Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rafał Filip | -- | 2543 | 2023-05-25 13:59:26 | | | |
| 2 | Jessie Wu | + 9 word(s) | 2552 | 2023-05-26 05:23:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Role of Antioxidants in Inflammatory Bowel Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/44843 (accessed on 07 February 2026).
Jarmakiewicz-Czaja S, Ferenc K, Filip R. Role of Antioxidants in Inflammatory Bowel Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/44843. Accessed February 07, 2026.
Jarmakiewicz-Czaja, Sara, Katarzyna Ferenc, Rafał Filip. "Role of Antioxidants in Inflammatory Bowel Disease" Encyclopedia, https://encyclopedia.pub/entry/44843 (accessed February 07, 2026).
Jarmakiewicz-Czaja, S., Ferenc, K., & Filip, R. (2023, May 25). Role of Antioxidants in Inflammatory Bowel Disease. In Encyclopedia. https://encyclopedia.pub/entry/44843
Jarmakiewicz-Czaja, Sara, et al. "Role of Antioxidants in Inflammatory Bowel Disease." Encyclopedia. Web. 25 May, 2023.
Copy Citation
Oxidative stress (OS) is defined as an imbalance between the induction of reactive oxygen species (ROS) and the antioxidant components of the body’s defence system. ROS are molecules composed of at least one oxygen atom and containing at least one unpaired electron. From a biochemical perspective, ROS are highly reactive compounds that interact reactively with cell organelles. ROS include hydrogen peroxide (H2O2), superoxide radicals (O2•−), hydroxyl radicals (•OH), and singlet oxygen (O2). They are mainly produced as by-products of oxygen metabolism processes, but there are external triggers that contribute to their increase.
antioxidants
inflammatory bowel disease
nutrients
oxidative stress
1. Oxidative Stress and Inflammatory Bowel Disease (IBD)
Many researchers argue that inflammatory bowel disease (IBD) is closely related to increased reactive oxygen species (ROS) production. Studies in animal models with induced colitis using dextran sulphate sodium (DSS) confirm the increased generation of ROS such as superoxide, hypochlorous acid, and hydrogen peroxide. At the same time, a reduction in the level of endogenous antioxidant compounds, including glutathione and superoxide dismutase, is observed [1]. Active and chronic inflammation of the mucous membrane is directly related to the generation of ROS, which serve as important signalling molecules in the context of the immune response [2][3]. Furthermore, studies show that ROS production in the microenvironment of inflammatory changes in the mucous membrane causes secondary damage, including extensive cellular and molecular damage. As a consequence, this can lead to the maintenance and consolidation of intestinal inflammation, as well as induce further cell damage [4]. Such damage increases the risk from pathogens (including through increased permeability of the intestinal barrier), which in turn may induce a renewed immune response that can initiate the development of IBD. Additionally, ROS overproduction alters intestinal absorption and disrupts intestinal peristalsis. Therefore, in recent years, the number of studies on oxidative stress as a major intermediate factor in the development of IBD has increased [5]. The protein complex acting as a transport factor known as the NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) is an important regulator in many diseases. Studies have shown that it is improperly activated in patients with IBD [6]. A study in mouse models has shown that an antioxidant drug that inhibits NF-kB activity can alleviate symptoms of colitis [7]. Recently, it has been observed that environmental factors such as high consumption of saturated fats and refined sugars, excessive use of antibiotics, or even stress resulting from daily life contribute to a high risk of IBD [8]. Cigarette smoke has been reported to greatly reduce endogenous antioxidant activity in the colon [9]. In IBD, oxidative stress is not limited to the digestive tract, but also has systemic effects in the form of extraintestinal manifestations [10][11]. Additionally, oxidative stress may be involved in the carcinogenesis process in patients with IBD [12]. A study reports that Helicobacter pylori may influence neutrophil induction to generate ROS, which ultimately contributes to the onset of gastric cancer [13].
2. Antioxidants
Antioxidants are substances that can remove damage caused by oxidative stress, or prevent or delay it [14]. Under physiological conditions, antioxidants regulate the production of free radicals [15]. Based on their occurrence, antioxidants can be divided into two groups: endo- and exogenic. The first group includes superoxide dismutase, glutathione peroxidase, catalase. Exogenous antioxidants include flavonoids, vitamins, and minerals, among others (Figure 1) [16].
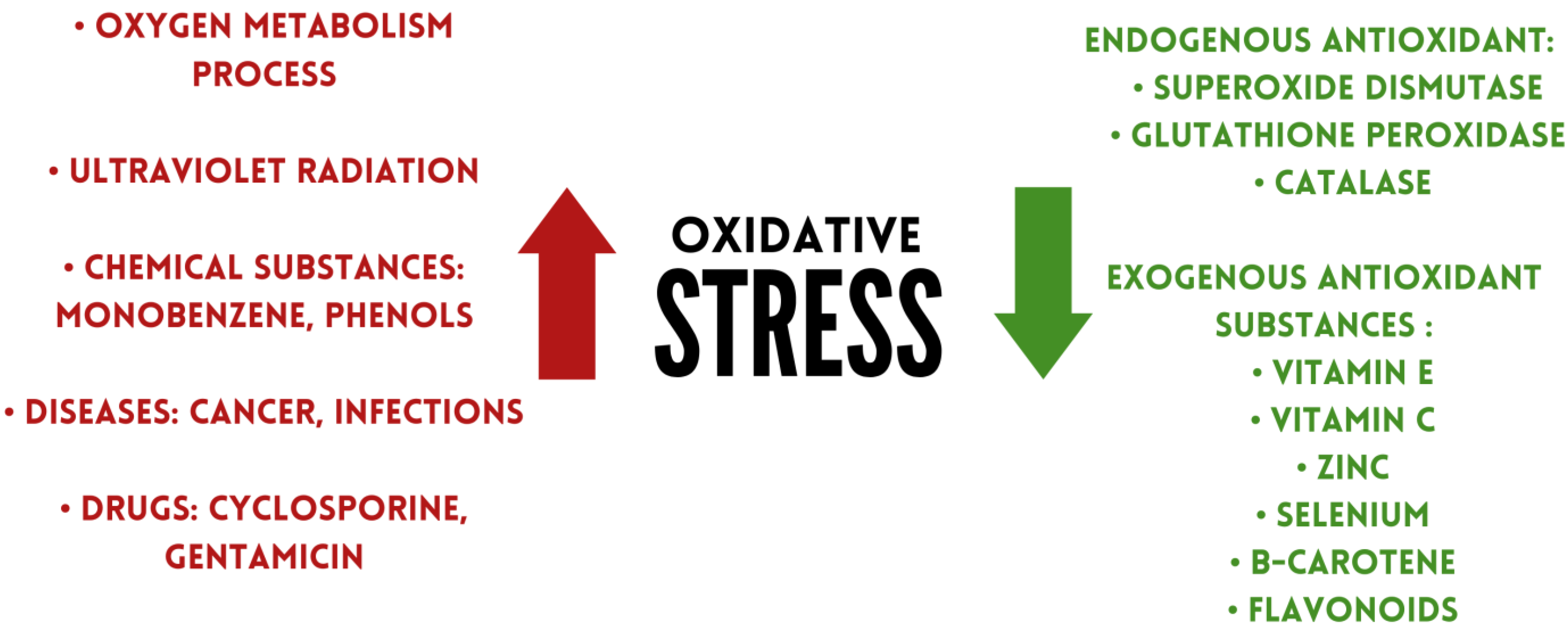
Figure 1. Factors affecting oxidative stress levels.
2.1. Exogenous Antioxidant Substances
2.1.1. Vitamin E
Vitamin E comprises four tocopherols (α, β, γ, δ) and four tocotrienols (α, β, γ, δ). The antioxidant properties of α-tocopherol are comparable to α-tocotrienol [17]. A-tocopherol is the best bioavailable form of vitamin E due to α-tocopherol transfer protein (α-TTP), which has a 100% affinity for α-tocopherol and is the main determinant of α-tocopherol concentration in plasma [18]. Vitamin E can modulate prostaglandin E2 (PGE2) production [19]. PGE2 is involved in enhancing cytokine signalling through gene regulation. It also facilitates Th1 differentiation and Th17 expansion [20]. Additionally, it promotes the production of IL-22 by Th22 cells. In contrast, the improvement of PGE2 inflammation occurs mainly through EP2 and EP4 receptors [21].
L-ascorbic acid is a compound that is not synthesised in the human body, so it must be supplied from exogenous sources. Vitamin C exhibits immunomodulatory and protective effects against ROS [22]. It can have a protective effect on the endothelium, among other things, by decreasing ROS in endothelial cells or neutralising the nitrate tolerance phenomenon [23]. It can act as a cofactor for mono and dioxygenase enzymes [24][25]. The hydroxyl groups of ascorbate in the lactate ring are electron donors and proton donors; they convert to the diketone moiety of dehydroascorbate (DHA), and therefore have a protective effect on cells. Such action of hydroxy groups is shown against superoxide radicals, singlet oxygen, and hydrogen peroxide [26]. Due to molecular stabilisation, the resulting ascorbyl radical is hardly reactive [27]. A high concentration of vitamin C has a protective effect on neutrophils against ROS. It also affects neutrophil leukocyte chemokinesis and chemotaxis [28]. In IBD, up to one fifth of patients with active inflammation may be vitamin C deficient, which may also be associated with feelings of increased fatigue and impaired wound healing [29]. The cause of vitamin C deficiency in patients may be due not only to the active form of the disease, but also to the avoidance of fruit and vegetable consumption [30]. A study by Miyake et al. showed that a higher intake of vitamin C and vegetables may be associated with a lower risk of UC [31]. Other researchers have reached similar conclusions [32]. Patients with Crohn’s disease (CD) also show reduced vitamin C intake [33]. Jo et al. studied the effect of vitamin C deficiency in induced inflammatory bowel disease by administering DSS to mice. They observed that deficiency of the compound caused a decrease in mucin, while it increased IL-6 production and oxidative stress [34]. The SLC23A1 polymorphism may result in a decreased activity of the ascorbate transporter and its reduced intracellular amount [35]. Good dietary sources of vitamin C include berries, citrus fruits, parsley [36].
2.1.2. Zinc
Zinc is a trace element that must be supplied to the body in order for it to function properly. Zinc deficiencies can lead to malfunctioning of T and B lymphocytes and to abnormal maturation and differentiation of them [37]. In addition, they can cause decreased phagocytosis and PMN (polymorphonuclear cells) chemotaxis, and also affect monocyte adhesion to the endothelium [38]. Zinc has also been shown to preserve redox metabolism. An example is the increase in intracellular zinc in granulocytes caused by H2O2. Zinc can also be released in increased amounts from metallothionein (MT) through ROS induction [39]. Zinc can increase IFN-γ (interferon gamma) secretion from peripheral blood mononuclear cells (PBMCs) [40]. Deficiency of the element can lead to increased production of TNFα and IL-6 [41]. The antioxidant activity of zinc occurs indirectly. Its antioxidant functions are multiple, including increasing glutathione (GSH) production or as a cofactor of antioxidant enzymes [42]. The element is also essential for maintaining normal intestinal barrier function, as its deficiency can reduce the function of the tight junction resulting in increased permeability. Additionally, the repair of the intestinal barrier requires the presence of zinc [43][44]. Zinc is also responsible for the proper functioning of intestinal alkaline phosphatase [45]. Deficiencies are more common in patients with IBD than in the general population [46]. In patients with IBD, microelement deficiencies can increase the risk of complications of the disease, as well as hospitalisation [47]. Therefore, screening is recommended, especially during disease exacerbations, to identify possible deficiencies [48].
2.1.3. Selenium
Selenium was discovered in 1817 [49]. Biologically, it is found in the form of 25 selenoproteins and occurs in humans as an element with immunomodulatory effects, among others. It mainly neutralises organic hydroperoxides and hydrogen oxides [50]. It has been shown to act on immune cells, such as NK cells and T lymphocytes, by affecting selected cell signalling pathways or antioxidant functions [51][52]. The element also modulates redox signalling and counteracts ROS [53]. The main compound in the selenoprotein group is glutathione peroxidase (GSH-Px). It consists of 4 units containing selenocysteine, which are antioxidant [54]. It can regulate free radical production when there is inflammation [55]. In addition, it can support immunoglobulin production [56]. Selenium is also essential for the metabolism of some intestinal microorganisms [57]. Cytoplasmic ROS activate the NF-κB signalling pathway and are subsequently involved in the expression of IL-2 and IFN-γ. Therefore, it is important to monitor selenium levels in IBD [58][59][60]. Yan et al. tested whether there was a correlation between serum selenium levels and disease activity in CD patients. After including 135 patients in the study, they observed that serum concentrations of the element were inversely correlated with the severity of the disease course, indicating that selenium could be a factor along with other factors for monitoring disease activity [61]. Some researchers indicate that it is possible to enhance the effect of a probiotic by adding selenium to it, which may also mitigate the inflammation that occurs [62][63][64]. This could be due, among other things, to an increase in SIRT1 gene expression [65]. Keshteli et al. in their study observed that a diet containing anti-inflammatory ingredients altered the composition of the intestinal microflora in patients with UC and led to metabolic changes, which consequently supported the maintenance of clinical remission [66]. In addition, adequate selenium levels can reduce the risk of cardiovascular disease in patients with IBD [67]. Short et al. in their study observed that selenoprotein P (SEPP1) has a significant role in the regulation of intestinal homeostasis and thus the occurrence of inflammation and indirectly colorectal cancer [68][69].
2.1.4. Betacarotene
Β-carotene is a vitamin A provitamin and belongs to the carotenoid group. It has a C40 in its structure including two β-ion rings [70]. By scavenging superoxide radicals and quenching singlet oxygen, it is considered a compound with antioxidant properties [71]. The antioxidant properties of the compound depend on its conformation. Hydrogen abstraction reactions are more exothermic in water compared to gaseous media [72]. Β-carotene shows positive effects in many diseases, such as diabetes and skin diseases [73][74]. Carotenoids also show beneficial effects on the gastrointestinal tract [75]. Honarbakhsh et al. investigated whether carotenoids can have a positive effect on improving intestinal dysfunction. They showed that in the presence of vitamin A deficiency, the administration of β-carotene can reduce intestinal ROS and levels of pro-inflammatory cytokines. In addition, the compound may also have the effect of reducing the permeability of the intestinal barrier [76]. Cheng et al., using epithelial cells in vitro, also observed an improvement in intestinal barrier function by enhancing tight junction function. They also found that with LPS- (lipopolysaccharide) induced colitis, β-carotene can reduce inflammation by down-regulating the toll-like receptor 4 (TLR4) pathway [77]. In addition, provitamin A can exhibit IL-6 and TNF-α lowering abilities [78]. Inflammatory bowel disease can also be alleviated by decreasing PGE2, nitric oxide (NO) production, and modulation of certain signaling pathways [79][80]. Other studies, in animal models, have shown that β-carotene administration can modulate the composition of the intestinal microbiota, which could significantly benefit patients with IBD [81][82]. Good dietary sources of β-carotene include vegetables (carrots, kale, parsley, chard) and fruits (apricots, melon) [36].
2.1.5. Flavonoids
Flavonoids are compounds made up of a benzopyrone ring that contains polyphenolic or phenolic groups. They have a variety of uses and actions [83]. The main groups of substances belonging to the category of flavonoids are: anticyanins (examples of bioactive substances: cyanidins, pelargonidins), flavanols (e.g., catechin, epicatechin), flavonols (e.g., quercetin, kaempferol), flavones (e.g., luteolin, apigenin), flavanones (e.g., naringenis, naringin), and isoflavones (e.g., daidzein, genistein) [84][85]. Due to the presence of a hydroxyl group in the β-ring and a double bond, flavonoids exhibit antioxidant abilities against peroxynitrite, superoxide, or hydroxyl radicals [86]. The antioxidant role of flavonoids is exerted by chelating metal ions, trapping reactive oxygen species, detoxifying enzymes, and increasing the production of antioxidant enzymes [87]. They also inhibit the expression of pro-inflammatory mediators such as the NF-κB cascade, and inhibit the release of pro-inflammatory cytokines [88]. In addition to their pro-inflammatory properties, the compounds show the ability to regulate tumour-associated macrophages (TAMs) [89]. The anti-inflammatory effects of flavonoids focus primarily on inhibiting the activation of intracellular protein complexes containing PRRs (pattern recognition receptors) and inflammatory molecules. This occurs by decreasing the expression of components of the inflammasome, resulting in inhibition of caspase-1 activation and the secretion of pro-inflammatory cytokines [90]. Flavonoids also show non-direct effects on the gut. In their work, Wang et al. show that citrus flavonoids can exert positive effects on maintaining normal intestinal barrier functions by regulating the expression of TJ (tight junction) expression. They mainly point to nobiletin as the bioactive component of flavonoids, which shows effects similar to those of an anti-inflammatory drug. In addition, citrus flavonoids show regulatory effects on mucin expression and secretion and on shaping the composition of intestinal microflora [91][92]. Due to their properties, flavonoids may exert beneficial effects on the course of IBD by, among other things, protecting against functional and morphological changes in the vascular endothelium [93]. Furthermore, they may counteract colonic inflammation by activating the AhR/Nrf2/NQO1 pathway as well as limiting the action of the NLRP3 (NLR family pyrin domain-containing-3) inflammasome [94]. Due to all these factors, antioxidants can reduce the disease activity index [95][96]. The main sources of flavonoids in food are herbs, vegetables, fruits, nuts, cereals, coffee, and tea [97].
2.2. Endogenous Antioxidant Substances
Despite the fact that excessive and uncontrolled oxidative stress has destructive properties for the digestive system, antioxidant defence systems can counteract the undesirable effects of ROS [98][99]. The main defence mechanism of the body involves the production of endogenous antioxidants, including superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) [100].
2.2.1. Superoxide Dismutase
Superoxide dismutase (SOD) is responsible for transforming superoxide radicals into hydrogen peroxide (H2O2) and molecular oxygen (O2) [101]. Hydrogen peroxide is subsequently converted by catalase and glutathione peroxidases [102]. The excessive and uncontrolled production of H2O2 can be potentially harmful to cells. In contrast, an optimal concentration of hydrogen peroxide may have a signalling effect [103]. Superoxide dismutase exists in three isoforms: SOD1, SOD2 and SOD3. SOD1 is present mainly in the cytosol of liver and kidney cells, as well as in the central nervous system and erythrocytes. SOD2 is predominantly found in mitochondria. SOD3 is found in blood serum, tissues, and body fluids (including synovial fluid and cerebrospinal fluid) [104].
2.2.2. Glutathione Peroxidase (GPX)
Glutathione peroxidase is a broad family of compounds with peroxidase activity [105]. GPX has the ability to catalyse the conversion of glutathione to oxidised glutathione (GSH) and can also reduce H2O2 to water molecules and lipid hydroperoxides to stable alcohols. Humans have eight GPX isoforms, many of which contain selenocysteine residues [106]. GSH, as a soluble antioxidant, has been shown to be less active in experimental mouse models of inflammatory bowel disease [107].
2.2.3. Catalase
Catalase is located mainly in peroxisomes [108]. CAT is responsible for breaking down H2O2 into water and molecular oxygen, thus preventing cell damage resulting from the Fenton reaction. In the Fenton reaction, which requires the presence of transition metal ions such as iron or copper, a highly reactive hydroxyl radical (HO) may be formed. In some cases, where catalase is absent, its functions can be performed by glutathione peroxidase [109]. Catalase can also act in a so-called peroxidative mode, in which its functions involve the breakdown of small substrates such as methanol or formate [110]. Another important function of catalase is apoptosis [111]. A study showed that CAT activity in erythrocytes increases in patients with UC [112]. In contrast, another analysis found persistent inhibition of CAT activity in mononuclear cells in patients with CD [113]. On the basis of this, Iborra et al. showed that the constant decrease in CAT observed in CD patients may be due to genetic changes. Various genetic mechanisms that inhibit this antioxidant may contribute to the pathophysiology of CD [114].
References
- Zhu, H.; Li, Y. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480.
- Pereira, C.; Grácio, D.; Teixeira, J.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417.
- Katsanos, K.; Papadakis, K. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver. 2017, 11, 455–463.
- Campbell, E.; Colgan, S. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120.
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194.
- Andresen, L.; Jørgensen, V.; Perner, A.; Hansen, A.; Eugen-Olsen, J.; Rask-Madsen, J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005, 54, 503–509.
- Naito, Y.; Takagi, T.; Ishikawa, T.; Handa, O.; Matsumoto, N.; Yagi, N.; Matsuyama, K.; Yoshida, N.; Yoshikawa, T.; Kotake, Y. alpha-Phenyl-N-tert-butylnitrone provides protection from dextran sulfate sodium-induced colitis in mice. Antioxid. Redox Signal. 2002, 4, 195–206.
- de Souza, H.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27.
- Guo, X.; Ko, J.; Mei, Q.; Cho, C. Aggravating effect of cigarette smoke exposure on experimental colitis is associated with leukotriene B(4) and reactive oxygen metabolites. Digestion 2001, 63, 180–187.
- Bourgonje, A.; von Martels, J.; Bulthuis, M.; van Londen, M.; Faber, K.; Dijkstra, G.; van Goor, H. Crohn’s Disease in Clinical Remission Is Marked by Systemic Oxidative Stress. Front. Physiol. 2019, 10, 499.
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179.
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601.
- Handa, O.; Naito, Y.; Yoshikawa, T. Helicobacter pylori: A ROS-inducing bacterial species in the stomach. Inflamm. Res. 2010, 59, 997–1003.
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715.
- Parveen, A.; Akash, M.; Rehman, K.; Kyunn, W. Recent Investigations for Discovery of Natural Antioxidants: A Comprehensive Review. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 143–160.
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237.
- Aggarwal, B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631.
- Arai, H.; Kono, N. α-Tocopherol transfer protein (α-TTP). Free. Radic. Biol. Med. 2021, 176, 162–175.
- Lewis, E.; Meydani, S.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494.
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421.
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606.
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211.
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615.
- Spoelstra-de Man, A.; Elbers, P.; Oudemans-Van Straaten, H. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255.
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 102117.
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501.
- Moritz, B.; Schmitz, A.; Rodrigues, A.; Dafre, A.; Cunha, M. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459.
- Elste, V.; Troesch, B.; Eggersdorfer, M.; Weber, P. Emerging Evidence on Neutrophil Motility Supporting Its Usefulness to Define Vitamin C Intake Requirements. Nutrients 2017, 9, 503.
- Gordon, B.; Galati, J.; Yang, S.; Longman, R.; Lukin, D.; Scherl, E.; Battat, R. Prevalence and factors associated with vitamin C deficiency in inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 4834–4845.
- Dunleavy, K.; Ungaro, R.; Manning, L.; Gold, S.; Novak, J.; Colombel, J. Vitamin C Deficiency in Inflammatory Bowel Disease: The Forgotten Micronutrient. Crohns Colitis 360 2021, 3, otab009.
- Miyake, Y.; Tanaka, K.; Nagata, C.; Furukawa, S.; Andoh, A.; Yokoyama, T.; Yoshimura, N.; Mori, K.; Ninomiya, T.; Yamamoto, Y.; et al. Japan Ulcerative Colitis Study Group. Dietary intake of vegetables, fruit, and antioxidants and risk of ulcerative colitis: A case-control study in Japan. Nutrition 2021, 91–92, 111378.
- Vahid, F.; Rashvand, S.; Sadeghi, M.; Hekmatdoost, A. The association between index of nutritional quality and ulcerative colitis: A case-control study. J. Res. Med. Sci. 2018, 23, 67.
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.; Hébuterne, X.; Schneider, S. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191.
- Jo, H.; Lee, D.; Go, C.; Jang, Y.; Chu, N.; Bae, S.; Kang, D.; Im, J.P.; Kim, Y.; Kang, J.S. Preventive Effect of Vitamin C on Dextran Sulfate Sodium (DSS)-Induced Colitis via the Regulation of IL-22 and IL-6 Production in Gulo(-/-) Mice. Int. J. Mol. Sci. 2022, 23, 10612.
- Chang, Y.L.; Rossetti, M.; Vlamakis, H.; Casero, D.; Sunga, G.; Harre, N.; Miller, S.; Humphries, R.; Stappenbeck, T.; Simpson, K.W.; et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019, 12, 457–467.
- Jarmakiewicz-Czaja, S.; Piątek, D.; Filip, R. The Influence of Nutrients on Inflammatory Bowel Diseases. J. Nutr. Metab. 2020, 2020, 2894169.
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68.
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285.
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222.
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273.
- Gammoh, N.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624.
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132.
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900.
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308.
- Islam, T.; Albracht-Schulte, K.; Ramalingam, L.; Schlabritz-Lutsevich, N.; Park, O.; Zabet-Moghaddam, M.; Kalupahana, N.; Moustaid-Moussa, N. Anti-inflammatory mechanisms of polyphenols in adipose tissue: Role of gut microbiota, intestinal barrier integrity and zinc homeostasis. J. Nutr. Biochem. 2022, 115, 109242.
- Soltani, Z.; Rafiei, F.; Ebrahim, A.; Rafiei, R. The Prevalence of Zinc Deficiency in Crohn’s Disease Patients. Maedica 2021, 16, 29–33.
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Milutinovic, A.S.; Milovanovic, T.; Stojkovic Lalosevic, M.; Stojanovic, M.; Pavlovic, S.; Popovic, D. Genetic Aspects of Micronutrients Important for Inflammatory Bowel Disease. Life 2022, 18, 1623.
- Moon, N.; Figgins, B.; Altshuler, E.; Pham, A.; Kamel, A. Concurrent zinc and vitamin B6 deficiencies in acutely exacerbated inflammatory bowel disease: Case reports. Nutr. Clin. Pract. 2022, 37, 203–208.
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 28, 11708.
- Mehdi, Y.; Hornick, J.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 13, 3292–3311.
- Huang, Z.; Rose, A.; Hoffmann, P. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743.
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203.
- Maggini, S.; Pierre, A.; Calder, P. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531.
- Kieliszek, M. Selenium Fascinating Microelement, Properties and Sources in Food. Molecules. 2019, 24, 1298.
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695.
- Saeed, F.; Nadeem, M.; Ahmed, R.; Nadeem, M.; Arshad, M.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229.
- Rayman, M. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14.
- Huang, L.J.; Mao, X.T.; Li, Y.Y.; Liu, D.D.; Fan, K.Q.; Liu, R.B.; Wu, T.T.; Wang, H.L.; Zhang, Y.; Yang, B.; et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 10, 1728–1744.
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581.
- Sahebari, M.; Rezaieyazdi, Z.; Khodashahi, M. Selenium and Autoimmune Diseases: A Review Article. Curr. Rheumatol. Rev. 2019, 15, 123–134.
- Yan, W.; Meihao, W.; Zihan, S.; Lingjie, H.; Haotian, C.; Qian, C.; Lianli, S. Correlation Between Crohn’s Disease Activity and Serum Selenium Concentration. Clin. Ther. 2022, 44, 736–743.
- Wu, Z.; Pan, D.; Jiang, M.; Sang, L.; Chang, B. Selenium-Enriched Lactobacillus acidophilus Ameliorates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice by Regulating Inflammatory Cytokines and Intestinal Microbiota. Front. Med. 2021, 31, 716816.
- Hu, Y.; Jin, X.; Gao, F.; Lin, T.; Zhu, H.; Hou, X.; Yin, Y.; Kan, S.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 effectively ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 2022, 5, 955112.
- Khattab, A.; Darwish, A.; Othman, S.; Allam, A.; Alqhtani, H. Anti-inflammatory and Immunomodulatory Potency of Selenium-Enriched Probiotic Mutants in Mice with Induced Ulcerative Colitis. Biol. Trace Elem. Res. 2023, 201, 353–367.
- Khazdouz, M.; Daryani, N.E.; Alborzi, F.; Jazayeri, M.H.; Farsi, F.; Hasani, M.; Heshmati, J.; Shidfar, F. Effect of Selenium Supplementation on Expression of SIRT1 and PGC-1α Genes in Ulcerative Colitis Patients: A Double Blind Randomized Clinical Trial. Clin. Nutr. Res. 2020, 26, 284–295.
- Keshteli, A.H.; Valcheva, R.; Nickurak, C.; Park, H.; Mandal, R.; van Diepen, K.; Kroeker, K.I.; van Zanten, S.V.; Halloran, B.; Wishart, D.S.; et al. Anti-Inflammatory Diet Prevents Subclinical Colonic Inflammation and Alters Metabolomic Profile of Ulcerative Colitis Patients in Clinical Remission. Nutrients 2022, 14, 3294.
- Castro Aguilar-Tablada, T.; Navarro-Alarcón, M.; Quesada Granados, J.; Samaniego Sánchez, C.; Rufián-Henares, J.Á.; Nogueras-Lopez, F. Ulcerative Colitis and Crohn’s Disease Are Associated with Decreased Serum Selenium Concentrations and Increased Cardiovascular Risk. Nutrients 2016, 8, 780.
- Short, S.; Whitten-Barrett, C.; Williams, C. Selenoprotein P in colitis-associated carcinoma. Mol. Cell. Oncol. 2015, 3, e1075094.
- Short, S.P.; Pilat, J.M.; Barrett, C.W.; Reddy, V.K.; Haberman, Y.; Hendren, J.R.; Marsh, B.J.; Keating, C.E.; Motley, A.K.; Hill, K.E.; et al. Colonic Epithelial-Derived Selenoprotein P Is the Source for Antioxidant-Mediated Protection in Colitis-Associated Cancer. Gastroenterology 2021, 160, 1694–1708.e3.
- Bohn, T.; Desmarchelier, C.; El, S.N.; Keijer, J.; van Schothorst, E.; Rühl, R.; Borel, P. β-Carotene in the human body: Metabolic bioactivation pathways—From digestion to tissue distribution and excretion. Proc. Nutr. Soc. 2019, 78, 68–87.
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087.
- Sandhiya, L.; Zipse, H. Conformation-dependent antioxidant properties of β-carotene. Org. Biomol. Chem. 2021, 20, 152–162.
- Kake, T.; Imai, M.; Takahashi, N. Effects of β-carotene on oxazolone-induced atopic dermatitis in hairless mice. Exp. Dermatol. 2019, 28, 1044–1050.
- Marcelino, G.; Machate, D.J.; Freitas, K.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 9, 5803.
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53.
- Honarbakhsh, M.; Malta, K.; Ericsson, A.; Holloway, C.; Kim, Y.K.; Hammerling, U.; Quadro, L. β-carotene improves fecal dysbiosis and intestinal dysfunctions in a mouse model of vitamin A deficiency. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2022, 1867, 159122.
- Cheng, J.; Balbuena, E.; Miller, B.; Eroglu, A. The Role of β-Carotene in Colonic Inflammation and Intestinal Barrier Integrity. Front. Nutr. 2021, 27, 723480.
- Grar, H.; Dib, W.; Gourine, H.; Negaoui, H.; Taleb, B.H.F.; Louaar, A.; Ouldhocine, S.; Kaddouri, H.; Kheroua, O.; Saidi, D. β-Carotene improves intestinal barrier function by modulating proinflammatory cytokines and improving antioxidant capacity in β-lactoglobulin-sensitize. J. Biol. Regul. Homeost. Agents 2020, 34, 1689–1697.
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544.
- Xu, G.; Ma, T.; Zhou, C.; Zhao, F.; Peng, K.; Li, B. β-Carotene Attenuates Apoptosis and Autophagy via PI3K/AKT/mTOR Signaling Pathway in Necrotizing Enterocolitis Model Cells IEC-6. Evid. Based Complement. Alternat Med. 2022, 17, 2502263.
- Zhu, L.; Song, Y.; Liu, H.; Wu, M.; Gong, H.; Lan, H.; Zheng, X. Gut microbiota regulation and anti-inflammatory effect of β-carotene in dextran sulfate sodium-stimulated ulcerative colitis in rats. J. Food Sci. 2021, 86, 2118–2130.
- Kuang, H.; Ma, Y.; Liu, Y. Protective effect of β-carotene on OVA-induced food allergy in mice by strengthening intestinal epithelial barrier function and regulating intestinal microflora. Food Funct. 2022, 13, 12330–12341.
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Dias, M.; Pinto, D.; Silva, A. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377.
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442.
- Bernatoniene, J.; Kopustinskiene, D. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 26, 965.
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628.
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419.
- Yi, Y. Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses. Mol. Nutr. Food Res. 2018, 62, e1800147.
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.; Li, S. Citrus flavonoids and the intestinal barrier: Interactions and effects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 225–251.
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370.
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The Potential Role of Phytonutrients Flavonoids Influencing Gut Microbiota in the Prophylaxis and Treatment of Inflammatory Bowel Disease. Front. Nutr. 2021, 14, 798038.
- Wang, K.; Lv, Q.; Miao, Y.; Qiao, S.; Dai, Y.; Wei, Z. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509.
- Hu, S.; Zhao, M.; Li, W.; Wei, P.; Liu, Q.; Chen, S.; Zeng, J.; Ma, X.; Tang, J. Preclinical evidence for quercetin against inflammatory bowel disease: A meta-analysis and systematic review. Inflammopharmacology 2022, 30, 2035–2050.
- Farzaei, M.H.; El-Senduny, F.F.; Momtaz, S.; Parvizi, F.; Iranpanah, A.; Tewari, D.; Naseri, R.; Abdolghaffari, A.H.; Rezaei, N. An update on dietary consideration in inflammatory bowel disease: Anthocyanins and more. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 1007–1024.
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. Dogaru. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Alzoghaibi, M. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547.
- Liu, P.; Li, Y.; Wang, R.; Ren, F.; Wang, X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines 2021, 10, 85.
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants 2018, 7, 159.
- Ferro, D.; Bakiu, R.; Pucciarelli, S.; Miceli, C.; Vallesi, A.; Irato, P.; Santovito, G. Molecular Characterization, Protein-Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila. Antioxidants 2020, 9, 949.
- Chovanová, K.; Böhmer, M.; Poljovka, A.; Budiš, J.; Harichová, J.; Szemeš, T.; Zámocký, M. Parallel Molecular Evolution of Catalases and Superoxide Dismutases-Focus on Thermophilic Fungal Genomes. Antioxidants 2020, 9, 1047.
- Marwicka, J.; Zięba, A. Antioxidants as a defence against reactive oxygen species. Aesth Cosmetol. Med. 2021, 10, 271–276.
- Xiao, B.H.; Shi, M.; Chen, H.; Cui, S.; Wu, Y.; Gao, X.H.; Chen, H.D. Glutathione Peroxidase Level in Patients with Vitiligo: A Meta-Analysis. Biomed. Res. Int. 2016, 2016, 3029810.
- Dayer, R.; Fischer, B.B.; Eggen, R.I.; Lemaire, S.D. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics 2008, 179, 41–57.
- Socca, E.A.; Luiz-Ferreira, A.; de Faria, F.M.; de Almeida, A.C.; Dunder, R.J.; Manzo, L.P.; Brito, A.R. Inhibition of tumor necrosis factor-alpha and cyclooxigenase-2 by Isatin: A molecular mechanism of protection against TNBS-induced colitis in rats. Chem. Biol. Interact. 2014, 25, 48–55.
- Weydert, C.; Cullen, J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66.
- Ekoue, D.; He, C.; Diamond, A.; Bonini, M. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 628–632.
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108.
- Kahl, R.; Kampkötter, A.; Wätjen, W.; Chovolou, Y. Antioxidant Enzymes and Apoptosis. Drug. Metab. Rev. 2004, 36, 747–762.
- Rana, S.; Sharma, S.; Prasad, K.; Sinha, S.; Singh, K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J. Med. Res. 2014, 139, 568–571.
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354.
- Iborra, M.; Moret, I.; Busó, E.; García-Giménez, J.L.; Ricart, E.; Gisbert, J.P.; Cabré, E.; Esteve, M.; Márquez-Mosquera, L.; García-Planella, E.; et al. The Genetic Diversity and Dysfunctionality of Catalase Associated with a Worse Outcome in Crohn’s Disease. Int. J. Mol. Sci. 2022, 23, 15881.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
709
Revisions:
2 times
(View History)
Update Date:
26 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




