of contaminants or fouling in/around the immobilized system. The magnitude of the retained activity difference between immobilized enzymes and dissolved enzymes (Ait − Adt) is determined by various parameters, such as inherent enzyme stability, support material properties, methods of immobilization, and application reaction conditions.
Therefore, immobilization not only provides more robust enzyme products against harsh catalytic conditions, but also provides high enzyme productivity that can enable commercial processes within the cost window allowed by the application [
60], such as the continuous production of high-fructose corn syrup using glucose isomerase in the form of immobilized granules in large packed-bed columns [
69]. Measuring biocatalytic longevity at lab scale may require special experimental setups for the targeted applications. For instance, lipase immobilized on cellulosic beads showed >700 h activity within a bench-scale reactor [
70], and carbonic anhydrase immobilized on fibrous textile structured packing [
71] retained 100% and 85% of the initial CO
2 capture performance after a 71-day longevity test and after 1 year of ambient dry storage, respectively, tested using a lab-scale gas scrubber.
3.3. Mass Transfer and Surface Property Considerations
In enzyme-catalyzed reactions, the overall observed catalytic efficiency depends on: (1) the rates at which the substrate and product diffuse into and away from enzyme catalytic sites, and (2) the rate at which the substrate is converted to a product by enzyme molecules [
72]. Enzyme immobilization can lead to changes in enzyme structural conformation, molecular steric hindrance, and changes in local charge density and pH near the surface that change the enzyme’s microenvironment and impact its activity [
73,
74]. Enzyme immobilization to a physical support can also change the accessibility of substrates as well as the diffusion of products, usually resulting in slower diffusion—referred to as ‘mass transfer (or mass transport) limitations’—due to a liquid boundary layer near solid surfaces that typically experiences lower turbulent flow and a lower concentration of reactants than the bulk liquid [
75]. Mathematical treatments of mass transfer phenomena that incorporate both diffusion and reaction have been developed [
76,
77,
78,
79], and elaborated in detail, depending on specific reactor configurations, such as for porous gas–liquid hollow fiber membrane contactors, where an enhancement factor (E = V
catalyzed/V
uncatalyzed) can be incorporated to account for improvements in mass transfer due to the (bio-catalyzed) chemical reaction [
80]. Simplified process metrics are reported when the detailed parameters needed for solving mathematical models are unknown [
81]. Kinetic parameters impacting immobilized catalysts may change for multiple reasons, such as steric exclusion between support materials and enzyme substrates, delayed diffusion to interior pores of porous media, and changes in driving forces, such as concentration gradients induced by mixing or flow, that deliver substrates to or separate products from the catalytic matrix [
74]. Models developed for immobilized enzymes with particulate form in packed-bed reactors [
82,
83] found that the smallest particle size that the reactor can handle, together with the highest enzyme loading on the particles, led to the best catalytic efficiency. Similar mass transfer variables and challenges occur for membrane reactors, where the requirement for flux through the membrane is an additional consideration that may require higher pore sizes (200–1400 nm) [
84] than the 10–100 nm pore size range that tends to perform best with particulate biocatalysts [
22]. Since reactive components and reaction configurations widely vary, fibrous membrane structures have strong potential to enhance substrate/product diffusion, because they have: tunable porosity and diversified surface chemistry (e.g., hydrophobicity, charges) for regulating enzyme loading, the potential to vary the curvature for decreasing denaturation and exposing enzymes to the reaction medium [
84], and versatile hierarchical micro- and macroscopic sizes, shapes, and geometries that can physically create and support reactive surfaces while optimizing membrane spacing [
75,
85]. A recent study using single-particle analysis elucidated that the localization and packing density of immobilized enzymes also have significant impact on the kinetics of the catalytic matrix [
86]. Such parameters can be controlled through various fiber formation techniques and immobilization approaches within membranes containing fibrous structures. Moreover, fibrous membrane physical flexibility and durability could enable unique reactor geometries that could even include self-standing catalytic reactors that facilitate the inlet and outlet of reactants and products.
As heterogeneous catalysts, the mass transfer processes for membrane-bound enzymes can be described by theoretical frameworks developed for general heterogeneous catalysis [
73,
76]. The book chapter by Dittmeyer and Emig [
87] illustrates the individual steps of a heterogeneous solid-liquid catalytic reaction on a porous catalyst. First, the substrate molecules (A
1) in the bulk liquid phase need to diffuse through a stagnant liquid film close to the external surface of the catalyst. Then, the substrate molecules need to diffuse through the interior pores to reach the active site surface, where a series of adsorption, transformation, and desorption processes occur. Subsequently, the product molecules (A
2) must diffuse back to the bulk liquid through pore and film diffusion. When the diffusion rate of the substrates from the bulk liquid to an immobilized enzyme’s active site is slower than the catalytic reaction rate, the observed rate, i.e., the apparent enzyme activity, is lower compared to the dissolved free enzyme. The rate of substrate flow in external mass transfer is often described by the product of a transport coefficient and the corresponding driving force, which is the gradient of the substrate concentration [
76]. When a membrane is used, the external mass transfer is also proportional to the surface area [
78].
The effectiveness factor (η = V/V
free) ratio was introduced as an analytical solution to represent the change in the enzyme reaction rate upon immobilization. It can be calculated by measuring the kinetic parameters for free enzymes and immobilized enzymes [
78,
88,
89]. This effectiveness factor is then used to determine the external mass transfer resistance for membrane immobilized enzymes and to determine the Nernst diffusion layer thickness [
89]. The rate of internal mass transfer is considered to proceed in parallel with the enzymatic reactions [
76]. Therefore, the change in the substrate conversion rate with immobilized enzymes is a sum of rate changes in diffusion and reaction inside membranes [
76]. Therefore, geometric and chemo-physical properties, such as, pore arrangement, hydrophilicity, and pore sizes, significantly impact the overall mass transfer in the reactions [
73].
A further dimensionless number, called Thiele modulus (
, has been introduced to quantify the effect of the mass transfer limitation on the overall reaction [
87]. It is defined as the square root of the ratio of the characteristic reaction rate in a bulk liquid phase over the effective diffusion rate at the external catalyst surface. In Equation (1), R is the radius (or thickness) of a typical porous pellet catalyst used for conventional reactors, k is the rate constant for an n-th order reaction, C
b is the concentration of the substrate in the bulk liquid, and D
e is the effective diffusion coefficient. Since the thickness of the catalyst layer on a membrane can be much thinner than typical pellet sizes, a Thiele modulus of as low as 1 is achievable in catalytic membrane reactors, signifying a complete utilization of the catalyst’s intrinsic activity [
90]. Moreover, regardless of what physical forms are used in immobilization, appropriate reactor selection and design for these heterogeneous catalysts characteristically helps to enhance the mass transfer rate of the system [
73].