Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mirjam Bonanno | -- | 1075 | 2023-05-23 08:34:31 | | | |
| 2 | Catherine Yang | Meta information modification | 1075 | 2023-05-23 08:44:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Militi, A.; Bonanno, M.; Calabrò, R.S. Stomatognathic Diseases in Neurological Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/44690 (accessed on 21 January 2026).
Militi A, Bonanno M, Calabrò RS. Stomatognathic Diseases in Neurological Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/44690. Accessed January 21, 2026.
Militi, Angela, Mirjam Bonanno, Rocco Salvatore Calabrò. "Stomatognathic Diseases in Neurological Disorders" Encyclopedia, https://encyclopedia.pub/entry/44690 (accessed January 21, 2026).
Militi, A., Bonanno, M., & Calabrò, R.S. (2023, May 23). Stomatognathic Diseases in Neurological Disorders. In Encyclopedia. https://encyclopedia.pub/entry/44690
Militi, Angela, et al. "Stomatognathic Diseases in Neurological Disorders." Encyclopedia. Web. 23 May, 2023.
Copy Citation
Patients affected by neurological disorders can develop stomatognathic diseases (SD) related to decreased bite force and quality of mastication, bruxism, severe clicking and other temporomandibular disorders (TMD), which deeply affect patients’ swallowing, masticatory and phonation functions and, therefore, their quality of life.
stomatognathic disease
temporomandibular disorders
neurological patients
1. Introduction
The stomatognathic system (SS) is defined as a functional complex including craniofacial structures with musculoskeletal and ligamentous components, the temporomandibular joint (TMJ), oral cavity, neck and masticatory muscles [1]. Patients affected by neurological disorders can develop stomatognathic diseases (SD) related to decreased bite force and quality of mastication, bruxism, severe clicking and other TMJ disorders (TMD), which deeply affect the patients’ quality of life [2]. In fact, the integrity of SS is fundamental in activating the neuromuscular chain that initiates the swallow reflex. On the other hand, SD can also cause myofascial pain that may irradiate in different regions, such as dental arches, ears, temples, forehead, occiput, cervical spine and shoulders [3] (Figure 1), resembling atypical headaches and facial pain.
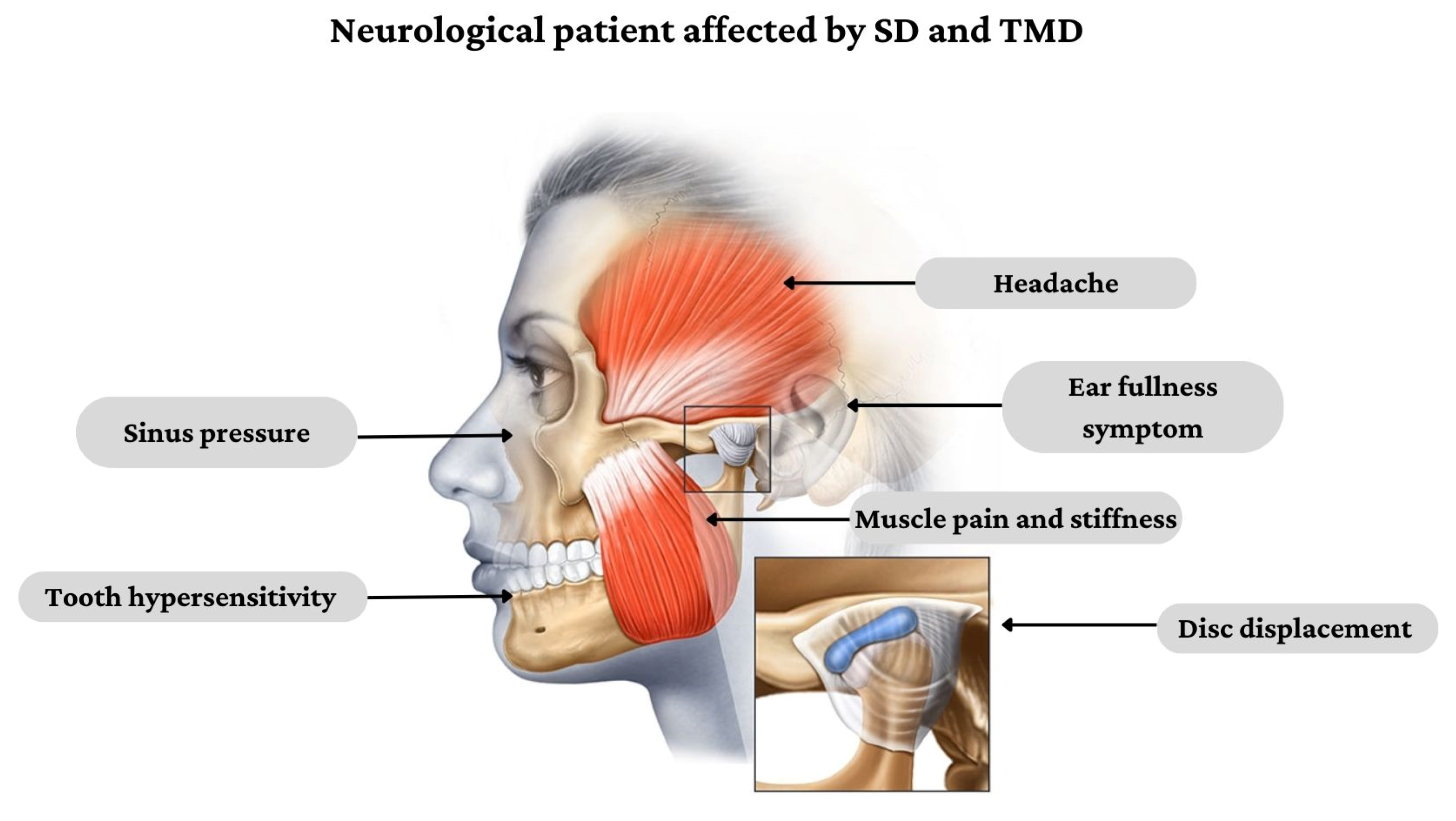
Figure 1. Clinical presentation of SD and TMD in people affected by neurological disorders.
Despite the presence of SD in neurological patients, functional training for SS and TMJ has not been commonly adopted in hospital settings as part of formal neurorehabilitation. Generally, the non-pharmacological treatment for SD and TMD aims to decrease pain, induce muscle release and stabilize muscle function and joint mobility through physical therapy (PT) and/or manual techniques (MT) [4].
2. Pathophysiology of Stomatognathic and Temporomandibular Joint Disorders in Neurological Disorders
The etiology of SD and TMD is linked to a wide range of functional, psychological and environmental factors, especially in neurological disorders in which the underlying pathology is complex. People affected by multiple sclerosis (MS) are more susceptible to developing TMD disorders [5][6]. Indeed, the concomitant presence of psychological disturbances (i.e., anxiety, depression, behavioral alteration) can exacerbate TMD disorders, as confirmed by a systematic review [5]. In this vein, it has been recently reported that patients who suffer from psychological distress are less responsive to conventional treatments for TMD, requiring a longer duration of therapy [7]. MS patients can manifest three common orofacial alterations: facial palsy, trigeminal neuralgia and/or paresthesia. However, Costa, C. et al. [8] described an unusual pattern of SD in a MS patient, which included tooth hypersensitivity, hyposalivation associated with caries, halitosis and bruxism. The latter tends to increase when occlusion is impaired, also contributing to head and neck pain. In this context, some studies hypothesized that the augmented mobility of cranial bones due to reduced bone mass density, especially in the temporal ones, expands and contracts during bruxism, increasing intracranial pressure, which can favor brain damage [9]. Another mechanism that can be involved in the pathogenesis of TMD or SD in MS patients is cerebellar dysfunction. In fact, cerebellar plaques and proprioceptive changes may lead to an increased propensity to fatigue of TMJ structures in addition to a lack of coordination of mandibular movements [5][10]. In a similar way, SD is present in patients with spinocerebellar ataxia (SCA), who often present dysarthric speech and swallowing difficulties. According to Ferreira et al. [11], SCA subjects showed a decreased bite force and hypotrophy of the masseter and temporalis muscles, with an augmented electromyographic activity. The underlying hypothesis for these electromyographic changes can be related to an increase in the amplitude and duration of motor unit action potentials, in addition to reduced muscle recruitment [12]. Moreover, the lack of coordination, especially during lateral mandibular movements in SCA patients, can be explained by the pathological alteration in cerebellum pathways, affecting the synchrony and precision of movements [13]. Furthermore, patients affected by Parkinson’s disease (PD) can manifest SD and TMD due to the presence of rigidity. Body muscle rigidity could also affect masticatory muscles in association with augmented muscle tone during sleep [14]. This condition could favor the repetitive jaw muscle activity and grinding of the teeth, named bruxism, which is considered a factor for developing TMD. Bruxism can occur both during sleep (sleep bruxism) and wakefulness (awake bruxism) [15], and its pathogenesis seems to be related to central nervous structure alterations. In fact, some antidepressant drugs, such as Selective Serotonin Reuptake Inhibitors (SSRI), can cause bruxism as a side-effect of inhibiting dopaminergic neurons [16]. This could explain why bruxism is frequent in PD patients due to the reduction of dopamine presence in basal ganglia [17]. TMD and SD were also found in other movement disorders, including dystonia. The term “dystonia” refers to prolonged or intermittent muscle contractions, causing repetitive and abnormal movements and/or postures [18]. In this context, oromandibular dystonia (OMD) is often misdiagnosed due to shared clinical features with TMD [19]. In fact, OMD is associated with masticatory disturbances such as limited mouth opening, orofacial pain and TMJ dislocations that can simulate an isolated TMD, overlooking the real etiology of the disturbance or pain. Today, six subtypes of OMD are recognized: jaw closing (i), opening (ii), deviation (iii), protrusion (iv), lingual (v) and lip (vi) dystonia. In particular, the jaw-closing subtype is related to a loss of reciprocal muscle inhibition that greatly limits mouth opening, especially during speaking or eating, worsening the patient’s quality of life. In addition, dystonia tends to expand to other muscles, including orbicularis oculi, neck and shoulder muscles [20]. The pathophysiology of SD in OMD patients is still unclear, although some studies found that functional movement disorders, as well as dystonia, present a hypoactivation of the supplementary motor area and abnormal connectivity of those brain areas designed to select or inhibit movements [21]. The onset of SD and TMD in stroke patients depends on the extent and site of the vascular lesion, which can affect cortical areas, or motor-neuron pools of cranial nerves in the brain stem, causing sensorimotor deficits in SS. The presence of facial and masticatory muscle dysfunctions has been demonstrated in post-stroke patients, including weakness and hypotonus of the masseter, orbicularis oris, mylohyoid and digastric with an increase of thickness in these muscles [22]. In detail, the most common TMD in post-stroke patients seems to be related to disc displacement, which alters the structural relationship with condyle, thus producing a click sound when the mouth opens due to translation movements. This alteration could be chronic as it interferes with the simple opening of the mouth during speech or eating, and the disc becomes progressively more dislocated. Another hypothesis is that forward head posture, due to inefficacy to maintain postural alignment, causes an overload in posterior cervical muscles that can influence the TMJ by changing the position of the mandibular condyle and, consequentially, its functioning [23][24][25].
References
- Zieliński, G.; Filipiak, Z.; Ginszt, M.; Matysik-Woźniak, A.; Rejdak, R.; Gawda, P. The Organ of Vision and the Stomatognathic System—Review of Association Studies and Evidence-Based Discussion. Brain Sci. 2022, 12, 14.
- Dai, R.; Lam, O.L.; Lo, E.C.; Li, L.S.; Wen, Y.; McGrath, C. Orofacial functional impairments among patients following stroke: A systematic review. Oral. Dis. 2015, 21, 836–849.
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.-Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain 2015, 16, 106.
- McNeely, M.; Armijo Olivo, S.; Magee, D. A systematic review of physical therapy intervention for temporomandibular dis-orders. Phys. Ther. 2006, 86, 710–720.
- Minervini, G.; Mariani, P.; Fiorillo, L.; Cervino, G.; Cicciù, M.; Laino, L. Prevalence of temporomandibular disorders in people with multiple sclerosis: A systematic review and meta-analysis. Cranio® 2022, 1–9.
- Manchery, N.; Henry, J.D.; Nangle, M.R. A systematic review of oral health in people with multiple sclerosis. Community Dent. Oral. Epidemiol. 2020, 48, 89–100.
- Jung, W.; Lee, K.-E.; Suh, B.-J. Influence of psychological factors on the prognosis of temporomandibular disorders pain. J. Dent. Sci. 2021, 16, 349–355.
- Costa, C.; Santiago, H.; Pereira, S.; Castro, A.R.; Soares, S.C. Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report. Diseases 2022, 10, 62.
- Williams, D.E.; Lynch, J.E.; Doshi, V.; Singh, G.D.; Hargens, A.R. Bruxism and Temporal Bone Hypermobility in Patients with Multiple Sclerosis. Cranio® 2011, 29, 178–186.
- Carvalho, L.; Matta, A.; Nascimento, O. Temporomandibular disorders (TMD) and multiple sclerosis (MS)(P1. 120). Cranio 2015, 84, 120.
- Ferreira, B.; Palinkas, M.; Gonçalves, L.; Da Silva, G.; Arnoni, V.; Regalo, I.H.; Vasconcelos, P.; Júnior, W.; Hallak, J.; Regalo, S.C.H.; et al. Spinocerebellar ataxia: Functional analysis of the stomatognathic system. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e165–e171.
- Liang, L.; Chen, T.; Wu, Y. The electrophysiology of spinocerebellar ataxias. Neurophysiol. Clin. 2016, 46, 27–34.
- Velázquez-Pérez, L.C.; Rodríguez-Labrada, R.; Fernandez-Ruiz, J. Spinocerebellar Ataxia Type 2: Clinicogenetic Aspects, Mechanistic Insights, and Management Approaches. Front. Neurol. 2017, 8, 472.
- Choi, H.-G.; Yoon, J.-H.; Chung, T.-H.; Min, C.; Yoo, D.-M.; Wee, J.-H.; Kang, S.-Y.; Choi, Y.; Hong, S.-J.; Byun, S.-H. Association between Temporomandibular Joint Disorder and Parkinson’s Disease. Brain Sci. 2021, 11, 747.
- Verhoeff, M.C.; Koutris, M.; Berendse, H.W.; van Dijk, K.D.; Lobbezoo, F. Parkinson’s disease, temporomandibular disorder pain and bruxism and its clinical consequences: A protocol of a single-centre observational outpatient study. BMJ Open. 2022, 12, e052329.
- Beers, E.; Van Grootheest, A.C. Bruxisme als bijwerking van serotonineheropnameremmers. Ned. Tijdschr. Tandheelkd. 2007, 114, 388–390.
- Verhoeff, M.C.; Lobbezoo, F.; Wetselaar, P.; Aarab, G.; Koutris, M. Parkinson’s disease, temporomandibular disorders and bruxism: A pilot study. J. Oral. Rehabil. 2018, 45, 854–863.
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873.
- Handa, S.; Shaefer, J.R.; Keith, D.A. Oromandibular dystonia and temporomandibular disorders. J. Am. Dent. Assoc. 2022, 153, 899–906.
- Yoshida, K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins 2022, 14, 282.
- Yoshida, K. Clinical Characteristics of Functional Movement Disorders in the Stomatognathic System. Front. Neurol. 2020, 11, 123.
- Schimmel, M.; Leemann, B.; Christou, P.; Kiliaridis, S.; Herrmann, F.R.; Müller, F. Quantitative assessment of facial muscle impairment in patients with hemispheric stroke. J. Oral. Rehabil. 2011, 38, 800–809.
- Ramos, M.A.; Moura, B.G.; Araujo, C.C.; Del Antonio, T.T.; Da Silva, J.K.M. Temporomandibular dysfunction in patients with a history of stroke. Man. Ther. Posturol. Rehabil. J. 2020, 17, 1–5.
- Lau, K.T.; Cheung, K.Y.; Chan, K.B.; Chan, M.H.; Lo, K.Y.; Chiu, T.T.W. Relationships between sagittal postures of thoracic and cervical spine, presence of neck pain, neck pain severity and disability. Man. Ther. 2010, 15, 457–462.
- Corrêa, E.C.; Bérzin, F. Temporomandibular disorder and dysfunctional breathing. Braz. J. Oral. Sci. 2004, 3, 498–502.
More
Information
Subjects:
Clinical Neurology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
23 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




