Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoshihisa Koyama | -- | 2690 | 2023-05-24 08:39:46 | | | |
| 2 | Fanny Huang | Meta information modification | 2690 | 2023-05-25 08:24:46 | | | | |
| 3 | Fanny Huang | Meta information modification | 2690 | 2023-05-25 08:33:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Koyama, Y.; Kobayashi, Y.; Kobayashi, H.; Shimada, S. Antioxidant Effects of the Si-Based Agent. Encyclopedia. Available online: https://encyclopedia.pub/entry/44750 (accessed on 07 February 2026).
Koyama Y, Kobayashi Y, Kobayashi H, Shimada S. Antioxidant Effects of the Si-Based Agent. Encyclopedia. Available at: https://encyclopedia.pub/entry/44750. Accessed February 07, 2026.
Koyama, Yoshihisa, Yuki Kobayashi, Hikaru Kobayashi, Shoichi Shimada. "Antioxidant Effects of the Si-Based Agent" Encyclopedia, https://encyclopedia.pub/entry/44750 (accessed February 07, 2026).
Koyama, Y., Kobayashi, Y., Kobayashi, H., & Shimada, S. (2023, May 24). Antioxidant Effects of the Si-Based Agent. In Encyclopedia. https://encyclopedia.pub/entry/44750
Koyama, Yoshihisa, et al. "Antioxidant Effects of the Si-Based Agent." Encyclopedia. Web. 24 May, 2023.
Copy Citation
Antioxidant therapy is an effective approach for treating diseases in which oxidative stress is involved in the onset of symptoms. This approach aims to rapidly replenish the antioxidant substances in the body when they are depleted due to excess oxidative stress. Importantly, a supplemented antioxidant must specifically eliminate harmful reactive oxygen species (ROS) without reacting with physiologically beneficial ROS, which are important to the body.
silicon
antioxidant
reactive oxygen species
hydrogen
1. Introduction
Reactive oxygen species (ROS) produced by respiration and immunoreactions are highly oxidizing. Under normal conditions, they are eliminated by in vivo antioxidant substances (superoxide dismutase, glutathione, and catalase). However, when excessive ROS production is caused by chronic diseases, viral or bacterial infection, and gluttony, the antioxidant mechanism in the body cannot eliminate ROS sufficiently. Consequently, important cellular components are oxidized by ROS, resulting in tissue damage and dysfunction. “Oxidative stress” is a pathological condition caused by a combination of overproduced ROS and depleted antioxidant substances in the body [1][2][3]. Oxidative stress is involved not only in the onset and symptomatic exacerbation of various diseases—such as inflammatory, metabolic, neurological, and ischemic diseases and cancer—but also in aging [1][2][3]. Accordingly, it is useful to administer exogenous antioxidants as replacements for depleted in vivo antioxidant substances [4]. Unfortunately, many antioxidants—such as vitamin C (VC), vitamin E (VE), polyphenols, and carotene—can have adverse effects, because they also remove useful biogenic ROS (superoxide, hydrogen peroxide, etc.) that are involved in normal physiological activities [5][6][7][8][9][10]. For example, overdoses of VC and VE induce nausea, vomiting, and increased cancer risk [11][12][13]. Moreover, when the effective doses of VC and VE, as determined in animal experiments, are converted to their human equivalents, the resulting doses are much higher than the upper tolerable limits for VC (2 g/day) or VE (1 g/day) recommended by the Food and Nutrition Board of the National Academic Medicine [14]. Paradoxically, excess vitamin C acts as a prooxidant and can worsen symptoms [15]. Thus, even if promising results are obtained through basic research, the clinical application of antioxidant therapy presents challenges. Given the above information, the discovery of hydrogen as an antioxidant in 2007 was highly significant [16].
Hydrogen selectively eliminates harmful ROS such as hydroxyl radicals and has known efficacy in alleviating the pathology of various diseases [17][18]. Moreover, with no reported side effects, hydrogen antioxidant therapy is safe [19]. However, the in vivo administration methods can be improved. Currently, there are two main methods: hydrogen gas inhalation and drinking water. The former is limited to specific settings because of the risk of explosion, whereas in the latter, only small amounts of hydrogen can be dissolved in water (1.6 ppm), which cannot sustain elevated hydrogen concentrations over long periods because of its excellent hydrogen permeability. In contrast, the Si-based agent reacted with water to generate hydrogen and made it possible to fill the rodent intestinal tract with a large amount of hydrogen via the oral administration of its agent-containing diet (Figure 1) [20][21]. So far, Si-based agents have alleviated the pathology of ROS-mediated diseases, such as model colitis (UC), Parkinson’s disease, renal failure, and facial paralysis [21][22][23]. Researchers believe that Si-based agents offer effective solutions to the above-mentioned challenges in hydrogen antioxidant therapy.

Figure 1. (A) Scanning electron micrographs of two different specimen positions of Si-based agent consisting of agglomerates of Si nanopowder which was fabricated via the bead-milling method and surface treatment using a hydrogen peroxide oxide solution. Small (left) and large (right) agglomerates. (B) Specially ordered diets fed to the experimental animals. AIN93M (left) and AIN93M containing 2.5% Si-based agent (right).
Previous reports have demonstrated the efficacy of Si-based agents for each disease. However, there have been no reports investigating the detailed action mechanism of the antioxidant effect and its various properties in Si-based agents. A cross-sectional analysis that transcends the framework of each paper has revealed an interesting hypothesis that Si-based agents have a variety of effects, and that these effects are exerted in various organs via the circulatory system. Researchers describe the characteristics and efficacy of Si-based agents in detail and discuss the potential of SI-based agents in treating ROS-mediated conditions and their hypothetical action mechanisms.
2. Development of a New Antioxidant Si-Based Agent
The Si-based agent was fabricated from polycrystalline Si powder (Osaka Titanium technologies Co., Ltd., Osaka, Japan; Si 5N Powder 2–45 µm). After bead-milling the Si powder in ethanol, a surface treatment was performed using a hydrogen peroxide solution to enhance the surface reactivity. To improve the safety of the Si-based agent, an agglomeration treatment was performed. The average crystallite size was 20–30 nm. Figure 1 shows the scanning electron micrographs of the Si-based agent. It was clearly seen that the Si-based agent consisted of agglomerates of Si nanopowder. The size of the agglomerates was 0.1–20 μm.
Figure 2 shows the volume of hydrogen generated by reaction of the Si-based agent with a pH 8.2 aqueous solution at 36 °C [20][21]. This hydrogen generation reaction continued for more than 24 h.
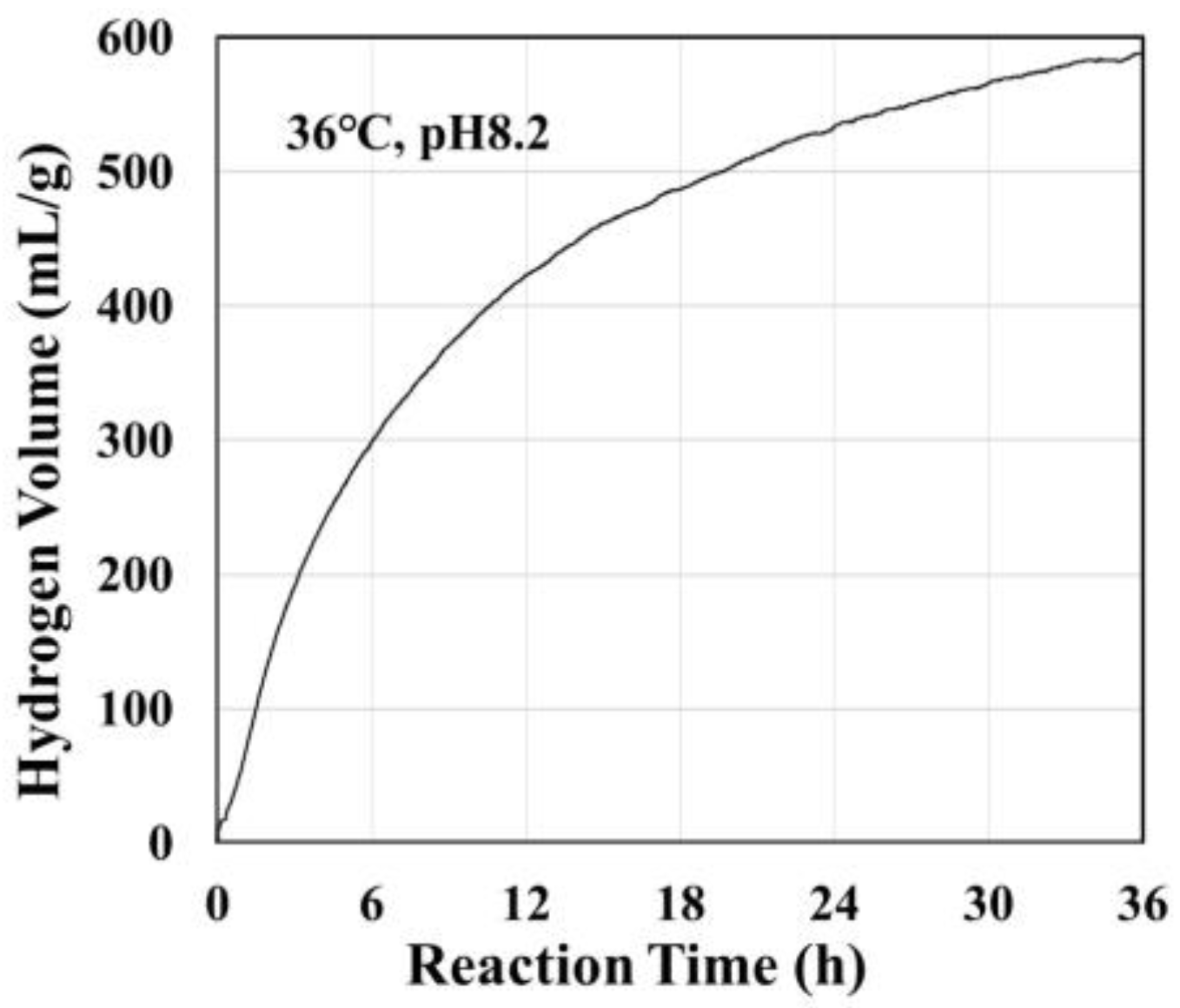
Figure 2. Volume of hydrogen generated by the reaction of Si-based agent with a pH 8.2 solution at 36 °C vs. the reaction time. The volume of generated hydrogen was determined by measurements of the dissolving hydrogen concentration using a potable dissolving hydrogen-meter. The solution was confined in a stainless container in which no gas phase was present, and therefore, all generated hydrogen dissolved in the solution.
It has been observed that the hydrogen generation rate significantly increases with the pH of the reacting solutions, while the pH of the reacting solutions themselves remains unchanged [20]. From these experimental results, researchers derived the following reaction formulas for hydrogen generation:
Si + 2OH− → SiO2 + 2H (or H2) + 2e (1)
2H2O + 2e → 2OH− + 2H (or H2) (2)
Si + 2H2O → SiO2 + 4H (or 2H2) (3)
Because the rate of Reaction (1) is much lower than that of Reaction (2), the overall hydrogen generation reaction (Reaction (3)) rate is determined by Reaction (1): In Reaction (1), OH− ions react with Si, and therefore, the reaction rate greatly increases with an increase in the concentration of OH− ions, i.e., pH. Additionally, in Reaction (1), OH- ions are consumed, while in Reaction (2), they are generated in an equivalent quantity, and hence, after the overall Reaction (3), the pH of the resulting solution is unchanged.
Figure 3 shows the mechanism of hydrogen generation using a Si-based agent. Before the reaction, the Si-based agent was covered with a silicon oxide layer 1–2 nm thick, and this thickness increased as the hydrogen generation reaction proceeded (cf. Reaction (3)) [24]. In the first reaction step, OH- ions move inward through the silicon oxide layer. The inward movement of OH- ions is enhanced by the electrical field induced by the OH- ions adsorbed on the surface of the silicon oxide layer [20]. In the second step, interface Reaction (3) proceeds when OH- ions reach the Si-based agent/silicon oxide interface. The interfacial reactions involve four elementary reactions [25].
2Si + OH− → Si2O + H + e (4)
Si2O + OH− → 2SiO + H +e (5)
2SiO + OH− → Si2O3 + H + e (6)
Si2O3 + OH− → SiO2 + H + e (7)
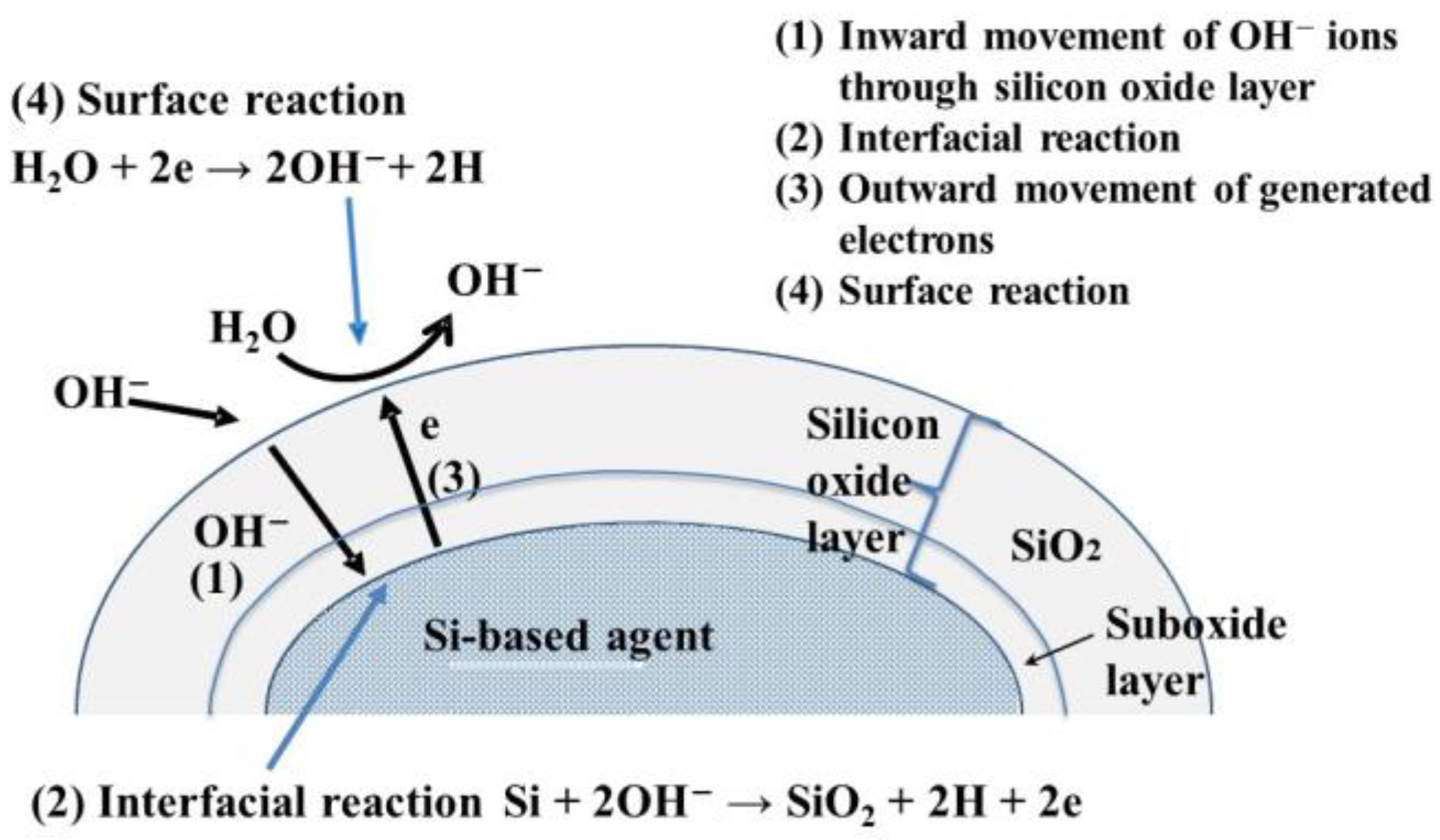
Figure 3. Mechanism of hydrogen generation by the reaction of Si-based agent with aqueous solutions, e.g., pancreatic juice and intestinal juice.
Electrons generated by Reactions (4)–(7) move outward and are trapped in the surface states (i.e., the characteristic energy levels in the bandgap at the surface). Then, water molecules accept these electrons, resulting in the formation of OH− ions and hydrogen. Thus, hydrogen is generated in the interfacial region and at the surface. Researchers observed that hydrogen atoms were bound to the surface, and interfacial Si atoms were present after the hydrogen generation reaction. It is highly probable that these hydrogen atoms are the source of the high reducing power of the Si-based agent. In animal experiments, researchers observed that the concentrations of reactive sulfur species increased with the administration of the Si-based agent, and it is thought that the hydrogen atoms on Si-based agents play an important role in their formation.
3. Antioxidant Effects of the Si-Based Agent
3.1. Antioxidant Action via Hydrogen
As described in the previous section, Si-based agents react with water to generate hydrogen [20]. Hydrogen is an antioxidant that specifically scavenges harmful ROS such as hydroxyl radicals. It has become clear that antioxidant therapy using hydrogen (i.e., hydrogen medicine) alleviates the pathology of many diseases, such as brain ischemia–reperfusion (IR) injury, Parkinson’s disease, and hepatitis [17][18]. Since no side effects have been reported thus far [19], hydrogen is expected to be an excellent antioxidant therapeutic agent. The antioxidant action of the Si-based agent resulted from hydrogen generated on the surface of the agent. Because the hydrogen generation reaction of the Si-based agent occurs under neutral to alkaline conditions, activity at the hydrogen generation site is influenced by the pH of the intestinal tract. The method researchers devised to measure mouse intestinal pH [26] demonstrated that the ileum (pH 7.3) to the rectum (pH 7.8) were somewhat alkaline, and the cecum was the most alkaline at pH 8.4 [21]. Surprisingly, the Si-based agent made the intestinal tract slightly more alkaline than in the normal state, facilitating the generation of hydrogen. The oral administration of the Si-based agent produced hydrogen in the mouse intestinal tract [21]. Moreover, the amount of hydrogen increased in the stomach, large intestine, and small intestine. Of course, the stomach is acidic (pH 3.7), and the results of in vitro experiments show that the Si-based agent does not generate hydrogen under acidic conditions; therefore, it is not conceivable that hydrogen is generated in the stomach. Because of its excellent permeability, hydrogen is thought to easily diffuse out of the intestinal tract from the site of generation [27]. However, because the amount of hydrogen generated by the Si-based agent may have exceeded the amount diffused outside the body, part of the generated hydrogen may have reached the stomach through the intestinal tract. Moreover, the Si-based agent generated a large amount of hydrogen in vitro [20]; however, the rate of increase in hydrogen generation after the Si-based agent was administered into the mouse intestinal tract is not very high [21]. As the capacity of the intestinal tract is fixed, it is conceivable that hydrogen is released outside the body owing to its permeability. In other words, the intestinal tract may have been saturated with hydrogen in the Si-based-agent-administered mice. Not only is it possible to respond to various pathological conditions by continuously transporting antioxidants from the stomach to the large intestine, but this process is also involved in the regulation of autonomic nerves such as hydrogen [28].
It has been reported that both VC and VE are depleted in patients with UC [29][30], and endogenous hydrogen is depleted in UC mouse models [21]. These findings suggest that the onset of UC causes excessive oxidative stress and that hydrogen is consumed and depleted as an antioxidant to relieve oxidative stress. The depletion of hydrogen in the livers of carbon-tetrachloride-induced hepatitis mouse models indicates that hydrogen, like other antioxidants, is used as an antioxidant when oxidative stress occurs [21]. Oxidative stress in the affected area is increased, which requires the elimination of many antioxidants. The administration of a Si-based agent alleviates the aggravation of disease conditions by replenishing the depleted hydrogen in affected areas, such as the large intestine and liver. Hence, Si-based agents are expected to constitute excellent antioxidant treatments for such conditions.
Lipid peroxides also oxidize other substances, and polyunsaturated fatty acids in particular cause ferroptosis, which is iron-dependent cell death [31]. The suppression of the generation and increase in lipid peroxides is necessary to control oxidative stress and cell death. According to previous reports, hydrogen tends to accumulate in the lipid bilayer of cell membranes [32]. Hydrogen is more abundant and soluble in unsaturated fatty acids (such as octanoic acid and linolenic acid) than in water. Calcium influx is inhibited in cells with cell membranes containing dissolved hydrogen. As calcium is a second messenger in cell signal transduction [33], it is possible that hydrogen regulates biological functions and exerts antioxidant effects through calcium signaling.
Because lipid peroxides are among the targets of hydrogen, Si-based agents may also play a major role in mitigating lipid oxidation. Si-based agents alleviate lipid peroxide accumulation in various diseases. The oxidative stress analysis based on lipid oxidation in blood showed that a Si-based agent alleviated systemic oxidative stress associated with pathological conditions in mouse models of UC, chronic renal failure, facial paralysis, and interstitial pneumonia [21][22][23][34]. In particular, researchers investigated the inhibition of lipid peroxide production in UC mouse models. Si-based agents suppress the increase in early products (hexanoyl-lysine) and lipid peroxides in the blood and increase 4-Hydroxy-2-nonenal (an index of the lipid peroxide production chain reaction) in the colon [21]. Moreover, Si-based agents inhibit the increase in malondialdehyde, the final product of lipid peroxidation, in chronic renal failure, renal ischemia–reperfusion (IR) injury, and IR injury during flap transplantation. Thus, it has been demonstrated that Si-based agents act at various stages of lipid oxidation.
Since Si-based agents inhibit the increase in urinary 8-hydroxy-2-deoxyguanosine in renal disease [22][35][36][37], these results indicate that Si-based agents eliminate various oxidative metabolites, especially lipid peroxides.
3.2. Activation of In Vivo Antioxidant Mechanisms
Si-based agents also affect the Nrf2-Keap1 system, an in vivo antioxidant mechanism [38]. Under non-oxidative stress, Nrf2 remains in the cytoplasm, where it binds with Keap1 and undergoes proteasome-dependent proteolytic repression. When cells are exposed to oxidative stress or electrophiles, the binding between Nrf2 and Keap1 is disrupted and Nrf2 is translocated to the nucleus, where it induces the expression of antioxidant genes such as Hmo-1 and cystathionine γ-lyase (Cth) [38]. Increased oxidative stress and decreased endogenous antioxidant factors were observed in the kidneys of chronic renal failure mouse models and the placentas of LPS-induced mother-to-child-infected mice; however, a Si-based agent suppressed the decrease in these factors and alleviated oxidative stress [35][39]. SIRT1, a histone deacetylase, activates Nrf2 [40]. Although SIRT1 expression was decreased in renal failure mouse models, this decrease was mitigated by the administration of a Si-based agent [35]. These findings suggest that Si-based agents directly activate the Nrf2-Keap1 antioxidant system.
Sulfur metabolites (e.g., hydrogen sulfide and glutathione) significantly affect the redox state of living organisms [41]. According to the sulfur index analysis, which exhaustively analyzes sulfur-metabolizing compounds, the large intestines of UC mouse models were oxidized, whereas when the same mice were administered a Si-based agent, their large intestines were in a reduced state. Thus, Si-based agents alleviate the oxidation associated with UC, according to redox indices based on sulfur metabolites [21]. This suggests that Si-based agents have a positive effect on the in vivo redox system involving sulfur metabolites. In particular, an increase in strongly reactive sulfur species (RSS) such as glutathione polysulfide was involved in the suppression of inflammation-associated oxidation in the large intestine in a UC mouse models treated with anSi-based agent. RSS, represented by cysteine persulfide—a molecule with an excess sulfur atom added to the thiol (SH) group of cysteine—is a bioactive substance with strong antioxidant and anti-inflammatory effects and the ability to regulate redox signals [42][43][44][45][46]. In particular, Si-based agents increase the levels of glutathione polysulfides. Glutathione and glutathione peroxidase degrade lipid peroxides into harmless lipid alcohols [47][48]. Therefore, Si-based agents can detoxify lipid peroxides through the glutathione/glutathione peroxidase system.
In infected mouse placenta, a Si-based agent also increased the expression of Cth, a cysteine synthesis enzyme, suggesting the possibility of activating not only RSS but also the entire sulfur metabolism pathway [21][39]. Furthermore, RNA sequencing of kidneys with IR injury revealed that the expression of oxidative-stress-related factors was decreased after the administration of a Si-based agent [37]. In fact, Si-based agents inhibit the decrease in levels of catalase associated with IR, an antioxidant enzyme that neutralizes hydrogen peroxide [49]. Taken together, these results suggest that Si-based agents affect NRF2-Keap1 and the glutathione/glutathione peroxidase systems involving RSS, activating the in vivo antioxidant mechanism.
In conclusion, Si-based agents eliminate oxidative stress by activating the antioxidant action of hydrogen and antioxidant mechanisms in the body, thereby reducing symptoms (Table 1).
Table 1. Antioxidant effects of Si-based agents against diseases.
| DISEASE | Administration of Si-Based Agent |
Refs. No. | |
|---|---|---|---|
| Pro-oxidant | The increase in oxidative stress in blood | Alleviation | [21][22][23][34] |
| The increase in LPO (HEL, 4-HNE, MDA) | Suppression | [21][35][37][50] | |
| The increase in urinary 8-OHdG | Inhibition | [22][35][36][37] | |
| The oxidation in large intestine | Alleviation | [21] | |
| Antioxidant | The decrease in Nrf2-Keap1 system-induced factors (Hmox-1, Cystationine-γ-lyase) | Inhibition | [35][39] |
| The decrease in RSS * | Alleviation | [21] | |
| The decrease in SIRT1 | Alleviation | [35] | |
| The decrease in Catalase | Alleviation | [37] |
* The increase via the administration of Si-based agent even in normal conditions.
References
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020, 48, 124–127.
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Yadav, A.; Kumari, R.; Yadav, A.; Mishra, J.P.; Srivatva, S.; Prabha, S. Antioxidants and its functions in human body. Res. Environ. Life Sci. 2016, 9, 1328–1331.
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001, 20, 464S–472S; discussion 473S–475S.
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084.
- Caraballoso, M.; Sacristan, M.; Serra, C.; Bonfill, X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev. 2003, 2, CD002141.
- Bjelakovic, G.; Nagorni, A.; Nikolova, D.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Meta-analysis: Antioxidant supplements for primary and secondary prevention of colorectal adenoma. Aliment. Pharmacol. Ther. 2006, 24, 281–291.
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst. Rev. 2008, 3, CD004183.
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet 2004, 364, 1219–1228.
- Bjelakovic, G.; Gluud, C. Surviving antioxidant supplements. J. Natl. Cancer Inst. 2007, 99, 742–743.
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46.
- Lawson, K.A.; Wright, M.E.; Subar, A.; Mouw, T.; Hollenbeck, A.; Schatzkin, A.; Leitzmann, M.F. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J. Natl. Cancer Inst. 2007, 99, 754–764.
- Hathcock, J.N.; Azzi, A.; Blumberg, J.; Bray, T.; Dickinson, A.; Frei, B.; Jialal, I.; Johnston, C.S.; Kelly, F.J.; Kraemer, K.; et al. Vitamins E and C are safe across a broad range of intakes. Am. J. Clin. Nutr. 2005, 81, 736–745.
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184.
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694.
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252.
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11.
- Xun, Z.M.; Zhao, Q.H.; Zhang, Y.; Ju, F.D.; He, J.; Yao, T.T.; Zhang, X.K.; Yi, Y.; Ma, S.N.; Zhao, P.X.; et al. Effects of long-term hydrogen intervention on the physiological function of rats. Sci. Rep. 2020, 10, 18509.
- Kobayashi, Y.; Matsuda, S.; Imamura, K.; Kobayashi, H. Hydrogen generation by reaction of Si nanopowder with neutral water. J. Nanopart. Res. 2017, 19, 176.
- Koyama, Y.; Kobayashi, Y.; Hirota, I.; Sun, Y.; Ohtsu, I.; Imai, H.; Yoshioka, Y.; Yanagawa, H.; Sumi, T.; Kobayashi, H.; et al. Author Correction: A new therapy against ulcerative colitis via the intestine and brain using the Si-based agent. Sci. Rep. 2022, 12, 15150.
- Kobayashi, Y.; Imamura, R.; Koyama, Y.; Kondo, M.; Kobayashi, H.; Nonomura, N.; Shimada, S. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci. Rep. 2020, 10, 5859.
- Koyama, Y.; Harada, S.; Sato, T.; Kobayashi, Y.; Yanagawa, H.; Iwahashi, T.; Tanaka, H.; Ohata, K.; Imai, T.; Ohta, Y.; et al. Therapeutic strategy for facial paralysis based on the combined application of Si-based agent and methylcobalamin. Biochem. Biophys. Rep. 2022, 32, 101388.
- Kobayashi, Y.; Kowada, Y.; Shirohata, T.; Kobayashi, H. Changes in structure and surface properties of Si-based agent during hydrogen generation reaction. Appl. Surf. Sci. 2021, 535, 147361.
- Kobayashi, Y.; Fujita, S.; Imamura, K.; Kobayashi, H. Structure and hydrogen generation mechanism of Si-based agent. Appl. Surf. Sci. 2021, 536, 147398.
- Sun, Y.; Koyama, Y.; Shimada, S. Measurement of intraluminal pH changes in the gastrointestinal tract of mice with gastrointestinal diseases. Biochem. Biophys. Res. Commun. 2022, 620, 129–134.
- Suzuki, A.; Yukawa, H. A Review for Consistent Analysis of Hydrogen Permeability through Dense Metallic Membranes. Membranes 2020, 10, 120.
- Sugai, K.; Tamura, T.; Sano, M.; Uemura, S.; Fujisawa, M.; Katsumata, Y.; Endo, J.; Yoshizawa, J.; Homma, K.; Suzuki, M.; et al. Daily inhalation of hydrogen gas has a blood pressure-lowering effect in a rat model of hypertension. Sci. Rep. 2020, 10, 20173.
- Buffinton, G.D.; Doe, W.F. Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic. Res. 1995, 22, 131–143.
- Fernandez-Banares, F.; Abad-Lacruz, A.; Xiol, X.; Gine, J.J.; Dolz, C.; Cabre, E.; Esteve, M.; Gonzalez-Huix, F.; Gassull, M.A. Vitamin status in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1989, 84, 744–748.
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193.
- Iuchi, K.; Imoto, A.; Kamimura, N.; Nishimaki, K.; Ichimiya, H.; Yokota, T.; Ohta, S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci. Rep. 2016, 6, 18971.
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6.
- Shimada, M.; Koyama, Y.; Kobayashi, Y.; Kobayashi, H.; Shimada, S. Effect of the new silicon-based agent on the symptoms of interstitial pneumonitis. Sci. Rep. 2023, 13, 5707.
- Imamura, R.; Kawamura, M.; Taniguchi, A.; Kobayashi, Y.; Nakazawa, S.; Kato, T.; Abe, T.; Uemura, M.; Kobayashi, H.; Nonomura, N. Efficacy of a Si-based agent against developing renal failure in a rat remnant kidney model. Biochem. Biophys. Res. Commun. 2020, 533, 698–703.
- Inagaki, Y.; Fukuhara, S.; Imamura, R.; Kobayashi, Y.; Kuribayashi, S.; Okada, K.; Sekii, Y.; Takezawa, K.; Kiuchi, H.; Uemura, M.; et al. Novel hydrogen-producing Si-based agent reduces oxidative stress, and improves sperm motility and in vitro fertilization rate in varicocoele. Andrology 2021, 9, 376–383.
- Kawamura, M.; Imamura, R.; Kobayashi, Y.; Taniguchi, A.; Nakazawa, S.; Kato, T.; Namba-Hamano, T.; Abe, T.; Uemura, M.; Kobayashi, H.; et al. Oral Administration of Si-Based Agent Attenuates Oxidative Stress and Ischemia-Reperfusion Injury in a Rat Model: A Novel Hydrogen Administration Method. Front. Med. 2020, 7, 95.
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733.
- Usui, N.; Togawa, S.; Sumi, T.; Kobayashi, Y.; Koyama, Y.; Nakamura, Y.; Kondo, M.; Shinoda, K.; Kobayashi, H.; Shimada, S. Si-Based Hydrogen-Producing Nanoagent Protects Fetuses From Miscarriage Caused by Mother-to-Child Transmission. Front. Med. Technol. 2021, 3, 665506.
- Huang, K.; Gao, X.; Wei, W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017, 361, 63–72.
- Tabassum, R.; Jeong, N.Y.; Jung, J. Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases. Neural Regen Res. 2020, 15, 232–241.
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611.
- Shinkai, Y.; Abiko, Y.; Ida, T.; Miura, T.; Kakehashi, H.; Ishii, I.; Nishida, M.; Sawa, T.; Akaike, T.; Kumagai, Y. Reactive Sulfur Species-Mediated Activation of the Keap1-Nrf2 Pathway by 1,2-Naphthoquinone through Sulfenic Acids Formation under Oxidative Stress. Chem. Res. Toxicol. 2015, 28, 838–847.
- Millikin, R.; Bianco, C.L.; White, C.; Saund, S.S.; Henriquez, S.; Sosa, V.; Akaike, T.; Kumagai, Y.; Soeda, S.; Toscano, J.P.; et al. The chemical biology of protein hydropersulfides: Studies of a possible protective function of biological hydropersulfide generation. Free Radic. Biol. Med. 2016, 97, 136–147.
- Akiyama, M.; Unoki, T.; Shinkai, Y.; Ishii, I.; Ida, T.; Akaike, T.; Yamamoto, M.; Kumagai, Y. Environmental Electrophile-Mediated Toxicity in Mice Lacking Nrf2, CSE, or Both. Environ. Health Perspect. 2019, 127, 67002.
- Saund, S.S.; Sosa, V.; Henriquez, S.; Nguyen, Q.N.; Bianco, C.L.; Soeda, S.; Millikin, R.; White, C.; Le, H.; Ono, K.; et al. The chemical biology of hydropersulfides (RSSH): Chemical stability, reactivity and redox roles. Arch. Biochem. Biophys. 2015, 588, 15–24.
- Sevanian, A.; Muakkassah-Kelly, S.F.; Montestruque, S. The influence of phospholipase A2 and glutathione peroxidase on the elimination of membrane lipid peroxides. Arch. Biochem. Biophys. 1983, 223, 441–452.
- Fujita, T.; Fujimoto, Y. Formation and removal of active oxygen species and lipid peroxides in biological systems. Nihon Yakurigaku Zasshi 1992, 99, 381–389.
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553.
- Otani, N.; Tomita, K.; Kobayashi, Y.; Kuroda, K.; Koyama, Y.; Kobayashi, H.; Kubo, T. Hydrogen-generating Si-based agent protects against skin flap ischemia-reperfusion injury in rats. Sci. Rep. 2022, 12, 6168.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
682
Revisions:
3 times
(View History)
Update Date:
25 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




