Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xianmin Zhang | -- | 1542 | 2023-05-24 04:28:19 | | | |
| 2 | Conner Chen | + 1 word(s) | 1543 | 2023-05-26 04:11:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yin, X.; Deng, L.; Ruan, L.; Wu, Y.; Luo, F.; Qin, G.; Han, X.; Zhang, X. Phthalocyanine Single-Molecule Magnets. Encyclopedia. Available online: https://encyclopedia.pub/entry/44742 (accessed on 07 February 2026).
Yin X, Deng L, Ruan L, Wu Y, Luo F, Qin G, et al. Phthalocyanine Single-Molecule Magnets. Encyclopedia. Available at: https://encyclopedia.pub/entry/44742. Accessed February 07, 2026.
Yin, Xiang, Li Deng, Liuxia Ruan, Yanzhao Wu, Feifei Luo, Gaowu Qin, Xiaoli Han, Xianmin Zhang. "Phthalocyanine Single-Molecule Magnets" Encyclopedia, https://encyclopedia.pub/entry/44742 (accessed February 07, 2026).
Yin, X., Deng, L., Ruan, L., Wu, Y., Luo, F., Qin, G., Han, X., & Zhang, X. (2023, May 24). Phthalocyanine Single-Molecule Magnets. In Encyclopedia. https://encyclopedia.pub/entry/44742
Yin, Xiang, et al. "Phthalocyanine Single-Molecule Magnets." Encyclopedia. Web. 24 May, 2023.
Copy Citation
Single-molecule magnets (SMMs) have attracted much attention due to their potential applications in molecular spintronic devices. Rare earth SMMs are considered to be the most promising for application owing to their large magnetic moment and strong magnetic anisotropy. Phthalocyanines (Pcs) are large rings with 18π electron conjugation and have a wide range of applications in spintronics.
single-molecule magnets
rare earth elements
phthalocyanines
1. Introduction
Single-molecule magnets (SMMs), with the slow relaxation of magnetization and quantum tunneling [1][2][3], are considered a significant discovery in the field of nanomagnetism [4]. SMMs are often used to fabricate nanoscale devices and high-density data storage media [5][6][7][8][9]. Notably, Mn12 [1] and Fe cluster [10] are the earlier discovered SMMs, which belong to 3d SMMs.
Since 2003, the introduction of lanthanide rare earth ions has allowed SMMs to enter a new stage. Rare earth SMMs exhibit magnetic bistability at a higher blocking temperature (TB) than 3d SMMs because lanthanide ions are f-orbital-based elemental ions with unparalleled single-ion anisotropy, TB is a key performance parameter of an SMM, one description of which refers the maximum temperature at which it is possible to observe hysteresis in the field-dependence of the magnetization, subject to the field sweep rate. Meanwhile, phthalocyanines (Pcs) are large rings with 18π electron conjugation and have a wide range of applications in spintronics. Therefore, LnPc2 SMMs have shown great potential for spintronics and device applications. The first example of the [TbPc2]− effective energy barrier (Ueff, that is the potential energy required for molecular magnetization (or magnetic moment) reversal) of 331 cm−1 broke the record of Ueff for multinuclear 3d SMMs [11]. Subsequently, scientists have shown great interest in studying not only mononuclear rare earth SMMs but also binuclear rare earth SMMs and multinuclear rare earth SMMs [12][13].
2. Phthalocyanine Single-Molecule Magnets
SMMs are nanosized molecules with a stable magnetization intensity coming from within a single molecule and therefore can be used as independent magnetic functional units. In essence, a maximum value of the imaginary part of the magnetization related to the external field frequency occurs when Alternating Current magnetization rate tests are performed at low temperatures [1][14]. The development stages of monomolecular magnets are as follows. First, are transition metal monomolecular SMMs (Mn12 and Fe clusters); then are rare earth single-molecule magnets, mainly lanthanide-based metal SMMs. Therefore, the use of rare earth metal ions, especially Tb and Dy ions, to construct SMMs is still an effective method to improve the performance of SMMs [15].
The 4f orbitals of lanthanide ions are inner orbitals and thus have strong spin-orbit coupling, which allows the crystal field interaction to be regarded as perturbative. Therefore, Ln-SMMs (Ln = Tb, Dy) have become an important part of the field of SMMs, are widely favored by researchers and have been reported far more than other metallic SMMs, occupying half of the field of molecular magnets.
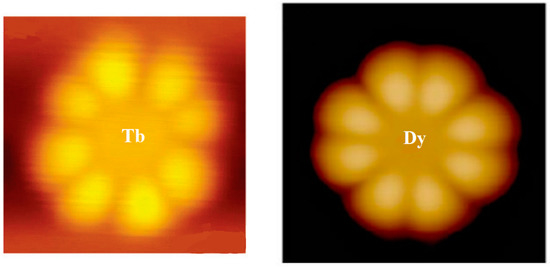
From Figure 1, it can be seen that the scanning tunneling microscopy (STM) images of LnPc2 (Ln = Tb, Dy) molecules observed by the experiment have the shape of eight lobes. However, DyPc2 is more regular than TbPc2 [16][17].
2.1. Structure and Category
Pc is an organic semiconductor with 18π electrons, and it has been demonstrated that Ln-Pc double- and triple-decker complexes are capable of forming [18][19][20][21][22][23][24][25][26][27][28][29]. The structures are shown in Figure 2 [30].
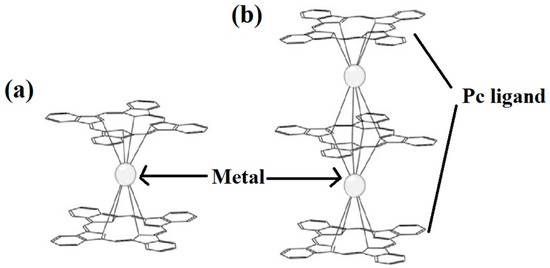
Figure 2. Structures of (a) double- decker metal Pcs, consisting of a metal ion sandwiched between two Pc ligands and (b) triple-decker metal Pcs, metal ions are stacked between sandwich-type Pc oligomers [30].
Compared to conventional magnetic particles composed of metals, metal alloys, or metal oxides at the nanoscale, SMMs have many important advantages: (1) SMMs consist of relatively independent molecular units, so they have a single size and a fixed structure [31]. (2) SMMs are generally soluble in organic solvents, which makes it possible to obtain magnetic materials that were previously available only under special conditions in chemical solutions under ordinary conditions. (3) The magnetic characteristics of SMMs can be refined through metal ions and Pcs and by improving the synthesis methods [32].
SMMs generally consist of an intrinsic metal nucleus surrounded by an organic ligand shell. Lanthanide elemental metal ions with a high spin ground state are good choices for the preparation of molecular materials with SMMs. However, designing the SMMs of such ions requires care to optimize the spatial distribution of ligand electrons with respect to the ion.
2.2. Double-Decker Pc of Tb/Dy
To date, more than one hundred Ln-SMMs have been discovered and studied. Owing to their excellent physical properties, Ln-Pcs (Ln = Tb, Dy) are widely favored by researchers [33][34][35][36][37]. The model of Ln-Pcs is shown in Figure 3a,b, the Ln3+ (Ln = Tb, Dy) ion is located in the center of the molecule with two parallel Pc rings to form a sandwich structure molecule. The double-decker Ln-Pcs (Ln = Tb, Dy) has D4d symmetry [33][34][38]. DyPc2 is similar in properties to TbPc2, which possesses an anisotropic Ueff of 410 cm−1 and a spin-orbit coupling quantum number of J = 6 [39].
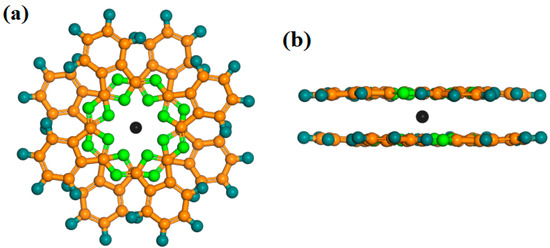
Figure 3. Diagrammatic sketch of [LnPc2]− (Ln = Tb, Dy), the angle between Pc ligands is 45°. (a) Top view; (b) side view. Colors: (Ln = Tb, Dy), black; N, green; C, orange; H, navy blue.
Rare earth Pcs were discovered by Kirin and Moskalev. Notably, double-decker Ln-Pcs could also be achieved at that time [40][41]. The crystal structure data of LnPc2 (Ln = Tb, Dy) are shown in Table 1 [16]. TbPc2 belongs to the same P212121 space group as DyPc2, and the crystal parameters are close in size.
Table 1. Crystal structure data of LnPc2 (Ln = Tb, Dy) [16].
| TbPc2 | DyPc2 | |
|---|---|---|
| Formula | C64H32N16Tb | C64H32N16Dy |
| Formula weight | 1183.99 | 1113.97 |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group | P212121(#19) | P212121(#19) |
| a (nm) | 0.88 | 0.89 |
| b (nm) | 1.06 | 1.06 |
| c (nm) | 5.08 | 5.08 |
| V (nm3) | 4.76 | 4.76 |
| Z | 4.00 | 4.00 |
| F(000) | 2372.00 | 2268.00 |
Dy ion-containing materials (such as magnetic resonance imaging, magnetostriction, and SMMs) have a wide range of promising applications in the magnetic field [42][43][44][45]. In SMMs, magnetic exchange interactions are important factors affecting the performance of SMMs, and early studies have shown that even very weak intermolecular magnetic exchange interactions can effectively suppress quantum tunneling effects and enhance the performance of SMMs [46][47]. Although the 4f electrons of rare earth ions are subject to the shielding effect of the outer electrons and the magnetic exchange between metal ions is relatively weak, this effect still has a significant impact on the properties of their SMMs.
Martínez-Flores et al. studied the geometries and electronic properties of LnPc2, as shown in Figure 4. They reported that unpaired electrons are transferred to Pc ligands [48], and the strong π-π interaction between intramolecular Pc rings becomes important for organic field effect transistors as intrinsic semiconductors compared to their monolayer analogs.
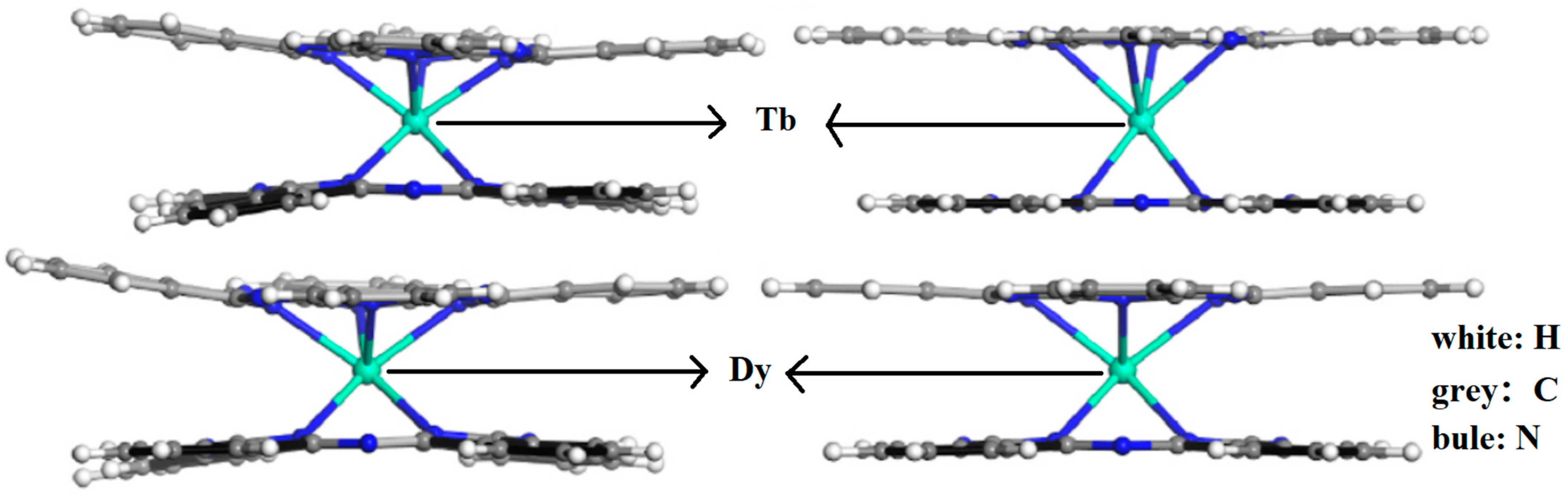
Figure 4. The structures of LnPc2 (Ln = Tb, Dy) compounds from X-ray diffraction (XRD) measurement (left) and density functional theory (DFT) calculation (right). In the DFT calculation, the PBE GGA correlation functional by Perdew-Burke-Ernzerhof (PBE) was the functional of choice, complemented by the empirical dispersion correction developed by Grimme [48].
The magnetic coupling of TbPc2 molecules was reported by Corradini and coworkers. They placed TbPc2, single-layer graphene, and an Au single-layer on top of a Ni(111) magnetic substrate. They found that the superexchange coupling leads to a change in the antiferromagnetic signal [49].
2.3. Multi-Decker Pc of Tb/Dy
Ln2-SMMs are SMMs containing two lanthanide element ions forming a large collection, and double-nuclear Pcs SMMs containing Dy and Tb are more common. The radially contracted nature of the 4f orbitals of rare earth ions tends to lead to extraordinarily weak intramolecular exchange coupling in multinuclear lanthanide complexes. Therefore, for most multinuclear Ln2-SMMs, the magnetic origin is mainly a single-ion effect.
There is another class of double nuclear Ln2-SMMs that are trilayer structured Pc SMMs, and the chemical general formula of these molecules is [PcLn(μ-Pc2)Ln(Pc3)] when the ligand Pc can be heterocyclic. The spacing between the Ln ions in the molecule is approximately 0.36 nm, which makes it possible to study the effect of intramolecular f-f interactions on the dynamic magnetic properties, and the lanthanide ions have a significant role in the physical properties of triple-decker Pc compounds [50].
Hellerstedt et al. reported a method to form Tb2Pc3 from TbPc2. The structures are shown in Figure 5a,b. The different colors (yellow and blue) of the densities represent the charge redistribution. The formation of Tb2Pc3 provides a novel way to investigate and control magnetic interactions [34].
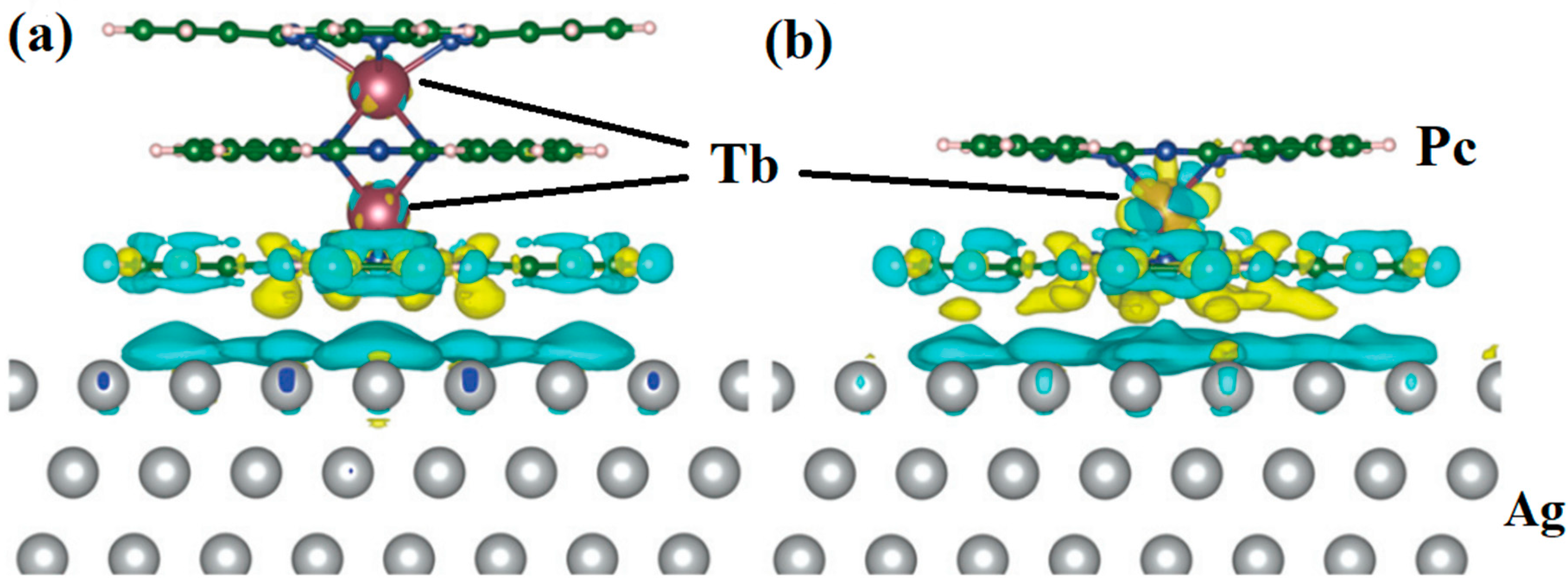
Figure 5. Calculated charge transfer between (a) Tb2Pc3 and (b) TbPc2 molecules and the Ag(111) surface obtained from DFT calculations. It used exchange correlation functional PBE + U with U = 5 eV for f-electrons of Tb and van der Waals interaction was approximated by the Tkatchenko-Scheffler dispersion correction method. The yellow and blue colors represent the accumulation and loss of density, respectively. The presence of blue density on the upper surface layer indicates substantial charge transfer from the metallic surface toward the molecule [34].
Ln3-SMMs can be divided into two main categories according to the structural arrangement of the metal ions: triangular and chain-like metal ion arrangements. Multinuclear Ln-SMMs are relatively rare in most rare earth elements because they are not easily synthesized due to their high nucleus numbers. Of course, Dy is the exception; the vast majority of rare earth SMMs with high nucleation numbers contain Dy, and the number of nuclei in Dy-SMMs can be as high as 50. However, in general, ligands for multinuclear Dy systems are not limited to Pcs.
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143.
- Wasielewski, M.R.; Forbes, M.D.E.; Frank, N.L.; Kowalski, K.; Scholes, G.D.; Yuen-Zhou, J.; Baldo, M.A.; Freedman, D.E.; Goldsmith, R.H.; Goodson, T., III; et al. Whaley. Exploiting chemistry and molecular systems for quantum information science. Nat. Rev. Chem. 2020, 4, 490–504.
- Bayliss, S.L.; Laorenza, D.W.; Mintun, P.J.; Kovos, B.D.; Freedman, D.E.; Awschalom, D.D. Optically addressable molecular spins for quantum information processing. Science 2020, 370, 1309–1312.
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-Molecule Magnet. MRS Bull. 2000, 25, 66–71.
- Wang, H.S.; Zhang, K.; Song, Y.; Pan, Z.Q. Recent advances in 3d-4f magnetic complexes with several types of non-carboxylate organic ligand. Inorg. Chim. Acta 2021, 521, 120318.
- Wang, H.L.; Liu, T.; Zhu, Z.H.; Peng, J.M.; Zou, H.H.; Liang, F.P. A series of dysprosium clusters assembled by a substitution effect-driven out-to-in growth mechanism. Inorg. Chem. Front. 2021, 8, 2136–2143.
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong exchange and magnetic blocking in N23−-radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542.
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186.
- Mannini, M.; Pineider, F.; Danieli, C.; Totti, F.; Sorace, L.; Sainctavit, P.; Arrio, M.A.; Otero, E.; Joly, L.; Cezar, J.C.; et al. Quantum tunnelling of the magnetization in a monolayer of oriented single-molecule magnets. Nature 2010, 468, 417–421.
- Sangregorio, C.; Ohm, T.; Paulsen, C.; Sesso, R.; Gatteschi, D. Quantum Tunneling of the Magnetization in an Iron Cluster Nanomagnet. Phys. Rev. Lett. 1997, 78, 4645–4648.
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695.
- Tang, J.K.; Hewitt, I.; Madhu, N.T.; Chastanet, G.; Wernsdorfer, W.; Anson, C.E.; Benelli, C.; Sessoli, R.; Powell, A.K. Dysprosium Triangles Showing Single-Molecule Magnet Behavior of Thermally Excited Spin States. Angew. Chem. Int. Edit. 2006, 45, 1729–1733.
- Rinehart, D.J.; Fang, M.; Evans, W.J.; Long, J.R. A N23− Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239.
- Gatteschi, D.; Sessoli, R. Molecular Nanomagnets; Oxford University: Oxford, UK, 2006.
- Hussain, B.; Savard, D.; Burchell, T.J.; Wernsdorfer, W.; Murugesu, M. Linking high anisotropy Dy3 triangles to create a Dy6 single molecule magnet. Chem. Commun. 2009, 9, 1100–1102.
- Katoh, K.; Yoshida, Y.; Yamashita, M.; Miyasaka, H.; Breedlove, B.K.; Kajiwara, T.; Takaishi, S.; Ishikawa, N.; Isshiki, H.; Zhang, Y.F.; et al. Direct Observation of Lanthanide(III)-Phthalocyanine Molecules on Au(111) by Using Scanning Tunneling Microscopy and Scanning Tunneling Spectroscopy and Thin-Film Field-Effect Transistor Properties of Tb(III)- and Dy(III)-Phthalocyanine Molecules. J. Am. Chem. Soc. 2009, 131, 9967–9976.
- Zhang, Y.J.; Wang, Y.F.; Liao, P.L.; Wang, K.; Huang, Z.C.; Liu, J.; Chen, Q.W.; Jiang, J.Z.; Wu, K. Detection and Manipulation of Charge States for Double-Decker DyPc2 Molecules on Ultrathin CuO Films. ACS Nano 2018, 12, 2991–2997.
- Avvisati, G.; Cardoso, C.; Varsano, D.; Ferretti, A.; Gargiani, P.; Betti, M.G. Ferromagnetic and antiferromagnetic coupling of spin molecular interfaces with high thermal stability. Nano Lett. 2018, 18, 2268–2273.
- Gruber, M.; Ibrahim, F.; Boukari, S.; Joly, L.; Costa, V.D.; Studniarek, M.; Peter, M.; Isshiki, H.; Jabbar, H.; Davesne, V. Spin-dependent hybridization between molecule and metal at room temperature through interlayer exchange coupling. Nano Lett. 2015, 15, 7921–7926.
- Javaid, S.; Bowen, M.; Boukari, S.; Joly, L.; Beaufrand, J.B.; Chen, X.; Dappe, Y.; Scheurer, F.; Kappler, J.P.; Arabski, J. Impact on Interface Spin Polarization of Molecular Bonding to Metallic Surfaces. Phys. Rev. Lett. 2010, 105, 077201.
- Ng, D.K.P.; Jiang, J. Sandwich-type heteroleptic phthalocyaninato and porphyrinato metal complexes. Chem. Soc. Rev. 1997, 26, 433–442.
- Kharisov, B.I.; Mendes-Rokhas, M.A.; Ganich, E.A. Traditional and electrochemical methods of synthesizing phthalocyanines and metal complexes on their base. Solvent effect. Russ. J. Coord. Chem. 2000, 26, 301–310.
- Nemykin, V.N.; Volkov, S.V. Mixed-ligand complexes of lanthanides with phthalocyanine and its analogues: Synthesis, structure, and spectroscopic properties. Russ. J. Coord. Chem. 2000, 26, 436–450.
- Buchler, J.W.; Ng, D.K.P.; Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Elsevier: Amsterdam, The Netherlands, 2000; pp. 245–294.
- Weiss, R.; Fischer, J.; Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Academic Press: New York, NY, USA, 2003; pp. 171–246.
- Kobayashi, N. Dimers, trimers and oligomers of phthalocyanines and related compounds. Coord. Chem. Rev. 2002, 227, 129–152.
- Jiang, J.; Liu, W.; Arnold, D.P. Sandwich complexes of naphthalocyanine with the rare earth metals. J. Porphyrins. Phthalocyanines 2003, 7, 459–473.
- Pushkarev, V.E.; Tomilova, L.G.; Tomilov, Y.V. Synthetic approaches to lanthanide complexes with tetrapyrrole type ligands. Russ. Chem. Rev. 2008, 77, 875–907.
- Jiang, J.Z.; Ng, D.K.P. A Decade Journey in the Chemistry of Sandwich-Type Tetrapyrrolato-Rare Earth Complexes. Acc. Chem. Res. 2009, 42, 79–88.
- Rizzini, A.L.; Krull, C.; Mugarza, A.; Balashov, T.; Nistor, C.; Piquerel, R.; Klyatskaya, S.; Ruben, M.; Sheverdyaeva, P.M.; Moras, P.; et al. Coupling of single, double, and triple-decker metal-phthalocyanine complexes to ferromagnetic and antiferromagnetic substrates. Surf. Sci. 2014, 630, 361–374.
- Christou, G. Single-molecule magnets: A molecular approach to nanoscale magnetic materials. Polyhedron 2005, 24, 2065–2075.
- Zhang, X.H.; Wang, S.P. 3d-4f Single Molecule-Magnets. Prog. Chem. 2010, 22, 1709–1719.
- Gonidec, M.; Davies, E.S.; McMaster, J.; Amabilino, D.B.; Veciana, J. Probing the Magnetic Properties of Three Interconvertible Redox States of a Single-Molecule Magnet with Magnetic Circular Dichroism Spectroscopy. J. Am. Chem. Soc. 2010, 132, 1756–1757.
- Hellerstedt, J.; Cahlik, A.; Svec, M.; de la Torre, B.; Moro-Lagares, M.; Chutora, T.; Papouskova, B.; Zoppellaro, G.; Mutombo, P.; Ruben, M.; et al. On-surface structural and electronic properties of spontaneously formed Tb2Pc3 single-molecule magnets. Nanoscale 2018, 10, 15553–15563.
- Ruan, L.X.; Tong, J.W.; Li, L.R.; Luo, F.F.; Zhang, R.; Qin, G.W.; Zhang, X.M. Magnetic relaxation dependences on the central ions for Ln (Ln = Tb, Dy, Er) phthalocyanines. Appl. Phys. Lett. 2020, 117, 072406.
- Thomas, A.L. Phthalocyanine Research and Application; CRC Press: Boca Raton, FL, USA, 1990.
- Herchel, R.; Zoufaly, P.; Nemec, I. The effect of the second coordination sphere on the magnetism of ·(18-crown-6) (Ln = Dy and Er). RSC Adv. 2019, 9, 569–575.
- Zhang, P.; Guo, Y.N.; Tang, J.K. Recent advances in dysprosium-based single-molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763.
- Ishikawa, N.; Sugita, M.; Tanaka, N.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Upward Temperature Shift of the Intrinsic Phase Lag of the Magnetization of Bis (phthalocyaninato) terbiumby Ligand Oxidation Creating an S = 1/2 Spin. Inorg. Chem. 2004, 43, 5498–5500.
- Kirin, I.S.; Moskalev, P.N.; Makashev, Y.A. Formation of Unusual Phthalocyanines of The Rare-Earth Elements. Russ. J. Inorg. Chem. 1965, 10, 1065–1066.
- Kirin, I.S.; Moskalev, P.N.; Makashev, Y.A. Production of unusual rare earth phthalocyanines. Russ. J. Inorg. Chem. 1965, 10, 369–372.
- Das, G.K.; Zhang, Y.; D’Silva, L.; Padmanabhan, P.; Heng, B.C.; Loo, J.S.C.; Selvan, S.T.; Bhakoo, K.K.; Tan, T.T.Y. Single-Phase Dy2O3:Tb3+ Nanocrystals as Dual-Modal Contrast Agent for High Field Magnetic Resonance and Optical Imaging. Chem. Mater. 2011, 23, 2439–2446.
- Eliseeva, S.V.; Bunzli, J.C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176.
- Norek, M.; Kampert, E.; Zeitler, U.; Peters, J.A. Tuning of the size of Dy2O3 nanoparticles for optimal performance as an MRI contrast agent. J. Am. Chem. Soc. 2008, 130, 5335–5340.
- Müller, M.; Montbrun, R.; Marz, M.; Fritsch, V.; Sürgers, C.; Löhneysen, H.V. Switching the Conductance of Dy Nanocontacts by Magnetostr. Nano Lett. 2011, 11, 574–578.
- Wernsdorfer, W.; Aliaga-Alcalde, N.; Hendrickson, D.N.; Christou, G. Exchange-biased quantum tunnelling in a supramolecular dimer of single-molecule magnets. Nature 2002, 416, 406–409.
- Hill, S.; Edwards, R.; Aliaga-Alcalde, N.; Christou, G. Quantum coherence in an exchange-coupled dimer of single-molecule magnets. Science 2003, 302, 1015–1018.
- Martínez-Flores, C.; Bolívar-Pineda, L.M.; Basiuk, V.A. Lanthanide bisphthalocyanine single-molecule magnets: A DFT survey of their geometries and electronic properties from lanthanum to lutetium. Mater. Chem. Phys. 2022, 287, 126271.
- Corradini, V.; Candini, A.; Klar, D.; Biagi, R.; De Renzi, V.; Rizzini, A.L.; Cavani, N.; del Pennino, U.; Klyatskaya, S.; Ruben, M.; et al. Probing magnetic coupling between LnPc2 (Ln = Tb, Er) molecules and the graphene/Ni (111) substrate with and without Au-intercalation: Role of the dipolar field. Nanoscale 2018, 10, 277–283.
- Li, Z.G.; Gao, F.; Xiao, Z.G.; Wu, X.Z.; Zuo, J.L.; Song, Y.L. Nonlinear optical properties and excited state dynamics of sandwich-type mixed (phthalocyaninato) (Schiff-base) triple-decker complexes: Effect of rare earth atom. Opt. Laser. Technol. 2018, 103, 42–47.
More
Information
Subjects:
Physics, Atomic, Molecular & Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
845
Revisions:
2 times
(View History)
Update Date:
26 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




