Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia A. Shnayder | -- | 3160 | 2023-05-24 14:55:08 | | | |
| 2 | Rita Xu | Meta information modification | 3160 | 2023-05-25 03:13:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nasyrova, R.F.; Shnayder, N.A.; Osipova, S.M. Antipsychotic Efflux Impairment via Blood-Brain Barrier. Encyclopedia. Available online: https://encyclopedia.pub/entry/44783 (accessed on 07 February 2026).
Nasyrova RF, Shnayder NA, Osipova SM. Antipsychotic Efflux Impairment via Blood-Brain Barrier. Encyclopedia. Available at: https://encyclopedia.pub/entry/44783. Accessed February 07, 2026.
Nasyrova, Regina F., Natalia A. Shnayder, Sofia M. Osipova. "Antipsychotic Efflux Impairment via Blood-Brain Barrier" Encyclopedia, https://encyclopedia.pub/entry/44783 (accessed February 07, 2026).
Nasyrova, R.F., Shnayder, N.A., & Osipova, S.M. (2023, May 24). Antipsychotic Efflux Impairment via Blood-Brain Barrier. In Encyclopedia. https://encyclopedia.pub/entry/44783
Nasyrova, Regina F., et al. "Antipsychotic Efflux Impairment via Blood-Brain Barrier." Encyclopedia. Web. 24 May, 2023.
Copy Citation
Antipsychotic (AP)—induced adverse drug reactions (ADRs) are a current problem of biological and clinical psychiatry. Despite the development of new generations of APs, the problem of AP-induced ADRs has not been solved and continues to be actively studied. One of the important mechanisms for the development of AP-induced ADRs is a genetically-determined impairment of AP efflux across the blood-brain barrier (BBB).
antipsychotic
transport protein
P-gp
BCRP
MRP1
adverse drug reaction
1. Introduction
Schizophrenia spectrum disorders (SSDs) are one of the serious and socially significant mental disorders, the prevalence of which reaches millions of cases in the world [1][2]. For the treatment of SSDs, psychotropic drugs of the antipsychotics (APs) group are widely used [3]. Despite the generation of new APs (Figure 1), the problem of AP-induced adverse drug reactions (ADRs) has not been solved [4]. The accumulated experience of predicting and purposeful prevention of ADRs caused by AP indicates that most of them can be prevented (for example, neurotoxic effects of AP) and/or significantly reduce the frequency and severity of symptoms of these ADRs (for example, cognitive disorders, psychoses, extrapyramidal disorders, etc.) [5][6][7]. This problem leads to: a decrease in the quality of life of patients; a decrease in adherence of patients with SSDs to chronic APs therapy; the development of SSDs’ pseudo-resistance to the prescribed APs; progression of SSDs [8][9]. The study of the mechanisms of development of AP-induced ADRs is based on changes in their metabolism and transport, depending on the following risk factors for ADRs [8][10][11]: (1) modifiable factors (choice of APs, dosage, dosing regimen, consideration of comorbid conditions, etc.) [8][10]; (2) non-modifiable factors (gender, age of patients, genetic predisposition) [8][10].
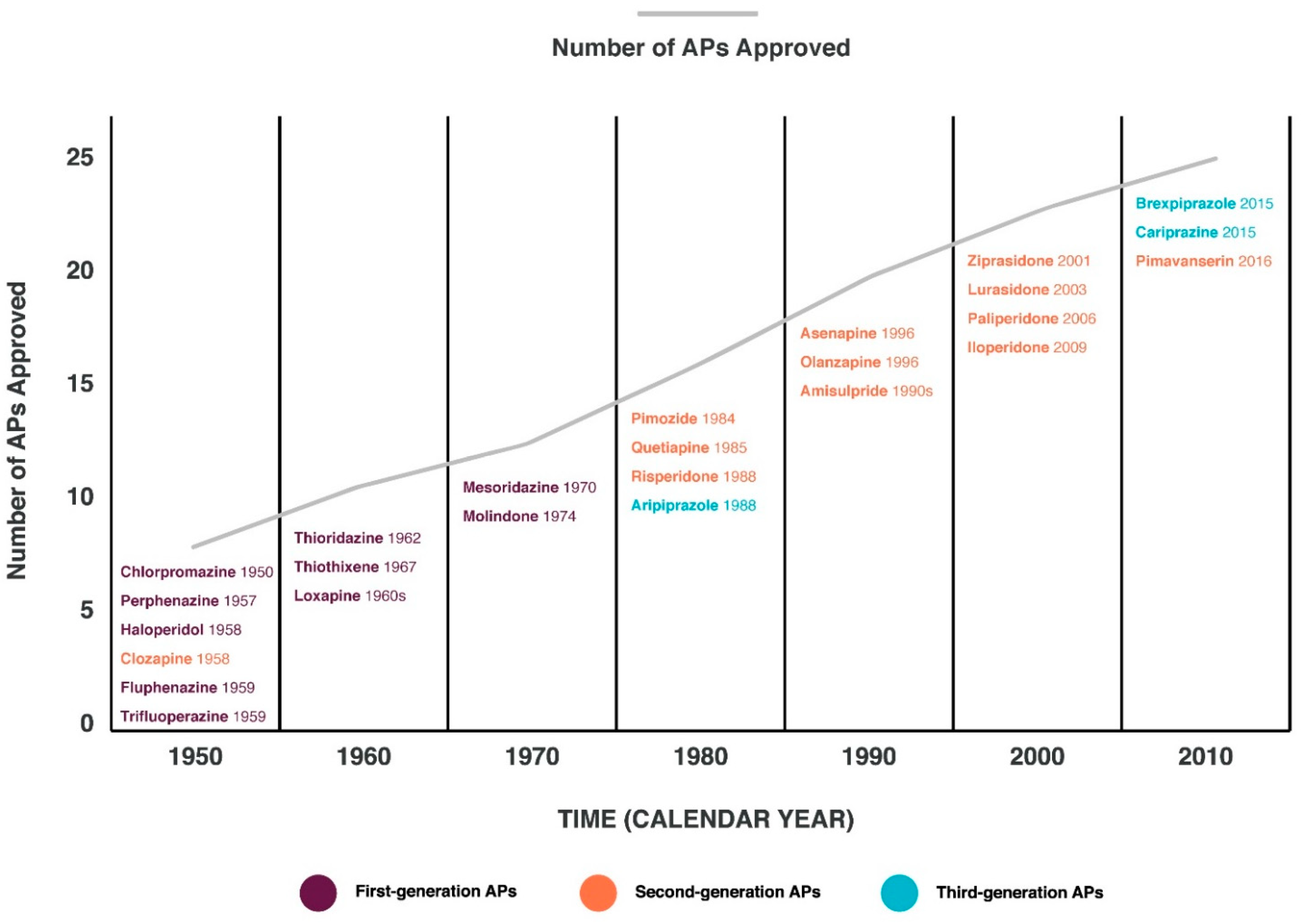
Figure 1. Timeline of antipsychotics (APs).
The study of genetic predisposition to the development of AP-induced neurotoxic ADRs is based on associative genetic studies and genome-wide studies of single nucleotide variants (SNVs) or polymorphisms of the candidate genes encoding key enzymes and proteins involved in the metabolism, transport, cumulation, excretion of APs and their active metabolites [8][10][11]. An important aspect of the efficacy and safety of SSD therapy, especially the prediction and prevention of the development of neurotoxic ADRs, is a genetically determined impairment of the efflux of APs across the blood-brain barrier (BBB) [12][13][14][15].
2. Blood-Brain Barrier
The BBB is a complex heterogeneous brain system with several levels of selective transport, regulation, and protection, capable of maintaining central nervous system (CNS) homeostasis and protecting the brain from potentially harmful endogenous and exogenous substances [16][17][18]. The BBB is a physical and metabolic barrier between the brain and the systemic circulation that serves to regulate and protect the brain microenvironment [19]. The structural units that make up the BBB perform not only protective, but also regulatory, nutritional and excretory functions. The main functional and anatomical elements of the BBB are brain capillary endotheliocytes, astrocytes, neurons, and pericytes, which are a “neurovascular unit” [20] (Figure 2).
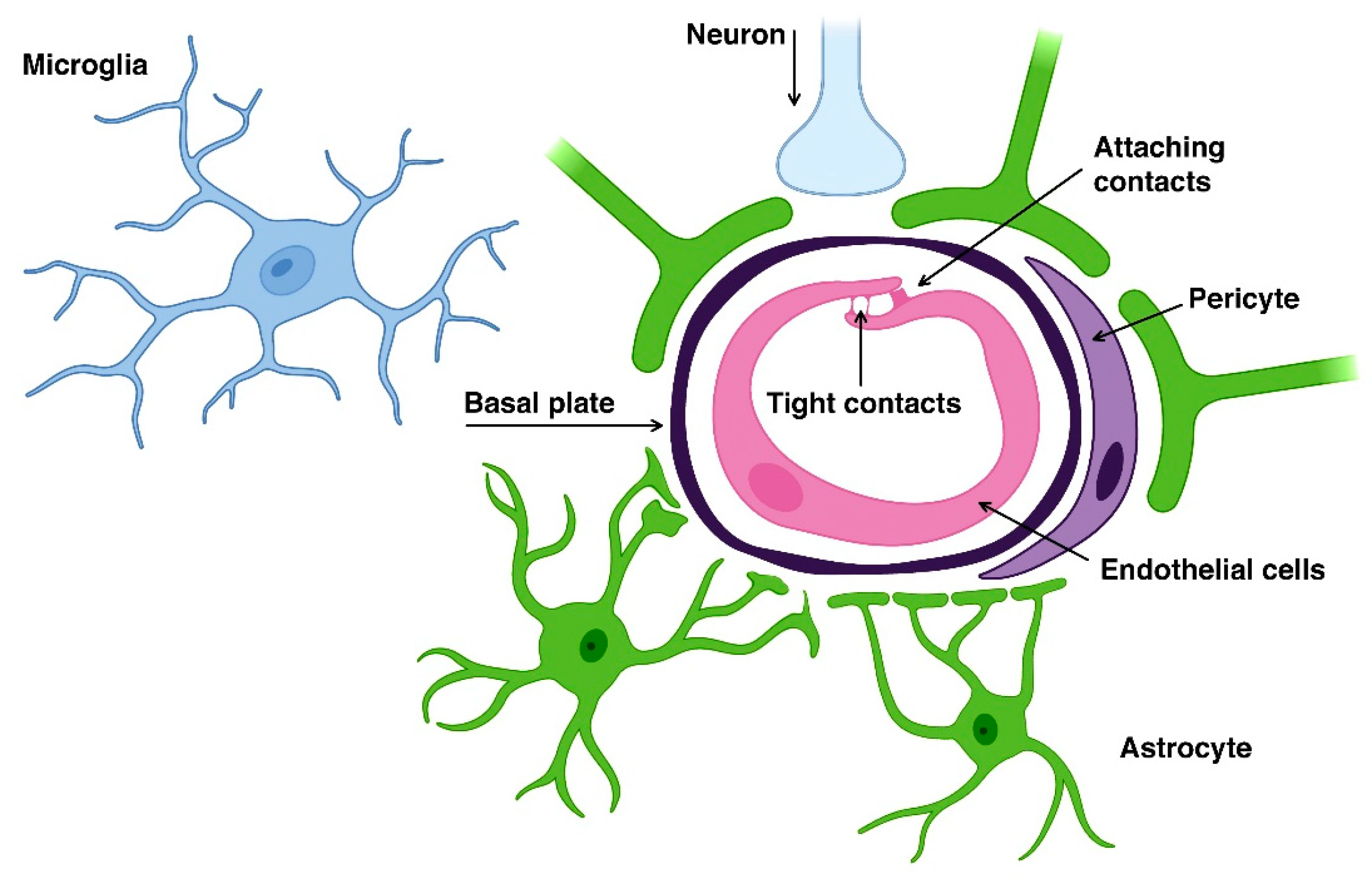
Figure 2. Neurovascular unit of the blood-brain barrier.
The BBB consists of a monolayer of endothelial cells in the capillaries of the brain [17][18][21]. The limited coverage of the BBB by the brain occurs due to the presence of tight contacts (occlusal zones) between neighboring endothelial cells and a relatively small number of fenestra and pinocytic vesicles in the endothelium of cerebral arterioles, capillaries, and venules [22]. The endothelial cells of the brain capillaries are surrounded by pericytes and astrocyte pedicels. Due to the presence of the BBB, circulating molecules do not have free access to the brain [17][18][21].
3. Transport Proteins of Antipsychotic Efflux via the Blood-Brain Barrier
The human ABC vector superfamily contains 49 members, which are subdivided into 7 subfamilies (from ABCA to ABCG) [23][24]. These transport proteins are localized on various membranes of cell organelles (with the exception of ABCE and ABCF), where they function as ATP-dependent and unidirectionally pump various endogenous and exogenous compounds transmembrane [24][25]. As outflow pumps, these transport proteins perform a wide range of physiological functions, including protective, excretory, and regulatory functions. They create barriers between the systemic circulation and many organs, such as the brain, cerebrospinal fluid (CSF), placenta, and testes, thereby limiting the penetration of APs and toxic metabolites, ensuring their active efflux (excretion) and, therefore, protecting these organs [26]. They are also expressed in the liver and kidneys, where they secrete xenobiotics and endogenous compounds. In addition, they limit the absorption of APs into the systemic circulation due to the release of APs into the gastrointestinal tract. Transport proteins of the ABCB family regulate many endogenous molecules that affect lipid and bile acid synthesis, antigen presentation, heme and iron homeostasis, transport and homeostasis of steroid hormones, and signaling molecules [24].
The most studied and clinically significant transport proteins that provide the efflux of AP across the BBB and the membrane of target neurons of AP action [23][27] (Figure 3):
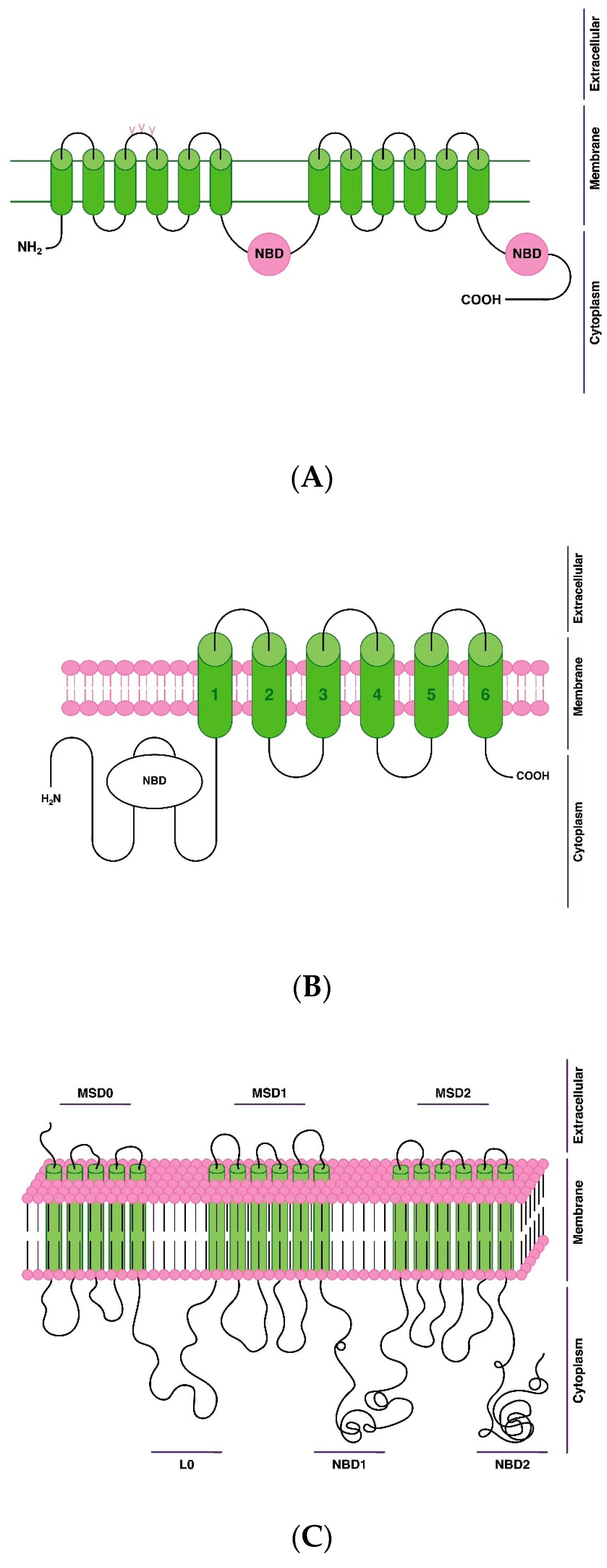
Figure 3. Scheme of the structure and localization of antipsychotic efflux transport proteins via blood-brain barrier on the cell membrane of endotheliocytes of the blood-brain barrier: (A)—P-glycoprotein (P-gp); (B)—breast cancer resistance protein (BCRP); (C)—multidrug resistance-associated protein 1 (MRP1). Note: L0—ligand 0; nucleotide binding domain—NBD; membrane spanning domain—MSD.
-
P-gp, or multidrug resistance protein 1 (MDR1);
-
breast cancer resistance protein (BCRP);
-
multidrug resistance-associated protein (MRP11).
Glycoprotein-P (P-gp) is a membrane transport protein with a wide range of endogenous and exogenous substrates. P-gp is located in hepatocytes, enterocytes and epithelial cells of the proximal renal tubules, neurons, and endotheliocytes of histo-hematic barriers, including the BBB. Increased activity of P-gp is associated with the development of drug-resistant forms of SSD, and reduced activity is associated with delayed AP efflux through the BBB and with the risk of the development of neurotoxic ADRs [12][26][28].
P-gp (or MDR1) is a member of the ATP-binding cassette (ABC) transporter superfamily and is an ATP-dependent efflux pump for drugs and other xenobiotics. ABC proteins transport various molecules across extracellular and intracellular membranes. The ABC family proteins are divided into seven subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP, GCN20, White). This protein is a member of the MDR/TAP subfamily and is encoded by the ABCB1 gene. Members of the MDR/TAP subfamily are involved in multidrug resistance [24][26].
The secondary structure of the P-gp protein is well known. This transport protein has a single polypeptide chain with N- and C-ends located inside the cytoplasmic region, and 12 transmembrane domains are located inside the plasma membrane. Also, P-gp contains 2 nucleotide binding domains (NBDs) that act as ATP binding sites. The first extracellular loop of P-gp contains three glycosylation sites [29].
P-gp expression in the brain is highest at the level of the frontal, medial and mediobasal cortex, in the hippocampus, tail, and also in other organs: adrenal glands, liver, gallbladder, small and large intestines, kidneys, ovaries, and fallopian tubes (in women) [30].
P-gp is encoded by the ABCB1 gene (chr7: 87,503,017-87,713,323 (GRCh38/hg38)). The molecular structure of the ABCB1 gene includes 28 exons and 28 introns [31]. The ABCB1 gene is highly polymorphic. The literature mentions about 100 SNVs/polymorphisms identified in different regions. However, only some of them are associated with the efflux of APs and lead to clinically significant changes in their transport [28][32][33][34]. The identification of low-functional and non-functional SNVs/polymorphisms of the ABCB1 gene is of clinical interest, since it is associated with an increased risk of AP-induced neurotoxic ADRs and a decreased safety of short-term and, especially, chronic psychopharmacotherapy.
BCRP is a membrane protein, ATP-binding cassette transporter. It is encoded by the ABCG2 gene. BCRP is one of five members of the human ABC protein superfamily G subfamily, along with ABCG1, ABCG4, ABCG5, and ABCG8. All members of the subfamily are semi-transporters and thus are thought to function as homo- or heterodimers or perhaps even as larger oligomeric structures [35].
Another feature of the ABC transporters G subfamily is that the nucleotide binding domain (NBD) is N-terminal to the transmembrane domain, while the opposite is true for other subfamilies of ABC transporters [36].
All members of the ABCG family, with the exception of BCRP, are known to be lipid transporters. In contrast, BCRP demonstrates perhaps the broadest substrate specificity and transports hydrophobic compounds, cations, anions, and drug conjugates [37]. It is known that this protein is involved in the transportation of APs and may play a role in the development of multidrug therapeutic resistance [35][36][37][38][39]. This protein consists of 655 amino acid residues, and contains one glycosylation site, one intramolecular disulfide bond and one intermolecular one. The intermolecular S–S bond provides stability of this transport protein dimer [36]. Basically, it is located on the cell membrane as a dimer, but can form oligomers up to a homododecamer (12 subunits) [35][39].
The BCRP expression in the brain is highest at the level of the frontal, medial and mediobasal cortex, in the hippocampus, tail, and also in other organs: seminal vesicles, testicles (in men), small and large intestines, placenta, lungs, thyroid gland, adrenal glands, myocardium [30].
The BCRP is encoded by the ABCG2 gene (chr4:88,090,150-88,231,628 (GRCh38/hg38)). The human ABCG2 gene consists of 16 exons and 15 introns [31]. At least 38 SNVs of the ABCG2 gene are mentioned in the literature, and in some sources 45–50 SNVs/polymorphisms of this gene [32][33][34].
The MRP1 is a protein associated with multidrug resistance 1, is encoded by the ABCC1 gene and is a member of the ABC superfamily [40]. It transports various molecules across extracellular and intracellular membranes. This transport protein is a member of the MRP subfamily that is associated with multidrug resistance. MRP1 is a peculiar member of the ABC transporter superfamily in several aspects. This transport protein has an unusually wide substrate specificity and is able to transport not only a wide range of neutral hydrophobic compounds, but also promote the efflux of numerous glutathione, glucuronate, and sulfate conjugates [40][41]. The transport mechanism of MRP1 is also complex: the composite substrate binding site provides both cooperativity and competition between different substrates [41]. This versatility and ubiquitous distribution in tissues make this transporter suitable for participation in various physiological functions, including the transport of various drugs, including APs [40].
This protein consists of five main domains (membrane spanning domains (MSD0, MSD1, MSD2)) and nucleotide-binding domains (NBD1 and NBD2) [41][42].
MRP1 expression in the brain is highest at the level of the frontal, orbitofrontal, and medial cortex, as well as in other organs: small and large intestines, liver, ovaries, fallopian tubes and endometrium (in women), adipose tissue, tonsils, lungs, pancreas, skin, bone marrow [30].
MRP1 is encoded by the ABCC1 gene (chr16:15,949,138-16,143,257 (GRCh38/hg38)). The ABCC1 gene contains 31 exons [31]. In studies of various populations, especially those of Asian and European origin, a large number of SNVs/polymorphisms of the ABCC1 gene have been found. Studies have shown that only a few of the 71 discovered SNVs/gene polymorphisms changed the amino acid sequence of the transport protein they encode, so ABCC1 is considered a highly conserved gene. Most of the genetic variations that have been described are found in intron sequences rather than exons [32][33][34].
4. Genetic Predisposition to Antipsychotic Efflux via the Brain-Blood Barrier
Genetic variants influence the effects of APs in terms of favorable and unfavorable results of psychopharmacological therapy, the development of ADRs, and the neurotoxicity of APs [43][44]. Many studies have shown that pharmacokinetics and neurotoxic ADRs induced by APs vary in patients with SSDs with certain genetic profiles. There is sufficient evidence of a clinically significant effect of patient genotype on the balance between benefit and risk of a wide range of first and new generation APs. Evidence-based guidelines with pharmacotherapeutic recommendations for combinations of specific APs and genotypes or predicted phenotypes in patients with SSDs are needed to implement the acquired knowledge of pharmacogenetics into daily clinical psychiatric practice [9][45].
In the case of a genetically determined decrease in the functional activity or expression of the P-gp, BCRP, and MRP1 transport proteins at the level of BBB endothelial cell membranes, the efflux of APs from the brain into the blood is disturbed to varying degrees (decreases significantly, insignificantly, or moderately) [23]. This, in turn, leads to an increase in the time of exposure of these APs to the brain, an increased risk of cumulation during chronic (long-term) psychopharmacotherapy, and an increased risk of developing serious AP-induced neurotoxic ADRs [26]. The accumulation of APs ultimately leads to a slowdown in their metabolism due to the enzymatic system, and therefore the phenotype of such patients with SSDs is more often referred to as a slow metabolizer rather than a slow transporter [10].
4.1. Phenotyping of Patients Depending on Antipsychotic Efflux Reduction via the Blood-Brain Barrier
Pharmacogenetic testing (PGx) has become a more popular laboratory diagnostic method over the past decade, but it is not yet a routine test in psychiatry. However, there is growing evidence that a significant proportion of neurotoxic ADRs in patients with SSDs are affected by a phenotype determined by homozygous or heterozygous carriage of full functional, low functional and non-functional allelic variants of the genes encoding transport proteins responsible for efflux of the first and new generations APs through the BBB [12][36][46][47].
There are several organizations such as the Dutch Pharmacogenetics Working Group (DPWG)), the Clinical Pharmacogenetics Implementation Consortium (CPIC) [48], the Canadian Drug Safety Pharmacogenomics Network (CPNDS) [49], the French National Network of Pharmacogenetics (Réseau) (RNPGx) [50], and the Russian Society of Pharmacogenetics, Pharmacokinetics and Personalized Therapy (SPPPT) [51], who develop clinical guidelines to help the clinician translate the results of previously performed associative genetic and genome-wide associative (GWAS) studies into clinical guidelines for treatment. In general, these organizations recommend pharmacogenetic testing in routine patient care if the clinical benefit to patients is considered significant, such as by reducing the risk of ADRs or the risk of drug treatment failure (therapeutic resistance or pseudo-resistance). However, most international and national clinical guidelines on clinical pharmacogenetics do not take into account the potential effect of SNVs/polymorphisms of the genes encoding transport proteins on the efflux of APs from the brain into the blood via the BBB, the strength of the interaction, or the complex interaction caused by the combination of SNVs/polymorphisms of the genes encoding the P-gp, BCRP, and MRP1 transport proteins and enzymes involved in the APs metabolism, when there are multiple biotransformation pathways. Not surprisingly, these recommendations sometimes contradict each other [52].
Additional sources of information about the pharmacogenetics of APs are the Summary Product Characteristics (SmPC) approved by the European Medicines Agency (EMA) [53] and other agencies, as well as instructions for prescribing APs approved by the US Federal Drug Agency (FDA) [54]. The number of APs with pharmacogenetic information in their SMPC or labels has increased over the years due to regulatory guidelines and policies. In addition, the PharmGKB website [55] is an open support tool for the pharmacogenetics of AP with collected and classified evidence for combinations of APs and other drugs and the genes.
As researchers better understand the influence of various genetic variants on the pharmacokinetics and levels of APs in the brain, possible personalized approaches for prediction and prevention of neurotoxic ADRs of AP therapy for SSDs become more and more clear.
Depending on the decrease in the functional activity of the key transport proteins that carry out the speed of the efflux of APs via the BBB from the brain into the blood [23][36][38], three phenotypes of patients with SSDs can be distinguished: poor transporter; intermediate transporter; extensive transporter.
4.1.1. Poor Transporter
Poor transporters (PT, also known as “poor metabolizers”—PM [56][57]) are patients whose transport proteins have a significantly reduced functional activity, resulting in a significantly reduced efflux of APs via the BBB. The PT phenotype occurs when both alleles of the ABCB1, ABCG2 and/or ABCC1 genes carry non-functional SNVs and give rise to the synthesis of transport protein with impaired functional activity or significantly reduce its expression in the BBB.
Typically, SSDs patients having this phenotype are homozygous for non-functional or low-functional SNVs/polymorphisms of the gene(es) ABCB1, ABCG2 and/or ABCC1 encoding one or more transport proteins (P-gp, BCRP, and/or MRP1, respectively). Accordingly, in such patients, APs accumulate in the brain in high concentrations, which leads to a significant increase in the risk of developing serious AP-induced neurotoxic ADRs.
4.1.2. Intermediate Transporter
Intermediate transporters (IT, also known as “intermediate metabolizers”—IM [56][57]) are patients whose transport proteins have a moderately reduced functional activity, resulting in a moderately reduced efflux of APs via the BBB. Typically, SSDs patients with this phenotype are heterozygous for non-functional or low-functional SNVs/polymorphisms of the gene (es) ABCB1, ABCG2 and/or ABCC1 encoding transport proteins (P-gp, BCRP, and/or MRP1, respectively). In such patients, the synthesis of a “defective” transport protein or a moderate decrease in the expression of a normal transport protein at the level of the BBB occurs, as a result of which the functional activity of the transport protein and the efflux of APs efflux via the BBB are reduced. Accordingly, with intermediate transporters, APs accumulate in the brain, which leads to a moderate increase in the risk of AP-induced neurotoxic ADRs.
4.1.3. Extensive Transporter
Extensive transporters (ET, also known as “extensive metabolizers”—EM [56][57]) are patients whose transport proteins have normal functional activity, which ensures a preserved (average) rate of efflux of APs via the BBB. SSDs patients with this transport protein phenotype are homozygous for a fully functional (“wildtype”) SNVs/polymorphisms of the genes) ABCB1, ABCG2 and/or ABCC1 encoding transport proteins (P-gp, BCRP, and/or MRP1, respectively). Accordingly, in these patients with SSDs, the risk of developing AP-induced neurotoxic ADRs is population average (mild).
4.2. Prediction of a Genetically Determined Decrease of Antipsychotic Efflux via the Blood Brain Barrier
The tactics of a psychiatrist who prescribes APs to patients with SSDs need a personal approach [7][36][38][47]. Thus, in carriers of risk alleles of non-functional or low-functional SNVs/polymorphisms of the ABCB1, ABCG2 and/or ABCC1 genes encoding transport proteins involved in the efflux of APs via the BBB, it is important to cumulatively evaluate as many previously studied non-functional and low-functional SNVs/polymorphisms of the genes encoding transport proteins involved in efflux through the BBB as possible, for APs of the first and new generations. Such a cumulative assessment of the genetic risk of speed decrease of the efflux of APs via the BBB and the development of AP-induced neurotoxic ADRs significantly modifies and significantly improves the currently existing personalized psychopharmacotherapy strategy for SSDs and explains the importance of developing PGx and its application in real clinical psychiatric practice [58].
In this regard, it is possible to conditionally divide the SNVs/polymorphisms into groups: low-functional and non-functional SNVs/polymorphisms of the ABCB1 gene; low-functional and non-functional SNVs/polymorphisms of the ABCG2 gene; low-functional and non-functional SNVs/polymorphisms of the ABCC1; proven in experimental (on an animal model) and clinical (in patients with mental disorders) studies, the effects of risk alleles of non-functional and low-functional SNVs/polymorphisms of these genes on changes in the activity of the transport proteins encoded by them and the level of their expression in the BBB in patients with SSDs.
To search and update the knowledge, information in the following open access databases may be used: (1) databases for evaluating the expression of transport proteins and their encoding genes (The Human Protein Atlas [30], The Human Gene Database (GeneCards [31], and databases “Gene” and “Protein” of the US National Library of Medicine [33]; (2) database of SNVs/polymorphisms of human genes (SNPedia) [32], Online Mendelian Inheritance in Man (OMIM) [59], SNP database of the US National Library of Medicine [33]; (3) pharmacogenetic database (PharmGKB) [34].
References
- McCutcheon, R.A.; Marques, R.T.; Howes, O.D. Schizophrenia—An overview. JAMA Psychiatry 2020, 77, 201–210.
- Orrico-Sánchez, A.; López-Lacort, M.; Muñoz-Quiles, C.; Sanfélix-Gimeno, G.; Díez-Domingo, J. Epidemiology of schizophrenia and its management over 8-years period using real-world data in Spain. BMC Psychiatry 2020, 20, 149.
- Katona, L.; Bitter, I.; Czobor, P. A meta-analysis of effectiveness of real-world studies of antipsychotics in schizophrenia: Are the results consistent with the findings of randomized controlled trials. Transl. Psychiatry 2021, 11, 510.
- Neznanov, N.G.; Nasyrova, R.F.; Shnayder, N.A. From classical to personalized psychoneurology. Pers. Psychiatry Neurol. 2022, 2, 1–3.
- Bahta, M.; Ogbaghebriel, A.; Russom, M.; Tesfamariam, E.H.; Berhe, T. Impact of adverse reactions to first-generation antipsychotics on treatment adherence in outpatients with schizophrenia: A cross-sectional study. Ann. Gen. Psychiatry 2021, 20, 27.
- Kaar, S.J.; Natesan, S.; McCutcheon, R.; Howes, J.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 2020, 172, 107704.
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356.
- Nasyrova, R.F.; Neznanov, N.G. (Eds.) Clinical Psychopharmacologenetics: Monography; DEAN Publishing House: St. Petersburg, Russia, 2019; pp. 1–405. ISBN 978-5-6043573-7-8.
- Neznanov, N.G. A paradigm shift to treat psychoneurological disorders. Pers. Psychiatry Neurol. 2021, 1, 1–2.
- Nasyrova, R.F.; Ivanov, M.V.; Neznanov, N.G. Introduction to Psychopharmacogenetics: Monography; DEAN Publishing House: St. Petersburg, Russia, 2015; pp. 1–272. ISBN 978-5-7452-0020-5.
- Xu, Q.; Wu, X.; Xiong, Y.; Xing, Q.; He, L.; Qin, S. Pharmacogenomics can improve antipsychotic treatment in schizophrenia. Front. Med. 2013, 7, 180–190.
- Alemayehu, D.; Melisie, G.; Taye, K.; Tadesse, E. The role of ABC efflux transporter in treatment of pharmaco-resistant schizophrenia: A review article. Clin. Pharmacol. Biopharm. 2019, 8, 189.
- Luptáková, D.; Vallianatou, T.; Nilsson, A.; Shariatgorji, R.; Hammarlund-Udenaes, M.; Loryan, I.; Andrén, P.E. Neuropharmacokinetic visualization of regional and subregional unbound antipsychotic drug transport across the blood–brain barrier. Mol. Psychiatry 2021, 26, 7732–7745.
- Eng, M.E.; Imperio, G.E.; Bloise, E.; Matthews, S.G. ATP-binding cassette (ABC) drug transporters in the developing blood–brain barrier: Role in fetal brain protection. Cell. Mol. Life Sci. 2022, 79, 415.
- Osipova, S.M.; Shnayder, N.A. Pharmacogenetic testing of antipsychotic transporter proteins: A case report in a 32-year-old woman with treatment-resistant schizophrenia. Pers. Psychiatry Neurol. 2022, 2, 98–106.
- Gupta, M.; Lee, H.J.; Barden, C.J.; Weaver, D.F. The blood–brain barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836.
- Benz, F.; Liebner, S. Structure and function of the blood–brain barrier (BBB). In Physiology, Pharmacology and Pathology of the Blood-Brain Barrier: Handbook of Experimental Pharmacology; Cader, Z., Neuhaus, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 12–23.
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The evolving concept of the blood brain barrier (BBB): From a single static barrier to a heterogeneous and dynamic relay center. Front. Cell. Neurosci. 2019, 13, 405.
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98.
- Gorbachev, V.I.; Bragina, N.V. Blood-brain barrier from the point of view of anesthesiologist. Review. Part 1. Ann. Crit. Care 2020, 3, 35–45.
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69.
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatr. 2018, 44, 131.
- Bruckmueller, H.; Cascorbi, I. ABCB1, ABCG2, ABCC1, ABCC2, and ABCC3 drug transporter polymorphisms and their impact on drug bioavailability: What is our current understanding. Expert Opin. Drug Metab. Toxicol. 2021, 17, 369–396.
- Szakács, G.; Homolya, L.; Sarkadi, B.; Váradi, A. MDR-ABC transporters. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 748–752.
- Thomas, C.; Tampé, R. Structural and mechanistic principles of ABC transporters. Annu. Rev. Biochem. 2020, 89, 605–636.
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316.
- Daood, M.; Tsai, C.; Ahdab-Barmada, M.; Watchko, J.F. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008, 39, 211–218.
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of genetic polymorphisms of ABCB1 (MDR1, P-glycoprotein) on drug disposition and potential clinical implications: Update of the literature. Clin. Pharmacokinet. 2015, 54, 709–735.
- Tulsyan, S.; Mittal, R.; Mittal, B. The effect of ABCB1 polymorphisms on the outcome of breast cancer treatment. Pharmgenomics Pers. Med. 2016, 9, 47–58.
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 15 August 2022).
- GeneCards: The Human Gene Database. Available online: https://www.genecards.org/ (accessed on 15 August 2022).
- SNPedia. Available online: https://www.snpedia.com/ (accessed on 15 August 2022).
- The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 August 2022).
- PharmGKB. Available online: https://www.pharmgkb.org/ (accessed on 15 August 2022).
- Mo, W.; Zhang, J.T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27.
- Sarkadi, B.; Homolya, L.; Hegedűs, T. The ABCG2/BCRP transporter and its variants–from structure to pathology. FEBS Lett. 2020, 594, 4012–4034.
- Jani, M.; Ambrus, C.; Magnan, R.; Jakab, K.T.; Beéry, E.; Zolnerciks, J.K.; Krajcsi, P. Structure and function of BCRP, a broad specificity transporter of xenobiotics and endobiotics. Arch. Toxicol. 2014, 88, 1205–1248.
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. 2019, 9, 659–674.
- Schwabedissen, H.E.; Kroemer, H.K. In vitro and in vivo evidence for the importance of breast cancer resistance protein transporters (BCRP/MXR/ABCP/ABCG2). Handb. Exp. Pharmacol. 2011, 201, 325–371.
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888.
- Hipfner, D.R.; Deeley, R.G.; Cole, S.P. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta (BBA)–Biomembr. 1999, 1461, 359–376.
- Wei, M.; Jing-Yuan, L.; Jian-Ting, Z. Biochemistry and pharmacology of human ABCC1/MRP1 and its role in detoxification and in multidrug resistance of cancer chemotherapy. Recent Adv. Cancer Res. Ther. 2012, 12, 371–404.
- Abdyrakhmanova, A.K.; Shnayder, N.A.; Nasyrova, R.F. A clinical case of late pharmacogenetic diagnosis of adverse reactions during psychopharmacotherapy in a patient with recurrent depressive disorder. Pharm. Pharm. 2021, 2, 21–23.
- Sychev, D.A. Clinical and pharmacological technologies of personalized medicine: What now and what will happen? V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2019, 4, 24–25.
- Dobrodeeva, V.S.; Shnayder, N.A.; Nasyrova, R.F. Pharmacogenetic aspects of the efficacy and safety of trazodone therapy. Pharm. Pharm. 2019, 1, 25–28.
- Girardin, F. Membrane transporter proteins: A challenge for CNS drug development. Dialogues Clin. Neurosci. 2006, 8, 311–321.
- Malla, S.; Muskiewicz, D.E.; Hussein, N.A.; Hall, F.S.; Tiwari, A.K. The role of ABC transporters in the actions of drugs of abuse. In Handbook of Substance Misuse and Addictions; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–18.
- The Clinical Pharmacogenetics Implementation Consortium (CPIC®). Available online: https://cpicpgx.org/ (accessed on 15 August 2022).
- The Canadian Pharmacogenomics Network for Drug Safety. Available online: https://cpnds.ubc.ca/ (accessed on 15 August 2022).
- Morningstar. Available online: https://www.morningstar.com/funds/xnas/rnpgx/quote (accessed on 15 August 2022).
- The Russian Society of Pharmacogenetics, Pharmacokinetics and Personalized Therapy (SPPPT). Available online: https://xn--80aaalkavmenpjw0bt.xn--p1ai/ (accessed on 15 August 2022).
- Abdullah-Koolmees, H.; van Keulen, A.M.; Nijenhuis, M.; Deneer, V.H.M. Pharmacogenetics guidelines: Overview and comparison of the DPWG, CPIC, CPNDS, and RNPGx guidelines. Front. Pharmacol. 2021, 11, 595219.
- The European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en (accessed on 15 August 2022).
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/ (accessed on 15 August 2022).
- Barbarino, J.M.; Whirl-Carrillo, M.; Altman, R.B.; Klein, T.E. PharmGKB: A worldwide resource for pharmacogenomic information. WIREs Syst. Biol. Med. 2018, 10, e1417.
- Hahn, M.; Roll, S.C. The influence of pharmacogenetics on the clinical relevance of pharmacokinetic drug–drug interactions: Drug–gene, drug–gene–gene and drug–drug–gene interactions. Pharmaceuticals 2021, 14, 487.
- Bousman, C.A.; Dunlop, B.W. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. Pharm. J. 2018, 18, 613–622.
- Shnayder, N.A.; Khasanova, A.K.; Strelnik, A.I.; Al-Zamil, M.; Otmakhov, A.P.; Neznanov, N.G.; Shipulin, G.A.; Petrova, M.M.; Garganeeva, N.P.; Nasyrova, R.F. Cytokine imbalance as a biomarker of treatment-resistant schizophrenia. Int. J. Mol. Sci. 2022, 23, 11324.
- OMIM: Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 15 August 2022).
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
724
Revisions:
2 times
(View History)
Update Date:
25 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




