Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong-Chien Ling | -- | 4314 | 2023-05-22 13:00:41 | | | |
| 2 | Jessie Wu | + 6 word(s) | 4320 | 2023-05-23 04:42:18 | | | | |
| 3 | Badal Kumar Mandal | Meta information modification | 4320 | 2023-06-01 19:21:03 | | | | |
| 4 | Badal Kumar Mandal | Meta information modification | 4320 | 2023-06-02 05:50:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mandal, B.K.; Ling, Y. Separation of Chlorophylls and Chlorophyllins in Food Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/44660 (accessed on 07 February 2026).
Mandal BK, Ling Y. Separation of Chlorophylls and Chlorophyllins in Food Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/44660. Accessed February 07, 2026.
Mandal, Badal Kumar, Yong-Chien Ling. "Separation of Chlorophylls and Chlorophyllins in Food Products" Encyclopedia, https://encyclopedia.pub/entry/44660 (accessed February 07, 2026).
Mandal, B.K., & Ling, Y. (2023, May 22). Separation of Chlorophylls and Chlorophyllins in Food Products. In Encyclopedia. https://encyclopedia.pub/entry/44660
Mandal, Badal Kumar and Yong-Chien Ling. "Separation of Chlorophylls and Chlorophyllins in Food Products." Encyclopedia. Web. 22 May, 2023.
Copy Citation
Chlorophyll is a natural green hue with a tetrapyrrole ring system with different substituents.
HPLC
HPLC/MS
non-target analysis
natural green colorants
chlorophylls
1. Introduction
Food products of different foodstuffs, food commodities, and beverages are available on the market. Chlorophyll derivatives, chlorophyllins, and their degradants could not be extracted from all green-colored foodstuffs and commodities using the same extraction procedure, because some foodstuffs are fatty, while others are non-fatty. Sometimes, a mixture of these foodstuffs may be present in food commodities. Hence, the extraction procedures of food colorants vary from one food type to another food type. It is important to first check the nature of the ingredients present in food commodities before accordingly selecting an extraction procedure for separation and identification.
2. Separation and Identification of Chlorophylls and Chlorophyllins in Food Products Using High-Performance Liquid Chromatography Methods
Cano (1991) developed an HPLC-PDA method for the determination of colorants in four collected kiwi fruits (Actinidia chinensis, Planch) and cultivars (Hayward, Abbot, Bruno, and Monty) by separating them through a Hypersil ODS stainless steel column (5 µm size × 10 cm length × 4.6 mm ID), with mobile phases of (A) methanol/water (75:25, v/v) and (B) ethyl acetate under gradient elution. The author detected chlorophyll a and b, and pheophytin a [1].
Yasuda et al. (1995) developed an RP-HPLC-PDA method for the analysis of chlorophylls and its derivatives in collected foodstuffs (boiled bracken, agar–agar, and chewing gum) after separation through a C18 RP-HPLC column, using a mobile phase of methanol:water (97:3, v/v) containing 1% acetic acid at a flow rate of 1 mL/min and a wavelength of 405 nm. The extraction of colorants was carried out at a pH of 3–4 using diethyl ether. The green colorants of the homogenised foodstuffs were extracted in ethyl ether at a pH of 3–4 adjusted with 0.1 N hydrochloric acid, and the organic solvent was evaporated. The residue was dissolved in methanol and used for HPLC analysis. The authors detected Cu-chlorin e6 and Cu-chlorin e4 in the Na-Cu-chlorophyllin-containing foodstuffs. Their results suggest that Cu-chlorin e6 is not stable under the heat and pH of the food manufacturing process, and hence the authors suggested the analysis of Cu-chlorin e4 as an indicator for the presence of Na-Cu-chlorophyllin in food commodities (boiled bracken, agar-agar and chewing gum) [2][3].
Nonomura et al. (1996) extracted chlorophyll a in spinach, and used it as a standard material for the preparation of Fe-chlorophyllins in inert and dark conditions to avoid molecular degradation. Then, they separated the components of Fe3+-chlorophyllin through an Inertsil ODS column, with a mobile phase of acetonitrile-phosphate buffer (pH 2) (60:40, v/v) containing tetramethyl ammonium chloride (0.01 M) and analyzed by RP-HPLC. They detected three major derivatives: Fe3+-pheophorbide a, Fe3+-chlorin e6, and Fe3+-chlorin e4. They also confirmed the presence of all three species using FAB-MS analysis [4].
Egner et al. (2000) analyzed chlorophyllin derivatives using HPLC, ESI/MS, and MS/MS techniques in human serum samples after oral consumption of Na-Cu-chlorophyllin, in Qidong, Jiangsu Province, People’s Republic of China. The authors found some green-colored serum and detected unreported Cu-chlorin e4 ethyl ester and Cu-chlorin e4. This finding suggested that chlorophyllin derivatives were bioavailable and absorbed into the bloodstream, creating the possibility of their chemopreventive activity [5].
Wang et al. (2004) initiated their study to monitor the green color of green tea infusions, as cold tea beverages in clear bottles are popular in different countries. They found chlorophylls to be the main component of the greenness of these tea infusions. In addition to chlorophylls, they detected flavonoids, catechins, and flavonols in green tea infusions, while quercetin was the main phenolic compound contributing to the greenness of the tea infusions [6]. Bohn et al. (2004) analyzed chlorophylls and their derivatives using HPLC equipped with a fluorescence detector. All the colorants were separated through an RP-C18 column (4 µm size × 25 cm length × 2 mm ID) with methanol for HPLC analysis. They identified chlorophyll a and a′, chlorophyll b and b′, and corresponding pheophytins [7].
Scotter et al. (2005) developed an HPLC-PDA and HPLC-Fluorescence method for determining the food color additives Cu-chlorophylls and Cu-chlorophyllins in foods and beverages. The authors found huge amounts of native chlorophylls in mint sauce samples. Food commodities containing significant amounts of emulsifiers (i.e., ice cream), gelatin, or fats were problematic during extraction; hence, further development of extraction regimes is desirable for such products. All of the samples analyzed with added E141 had estimated total copper chlorophyllin contents of below 15 mg/kg (range 0.7–13.0) [8] (Table 1).
Roca et al. (2010) developed an HPLC-PDA method to monitor the adulteration of olive oils, which is used to make their green coloration. The separation was carried out using a stainless steel C18 column (3 µm size x 20 cm length × 4.6 mm ID) with the mobile phases (A) water/ion pair reagent/methanol (1/1/8, v/v/v) and (B) methanol/acetone (1:1, v/v). A mixture of 0.05 M tetrabutylammonium and 1.0 M ammonium acetate in water was used as the ion-pair reagent. They detected pheophytins (a and b) in the collected samples adulterated with E141ii, but did not find them in the samples that contained colorant E141i, indicating the capability of this method to monitor the adulteration of vegetable oils with E141ii. The authors suggested selecting a λmax of 654 nm for Cu-pyropheophytin a, and of 633 nm for Cu-pyropheophytin b, during the screening of the studied adulterated olive oil samples [9].
Loranty et al. (2010) studied the fate of chlorophylls and carotenoids in commercial dry herbal and fruit teas, as well as in infusions made from these teas. They developed an HPLC-PDA method for this study. The colorants were separated using a Phenomenex Luna C18 column (5 µm size × 25 cm length × 4.6 mm ID), with mobile phases of (a) acetonitrile:water (90:10, v/v) and (b) ethyl acetate, under gradient elution at a flow rate of 1 mL/min. The authors detected complex chlorophyll and related pigment profiles in all of the evaluated commercial dry teas, whereas lutein was the main component in the infusion [10].
Baskan et al., (2013) analyzed chlorophyll-related colorants in fresh spinach (Spinacia oleracea), carrot (Daucus carota) and tomato (Lycopersicon esculentum), and in the wastes of tomato paste and orange juice manufacturers, using the HPLC-PDA method. They used a Waters YMC C30 HPLC column (5 µm size × 25 cm length × 4.6 mm size) and eluted using mobile phases (a) MeOH:MeCN (50:50, v/v) with 0.1% (v/v) TEA and (b) acetone. The injection volume was 20 µL and the flow rate was 1.5 mL/min, with a run time of 40 min at 35 °C, within a wavelength range of 200–800 nm. They detected only chlorophyll a and chlorophyll b [11].
Kenner et al. (1973) analyzed chlorophyll a and chlorophyll b using the HPLC-UV-Vis method. In this study, the authors used an isocratic mobile phase CHCl3-MeOH (20:1, v/v) and identified different chlorophyll derivatives such as Pheophytin a, Mesopurpurin-7 trimethyl ester, Purpurin-18 methyl ester, Mesopurpurin-18 methyl ester, Rhodoporphyrin-XV dimethyl ester, Chlorin-p6 trimethyl ester, Purpurin-7 trimethyl ester, and Methyl mesopyrophaeophorbide-a [12].
Fang et al. (2015) developed a chromatographic method using UHPLC-PDA. Within this method, an inertSustain C18 RP-HPLC column (2 μm size × 10 cm length × 2.1 mm ID) was used for the separation of colorants after elution, using a gradient system comprising mobile phases (a) 1 M ammonium acetate/MeOH (2/8, v/v) (b) MeCN, (c) MeOH, and (d) H2O. The flow rate was 0.25 mL/min, and the analysis was monitored at a λmax of 430 nm. They identified different colorants such as Cu-pyropheophytin a, Cu-pheophytin a and a′, Cu-pyropheophytin b, and Cu-152-Methyl-phytol-rhodin g7 ester (Cu-rhodin g7) [13].
Furuya et al. (1988) studied the fate of pheophytinato a nickel(ll) and pheophytinato b nickel(II) after fortification using the HPLC-UV-Vis method, after separation through a Inertsil ODS-2 HPLC column (5 μm size × 15 cm length × 4.6 mm ID). They used a mobile phase of Acetone-MeOH (50:50, v/v) and eluted at a flow rate of 1.4 mL/min, maintained at 20–30 °C, and a λmax of 420 or 428 nm. Only pheophytinatonikel(II) was identified [14].
Viera et al. (2021) analyzed fiber-rich vegetable puree, fat-rich virgin olive oil, and fruit juice for chlorophyll-based colorants using an HPLC-UV-Vis method. The separation was carried out using a Mediterranean Sea18 HPLC column (3 μm size × 20 cm length × 4.6 mm ID), using mobile phases (a) H2O/0.05 M ammonium acetate/MeOH (1/1/8, v/v/v) and (b) MeOH/acetone (1/1, v/v), within a wavelength range (λ-range) of 350 to 800 nm. They found different chlorophyll derivatives such as chlorins, rhodins, pheophorbides, chlorophylls, pheophytins, 132-OH-pheophorbides, 132-OH-chlorophylls, 132-OH-pheophytins, 151-OH-lactone-pheophorbides, 151-OH-lactone-pheophytins, and pyropheophytins [15].
Laddha et al. (2020) monitored the fate of chlorophyllins after intake by rats [16]. For this study, the authors collected rat plasma and analyzed it using HPLC-PDA after separation through a Luna® C18 RP-HPLC column (100 Å 4.5 μm size × 25 cm length × 4.6 mm ID), using a mobile phase of MeOH:10 mM ammonium acetate (90:10, v/v) at a flow rate of 1 mL/min. The injection volume was 20 μL, and the run time was 20 min. They detected Na-Cu-chlorophyllin in the rat plasma [17].
Suzuki et al. (2016) developed an analytical technique based on HPLC-UV-Vis, and separated different colorants from processed foods (seaweed, pickled leaf, chewing gum, fried fish cake, white chocolate, mugwort-flavored rice cake) using an Inertsil ODS-3V RP-HPLC column (5 µm size × 15 cm length × 4.6 mm ID). The colorants were eluted using mobile phases (a) 1.0 mmol/L ammonium acetate:MeOH (20:80, v/v) and (b) MeOH:acetone (80:20, v/v) at a flow rate of 1 mL/min, maintaining a temperature of 40 °C. The injection volume was 10 µL, and the run time was 30 min, monitoring at a wavelength of 405 nm. The authors detected Cu-chlorophylls and Na-Cu-chlorophylls in their samples [18].
Chong et al. (2018) determined Na-Cu-chlorophyllin in water-soluble and fat-soluble food samples by using an HPLC-PDA method after separation through an Inertsil ODS-2 (5 μm size × 25 cm length × 4.6 mm ID), using a mobile phase of MeOH:H2O (97:3, v/v) including 1% acetic acid, at a flow rate of 1 mL/min, for a run time of 20 min. The injection volume was 10 µL, and the column temperature was maintained at 35 °C; the analysis was carried out at a λmax of 405 nm. The authors detected Cu-isochlorin e4, Cu-chlorin p6, and Cu-chlorin e6 in their samples [19].
In another study, Chong et al. (2019) used an HPLC-PDA method to monitor the fate of chlorophyll-based colorants in food samples fortified with Na-Fe-chlorophyllin, after separation through an Inertsil ODS-2 HPLC column (5 μm size × 25 cm length × 4.6 mm ID), using a mobile phase of MeOH:H2O (80:20, v/v) including 1% acetic acid, at a flow rate of 1 mL/min, for a run time of 20 min. The injection volume was 10 µL; the column was maintained at 35 °C and monitored at a λmax of 390 nm [20].
Table 1. Separation and identification of green colorants in foodstuffs and beverages using HPLC methods.
| S. No. |
Sample Type | Instrument Used | Stationary Phase | Mobile Phase, Inj. Volume, Flow Rate (mL/min), Run Time (min) | Analyzed Colourants | Reference |
|---|---|---|---|---|---|---|
| 1 | Green table olives with E-141(ii) colourant |
HPLC-PDA | C-18 stainless steel column (3 µm size × 20 cm length × 0.46 cm ID) | Mobile phases: (a) water/ion pair reagent/methanol (1/1/8, v/v/v) and (b) methanol/acetone (1/1, v/v). | Pheophorbide a Pyropheophorbide a 15-G-chlorophyll b 15-G-pheophytin b 15-G-chlorophyll a 15-G-pheophytin a Chlorophyll b Chlorophyll b’ 132-OH-chlorophyll b 15-F-chlorophyll b Chlorophyll a Chlorophyll a’ 132-OH-chlorophyll a 15-F-chlorophyll a Pheophytin b Pheophytin b’ Pheophytin a Pheophytin a’ Pyropheophytin a (note: G: glyoxylic acid, F: Formyl) |
[21] |
| 2 | Food colour additives Cu-chlorophylls and Cu-chlorophyllins in foods and beverages | HPLC-PDA and HPLC-Fluorescence | Vydac 201TP54 C18 cokumn (5 µm size × 25 cm length × 4.6 mm ID) |
Mobile phases: (a) MeOH: 1.0 M ammomium acetate (80:20, v/v) and (b) MeOH:acetone (60: 40, v/v), 50 µL, 1 mL/min, 60 min | Chlorophyll, Cu-chlorin e6 |
[8] |
| 3 | Adulterated green coloured olive oils with Cu-chlorophyll (E-141i) | HPLC-PDA | C18 stainless steel column (3 µm size × 20 cm length × 4.6 mm ID) | Mobile phases: (a) water/ion pair reagent/methanol (1/1/8, v/v/v) and (b) methanol/acetone (1:1, v/v); 1.25 mL/min, 40 min | Cu-pyropheophytin a, Pheophytin b/b’, Pheophytin a/a’, Pyro-pheophytin a, Cu-132-OH-pheophorbide a, Cu-pyro-pheophorbide a/b |
[9] |
| 4 | Fresh spinach (Spinacia oleracea), carrot (Daucus carota) and tomato (Lycopersicon esculentum), wastes of tomato paste and orange juice manufacturers | HPLC-PDA | Waters YMC C30 column (5 µm size × 25 cm length × 4.6 mm ID) | MeOH:MeCN (50:50, v/v) with 0.1% (v/v) TEA and acetone | Fresh spinach (Spinacia oleracea), carrot (Daucus carota) and tomato (Lycopersicon esculentum), wastes of tomato paste and orange juice industries | [11] |
| 5 | Chlorophyll a and chlorophyll b | HPLC-UV-Vis | NA | CHCl3-MeOH (20:1, v/v) | Pheophytin a, Mesopurpurin-7 Trimethyl Ester, Purpurin- 18 Methyl Ester, Mesopurpurin- 18 Methyl Ester, Rhodoporphyrin-XV Dimethyl Ester, Chlorin-p6, Trimethyl Ester, Purpurin-7 Trimethyl Ester, Methyl mesopyrophaeophorbide-a, |
[12] |
| 6 | 29 Edible oils (olive oil, grapeseed oil and blended oil) | UHPLC-PDA | InertSustain C18 column (2 µm size × 10 cm length × 2.1 mm ID) | Mobile phases: (a) 1 M ammonium acetate/MeOH (2/8, v/v) (b) MeCN (c) MeOH (d) H2O, 0.25 mL/min | Cu-pyropheophytin a, Cu-pheophytin a and a′, Cu-pyropheophytin b, Cu-152-Methyl-phytol-rhodin g7 ester (Cu-rhodin g7) |
[13] |
| 7 | Synthesized and fortified sample with Pheophytinato a nickel(ll) and Pheophytinato b nickel(II) | HPLC-UV-Vis | Inertsil ODS-2 C18 column (5 µm size × 25 cm × 4.6 mm ID) |
Mobile phase: Acetone-MeOH (50:50, v/v), 1.4 mL/min, at 20–30 °C and a λmax of 420 or 428 nm. |
Pheophytinatonikel(II) | [14] |
| 8 | Fiber-rich vegetable puree, fat-rich virgin olive oil, and fruit juice | HPLC-UV-Vis | Mediterranea Sea18 column (3 µm size × 20 cm length × 4.6 mm ID) | Mobile phases: (a) H2O/0.05 M ammonium acetate/MeOH (1/1/8, v/v/v) and (b) MeOH/acetone (1/1, v/v). λ-range: 350 to 800 nm | Chlorins, Rhodins, Pheophorbides, Chlorophylls, Pheophytins, 132-OH-pheophorbides, 132-OH-chlorophylls, 132-OH-pheophytins, 151-OH-lactone-pheophorbides, 151-OH-lactone-pheophytins, Pyropheophytins |
[15] |
| 9 | Rat plasma | HPLC-PDA | Luna C18 RP-HPLC column (100 Å 4.5 µm size × 25 cm length × 4.6 mm ID) | Mobile phase: MeOH:10 mM ammonium acetate (90:10, v/v), 20 µL, 1 mL/min, 20 min | Na-Cu-chlorophyllin | [17] |
| 10 | Processed foods (seaweed, pickled leaf, chewing gum, fried fish cake, white chocolate, mugwort-flavored rice cake) | HPLC-UV-Vis | Inertsil ODS-3V column (5 µm size × 15 cm length × 4.6 mm ID) | Mobile phases: (a) 1.0 mmol/L ammonium acetate:MeOH (20:80, v/v) and (b) MeOH:acetone (80:20, v/v), 10 µL, 1 mL/min, 30 min at 40 °C and 405 nm | Cu-chlorophylls, Na-Cu-chlorophylls |
[18] |
| 11 | Na-Cu-chlorophyllin in water-soluble and fat-soluble food samples | HPLC-PDA | Inertsil ODS-2 C18 column (5 µm size × 25 cm × 4.6 mm ID) |
Mobile phase: MeOH:H2O (97:3, v/v) including 1% acetic acid, 10 µL, 1 mL/min, 20 min at 35 °C and a λmax of 405 nm |
Cu-isochlorin e4, Cu-chlorin p6, Cu-chlorin e6 |
[19] |
| 12 | Fortified candy samples with Na-Fe-chlorophyllin and Na-Cu-chlorophyllin | HPLC-PDA | Inertsil ODS-2 C18 column (5 µm size × 25 cm × 4.6 mm ID) |
Mobile phase: MeOH:H2O (97:3 and 80:20, v/v) containing 1% acetic acid, 1 mL/min, 30 min at a λmax of 395 nm | Na-Fe-chlorophyllin, Na-Cu-chlorophyllin, Fe-Isochlorin e4, Cu-Isochlorin e4 |
[20] |
| 13 | Grapes and Port wines | HPLC-DAD | Nova-Pak C18 RP HPLC column (60 Å 4 µm size × 30 cm length × 3.9 mm ID) | Mobile phase: (a) 100% ethyl acetate and (b) 90% MeCN in H2O (9:1, v/v), 20 µL, 1 mL/min, 45 min at a λmax of 447 nm |
Chlorophyll b, Pheophytin a/b |
[22] |
| 14 | Na-Cu-chlorophyllin and CuSO4 as additives in 16 table olives | HPLC-DAD | Alltech Prontosil C30 RP HPLC column (200 Å 5µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) Methanol:distilled water: Acetic acid (90:10:0.5 v/v/v) and (b) tert-butylmetyl ether:Methanol:Acetic acid (100:10:0.5 v/v), 1 mL/min, 45 min | Chlorin e6, Cu-rhodin g7, Cu-chlorin e6, Cu-chlorin p6, Pheophorbide a, Cu-isochlorin e4, Isochlorin e4, Cu-151-OH-lactone-pheophytin a, Pheophytin a/b, Cu-pyropheophorbide a, Chlorophyll a/b, Pheophorbide a, Cu-rhodochlorin |
[21] |
3. Separation and Identification of Chlorophylls and Chlorophyllins in Food Products Using High-Performance Liquid Chromatography (HPLC)-MS Methods
Mendes-Pinto et al. (2005) analyzed carotenoids and chlorophyll-derived compounds in grapes and Port wines using HPLC-DAD and HPLC-DAD-MS (ESP+) analysis. They detected 13 carotenoid and chlorophyll-derived compounds in grapes, whereas pheophytins a and b were unknown. They also found 19 compounds with carotenoid or chlorophyll-like structures in Port wines. Their observation was that chlorophyll derivatives degraded faster than carotene and lutein [22].
Mortensen and Geppel developed an HPLC-PDA method for the detection of Na-Cu-chlorophyllin and its derivatives in the collected five commercial Na-Cu-chlorophyllin samples and one green food colorant. Additionally, they used an MS detector for the authentication of the separated colorants. Based on their absorption spectra and mass data, three of the collected standards contained Cu-chlorin e6, Cu-chlorin p6, and Cu-isochlorin e4. The other two samples contained a low amount of Cu-chlorin e6, but Cu-chlorin p6 was absent. The majority of samples contained porphyrins, but no samples contained chlorins derived from chlorophyll b [23].
Gandul-Rojas et al. (2012) studied the pattern of color adulteration in table olives using the non-permitted semi-synthetic green colorant Na-Cu-chlorophyllin (E141ii), using the HPLC-DAD method [21]. For the HPLC analysis, the colorants were extracted as per the method of Mínguez-Mosquera and Garrido-Fernández (1989) [24]. The colorants in the extract were analyzed using the HPLC-PDA method after separating through a C-18 stainless steel column (3 µm size × 20 cm length × 0.46 cm ID) with mobile phases consisting of (A) water/ion pair reagent/methanol (1/1/8, v/v/v), and (B) methanol/acetone (1/1, v/v). A mixture of tetrabutylammonium (0.05 M) and ammonium acetate (1.0 M) in water was used as the ion-pair reagent. Cu-chlorophyllin complexes were found in the extract. The results of this study suggested the fraudulent practices of vendors in their achievement of a green color in the served table olives [21].
Yoshioka and Ichihashi (2008) developed a chromatographic technique using RP-HPLC equipped with a PDA detector for the analysis of 40 synthetic food colors in drinks and candies collected from Japanese local markets. The authors separated the colorants using a ZORBAX Eclipse XDB-C18 Rapid Resolution HT (1.8 µm size × 5 cm length × 4.6 mm ID) with gradient elution, using a mobile phase solvent A (0.1 mol/L of ammonium acetate aqueous solution, pH 6.7) and solvent B (1:1 methanol–acetonitrile, v/v) at a flow rate of 1.5 mL/min [25].
Huang et al. (2008) developed an HPLC-APCI-MS method to monitor chlorophylls and their derivatives in a traditional Chinese herb Gynostemma pentaphyllum Makino. They used a HyPURITY C18 column for the separation of chlorophyll-based colorants in the sample, with a quaternary solvent system of hexane–acetone–ethanol–toluene (10:7:6:7, v/v/v/v) under gradient elution. They quantified chlorophyll a and a′, chlorophyll b and b′, pheophytin a and a′, pheophytin b and b′, hydroxypheophytin a and a′, pyropheophytin a, hydroxychlorophyll a and b, and hydroxypheophytin b and b′ [26].
Aparicio-Ruiz et al. (2010) checked the degradation kinetics of chlorophyll a-series pigments at varying temperatures in the collected three virgin olive oils. They found that the isocyclic ring alteration formed pheophytin, pyropheophytin, 132-OH-pheophytin, and 151-OH-lactone-pheophytin, whereas the porphyrin ring alteration resulted in colorless compounds. In addition, the authors did not find any matrix effect on 151-OH-lactone-pheophytin conversion, but 132-OH-pheophytin conversion was affected by the oil matrices [27].
Kao et al. (2011) developed an HPLC-DAD-APCI-MS method to determine chlorophyll and its derivatives in hot-air-dried and freeze-dried Chinese herb Rhinacanthus nasutus (L.) Kurz samples. The authors separated different colorants using an Agilent Eclipse XDB-C18 column, with a mobile phase of (A) methanol/N,N-dimethylformamide (97:3, v/v) and (B) acetonitrile under gradient elution. They identified chlorophyll a and a′, hydroxychlorophyll a and b, 15-OH-lactone chlorophyll a, chlorophyll b and b′, pheophytin a and a′, hydroxypheophytin a and a′, and pheophytin b in hot-air-dried Rhinacanthus nasutus, but the freeze-dried Rhinacanthus nasutus contained only chlorophyll a and a′, chlorophyll b and pheophytin a. Zinc-phthalocyanine was found to be an appropriate internal standard to quantify all the chlorophyll compounds. The results suggested that chlorophyll a and pheophytin a were the most abundant in the hot-air-dried samples, while chlorophyll a and chlorophyll b were the main colorants in freeze-dried samples [28] (Table 2).
Fu et al. (2012) developed an HPLC-UV-MSE method for the analysis of targeted pigments of carotenoid and chlorophyll species in Dunaliella salina samples. The separation of the pigments was carried out through an ACQUITY UPLC HSS T3 column (1.8 µm size × 15 cm length × 2.1 mm ID) (Waters, Manchester, UK) with mobile phases of (A) acetonitrile:methanol:MTBE (70:20:10, v/v/v) and (B) 10 mM ammonium acetate, under gradient elution at a flow rate of 0.5 mL/min, and at 45 °C. They identified 37 pigments, including 19 carotenoid species and 18 chlorophyll species (chlorophyll a and b, chlorophyll a and b derivatives), and carried out quantification of seven targeted compounds. The limit of detection for lutein was 0.01 ng/mL, and that of chlorophyll a was 0.24 ng/mL [29].
Isakau et al. (2007) tried to analyze the tetrapyrrolic compound chlorin e6 and its degradants, after its uses as a photolon formulation for photodynamic therapy of various diseases. The authors developed an HPLC-PDA-MS-based chromatographic method for this study, and identified several degradants such as chlorin e6 174-ethyl ester, chlorin e4, 15-hydroxyphyllochlorin, rhodochlorin, 151-hydroxymethylrhodochlorin δ-lactone, rhodochlorin-15-oxymethyl δ-lactone, rhodochlorin-15-oxymethyl δ-lactone 174-ethyl ester, 151-hydroxymethylrhodoporphyrin δ-lactone, rhodoporphyrin-15-oxymethyl δ-lactone, and purpurin 18. They used an analytical HPLC column (3.5 µm size × 15 cm length × 4.6 mm ID) and a semi-preparative column (5 µm size × 15 cm length × 10 mm ID) packed with XTerra RP-18, using a mobile phase A (0.1% TFA in water) and B (acetonitrile) under gradient elution [30].
Loh et al. (2012) analyzed the Chinese herb Taraxacum formosanum, considering its different medicinal values, as an essential component of different drug formulations. Chlorophylls were extracted in 30 mL of hexane/ethanol/acetone/toluene (10:6:7:7, v/v/v/v), the upper layer was collected and evaporated to dryness, and the residue was dissolved in 5 mL of acetone, filtered, and stored for HPLC analysis. For chlorophyll derivatives, the authors used column chromatography for separation, after dissolving 10 g of the herb sample in 80 mL of hexane/ethanol/acetone/toluene (10:6:7:7, v/v/v/v) for 1 h at room temperature. Finally, the supernatants were evaporated to dryness and the residue was dissolved in 5 mL of acetone, filtered and stored for analysis. A HyPURITY C18 column (5 μm size × 15 cm length × 4.6 mm ID) was used for the separation of chlorophyll and its derivatives, with a quaternary mobile phase of (a) water, (b) methanol, (c) acetonitrile, and (d) acetone, under gradient elution. They determined chlorophylls a and a′, chlorophylls b and b′, pheophytins a and a′, hydroxychlorophyll b, hydroxychlorophylls a and a′, and chlorophyllides a and a′ in the herb extract. The authors found chlorophyllide b, pyropheophorbide b, hydroxypheophytin a, and hydroxypheophytin a′ in the extract collected from the column, which accounted for 63% of the total content, suggesting more investigation is needed before the use of this herb in any drug formulation [31].
Lafeuille et al. (2014) studied the effect of five different drying treatments on the green colorants of 50 collected samples of culinary aromatic herbs in Turkey and Egypt. Different drying methods such as sun-drying, freeze-drying, oven-drying, DP1 (a modified traditional sun-drying process), and DP2 (a specially designed drying process to preserve the green colorants of aromatic herbs) were applied for drying. They used a standard extraction procedure for the extraction of green colorants from the collected samples. Briefly, 1 g of the fresh or dry herb were mixed with 100 mL of an 80:20 acetone:sodium citrate solution (0.1 M). The solution was filtered and stored for analysis. For this study, they developed an HPLC-PDS-MS method after separating through a Kinetex stainless-steel HPLC C18-column (6 μm size × 10 cm length × 4.6 mm ID) with a mobile phase of acetone:methanol (80:20, v/v) containing 0.5 M of NH4OAc. They detected 24 pigments (2 original chlorophyll a and b, 22 different degradants). Among the degradants, chlorophyllide, pyrochlorophyll, pheophytin, pyropheophytin, and pheophorbides were identified [32].
Based on literature survey and findings of different researchers, it is evident that there are various degradants of natural green chlorophylls found under different food processing conditions. Based on this, researchers can generalize chlorophyll and chlorophyllins’ structures as well as their degradants. Three structures are based on chlorin-skeleton (str-1-3)-related, and another three are based on porphyrin-skeleton (str-4-6)-related colorants (Figure 1). Depending on M (any metal cation), R (H, CH3), R1 (Phytyl group, H), R2 (H, OH, COOCH3) and R3 (H, OH, COOCH3), with or without an intact isocyclic ring, researchers can obtain different chlorophylls, chlorophyllins and their derivatives. Although various chlorin-skeleton-based colorants have been detected by different researchers, porphyrin-skeleton-based colorants could be reported in the near future.
Table 2. Separation and identification of green colorants in foodstuffs and beverages using HPLC-MS methods.
| S. No. |
Sample Type | Instrument Used | Stationary Phase | Mobile Phase, Flow Rate (mL/min), Run Time (min) | Analyzed Colourants | Reference |
|---|---|---|---|---|---|---|
| 1 | Five commercial Na-Cu-chlorophyllin samples |
HPLC-PDA and HPLC-APCI/ESI-MS | Waters YMC C30 column (5 µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) MeOH:H2O:AcOH (90:10:0.5, v/v/v) and (b) tert-butyl methyl ether:MeOH:AcOH (100:10:0.5, v/v/v), 10µL (PDA)/100 µL (MS), 1.1 mL/min, 45 min | Cu-chlorin e6, Cu-chlorin p6, Cu-isochlorin e4, Chlorin e6, Cu-pyropheophorbide a, Cu-purpurin 7, Cu-rhodin g7, Rhodin, Cu-rhodin, Cu-rhodochlorin, Cu-porphyrin |
[23] |
| 2 | Spinach-extracted chlorophyll a derived Fe-chlorophyllins | RP-HPLC-FAB-MS | Inertsil ODS C18 column (5 µm size × 25 cm length × 4.6 mm ID) | MeCN-phosphate buffer (pH 2) (60:40, v/v) containing tetramethyl ammonium chloride (0.01 M), | Fe(III)-pheophorbide a Fe(III)-chlorin e6 Fe(III)-chlorin e4 |
[4] |
| 3 | Serum samples | HPLC, ESI/MS, and MS/MS | (a) Prodigy C18 column (5 µm size × 25 cm length × 4.6 mm ID) (b) Vydac C18 column |
Mobile phases: (a) 0:20, v/v) with 1% (v/v) AcOH and (b) MeOH, 1 mL/min, | Chlorin e4 Ethyl Ester | [5] |
| 4 | 29 Edible oils (olive oil, grapeseed oil and blended oil) | UHPLC-APCI(-)-Q-Orbitrap-MS-MS | Halo C18 column (2.7 µm size × 10 cm length × 4.6 mm ID) | Mobile phases: (a) MeCN and (b) MeOH, 0.8 mL/min, at 30 °C | Cu-chloropheophytin a (m/z = 535) | [13] |
| 5 | Na-Cu-chlorophyllin in water-soluble and fat-soluble food samples | ESI-LC-TOF-MS | Acquity UPLC® BEH C-18 (1.7 μm size × 10 cm length × 2.1 mm ID) |
Mobile phases: (a)Water and (b) MeCN (A:B = 62.5:37.5), 5 uL, 0.35 mL/min, 12 min at 35 °C | Cu-isochlorin e4, Cu-chlorin p6, Cu-chlorin e6 |
[19] |
| 6 | Fortified food samples with Na-Fe-chlorophyllin | ESI-LC-TOF-MS | Acquity UPLC® BEH C-18 (1.7 μm size × 10 cm length × 2.1 mm ID) |
A: Water and B: MeCN (A:B = 62.5:37.5), 5µL, 0.35 mL/min, 12 min at 35 °C. | Fe(III)-isochlorin e4, Fe(III)-chlorin e4 |
[20] |
| 7 | Grapes and Port wines | HPLC-DAD-MS (ESP+) | Waters YMC C30 column (5 µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) H2O, (b) MeOH, and (c) tert-butyl methyl ether, 1 mL/min, acquisition of the mass data between m/z 100 and 700 |
Pheophorbide b, Pheophytin a/b, Pheophytin a/b like compound, Unknown chlorophyll-derived compound |
[22] |
| 8 | Photolon formulation | HPLC-PDA-MS | C-18 RP-HPLC column (3.5 µm size × 15 cm length × 4.6 mm ID) and a semi-preparative column (5 µm size × 15 cm length × 10 mm ID) | Mobile phases: (a) (0.1% TFA in water) and (b) (MeCN), 10 µL, 1 mL/min, 30 min | chlorin e6 174-ethyl ester, chlorin e4, 15-hydroxyphyllochlorin, Rhodochlorin, 151-hydroxymethylrhodochlorin δ-lactone, Rhodochlorin-15-oxymethyl δ-lactone, Rhodochlorin-15-oxymethyl δ-lactone 174-ethyl ester, 151-hydroxymethylrhodoporphyrin δ-lactone, Rhodoporphyrin-15-oxymethyl δ-lactone, Purpurin 18 |
[30] |
| 9 | Hot-air-dried and freeze-dried Chinese herb Rhinacanthus nasutus (L.) Kurz samples | HPLC-DAD-APCI-MS | Agilent Eclipse XDB C18 column (5 µm size × 15 cm length × 4.6 mm ID) | Mobile phases: (a) MeOH/N,N-dimethylformamide (97:3, v/v) and (b) MeCN under gradient elution, 1 mL/min, 2 min at a λmax of 600 nm | Chlorophyll a/a′, Hydroxychlorophyll a/b, 15-OH-lactone chlorophyll a, Chlorophyll b/b′, Pheophytin a/a′, Hydroxypheophytin a/a′, Pheophytin b |
[27] |
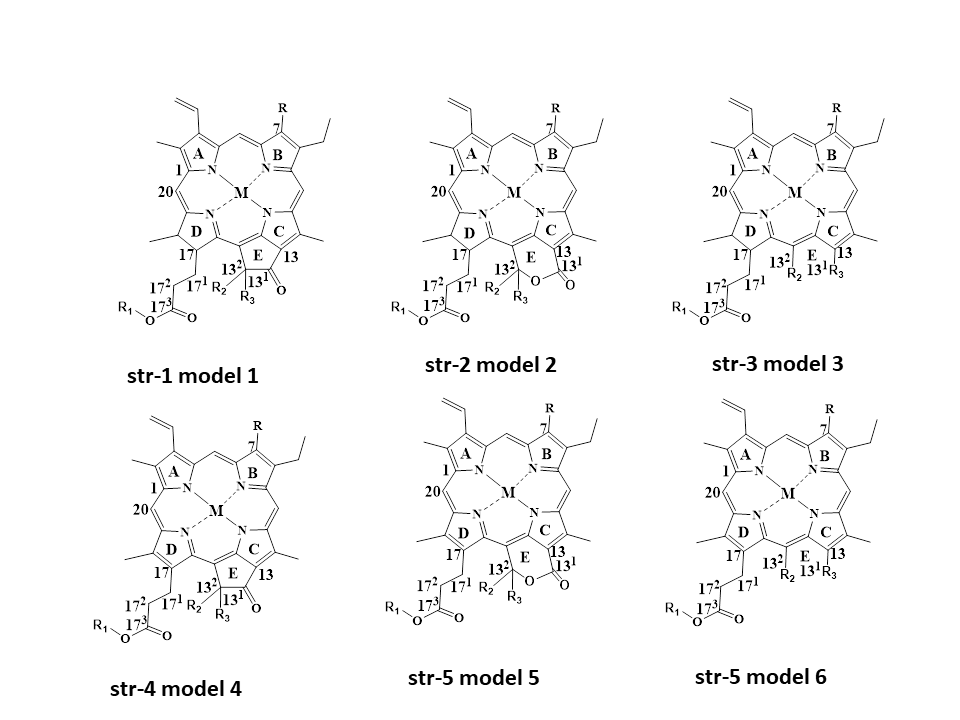
Figure 1. Common model structures of chlorophyll and chlorophyllins with a chlorin-based skeleton and a porphyrin-based skeleton.
References
- Cano, M.P. HPLC Separation of Chlorophyll and Carotenoid Pigments of Four Kiwi Fruit Cultivars. J. Agric. Food. Chem. 1991, 39, 1786–1791.
- Yasuda, K.; Tadano, K.; Ushiyama, H.; Ogawa, H.; Kawai, Y.; Nishima, T. Investigation to find an indicator substance for the analysis of sodium copper chlorophyllin in foods. J. Food Hyg. Soc. Jpn. 1995, 36, 710–716.
- Ushiyama, H.; Nishijima, M.; Yasuda, K.; Kamimura, H.; Tabata, S.; Nishima, T. Determination of sodium copper chlorophyllin in foods. J. Food Hyg. Soc. Jpn. 1986, 27, 417–420.
- Nonomura, Y.; Yamaguchi, Y.; Hara, K.; Furuya, K.; Yoshioka, N.; Inoue, H. High-performance liquid chromatographic separation of iron(III) chlorophyllin. J. Chromatogr. A 1996, 721, 350–354.
- Egner, P.A.; Stansbury, K.H.; Snyder, E.P.; Rogers, M.E.; Hintz, P.A.; Kensler, T.W. Identification and characterization of chlorin e4 ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem. Res. Toxicol. 2000, 13, 900–906.
- Wang, L.F.; Park, S.C.; Chung, J.O.; Baik, J.H.; Park, S.K. The Compounds Contributing to the Greenness of Green Tea. J. Food Sci. 2004, 69, S301–S305.
- Bohn, T.; Walczyk, T. Determination of chlorophyll in plant samples by liquid chromatography using zinc–phthalocyanine as an internal standard. J. Chromatogr. A 2004, 1024, 123–128.
- Scotter, M.J.; Castle, L.; Roberts, D. Method development and HPLC analysis of retail foods and beverages for copper chlorophyll (E141 ) and chlorophyllin (E141 ) food coloring materials. Food Addit. Contam. 2005, 22, 1163–1175.
- Roca, M.; Gallardo-Guerrero, L.; Mínguez-Mosquera, M.I.; Gandul Rojas, B.B. Control of Olive Oil Adulteration with Copper-Chlorophyll Derivatives. J. Agric. Food. Chem. 2010, 58, 51–56.
- Loranty, A.; Rembiałkowska, E.; Rosa, E.A.; Bennett, R.N. Identification, quantification and availability of carotenoids and chlorophylls in fruit, herb and medicinal teas. J. Food Compos. Anal. 2010, 23, 432–441.
- Baskan, K.S.; Tutem, E.; Ozer, N.; Apak, R. Spectrophotometric and Chromatographic Assessment of Contributions of Carotenoids and Chlorophylls to the Total Antioxidant Capacities of Plant Foods. J. Agric. Food Chem. 2013, 61, 11371–11381.
- Kenner, G.W.; McCombie, S.W.; Smith, K.M. Pyrroles and Related Compounds. Part XX1V.l Separation and Oxidative Degradation of Chlorophyll Derivatives. J. Chem. Soc. Perkin Trans. 1973, 1, 2517–2523.
- Fang, M.; Tsai, C.-F.; Wu, G.-Y.; Tseng, S.H.; Cheng, H.-F.; Kuo, C.-H.; Hsua, C.-L.; Kao, Y.M.; Shih, D.Y.C.; Chiang, Y.-M. Identification and quantification of Cu-chlorophyll adulteration of edible oils. Food Addit. Contam. Part B Surveill. 2015, 8, 157–162.
- Furuya, K.; Ohki, N.; Inoue, H.; Shirai, T. Determination of Pheophytinatonickel (II) by Reversed-Phase HighPerformance Liquid Chromatography. Chromatographia 1988, 25, 319–323.
- Viera, I.; Herrera, M.; Roca, M. In Vitro Bioaccessibility Protocol for Chlorophylls. J. Agri. Food Chem. 2021, 69, 8777–8786.
- Mathiyalagan, S.; Mandal, B.K. A review on assessment of acceptable daily intake for food additives. Biointerf. Res. Appl. Chem. 2020, 10, 6033–6038.
- Laddha, A.P.; Nalawade, V.V.; Gharpure, M.; Kulkarni, Y.A. Development and Validation of HPLC Method for Determination of Sodium Copper Chlorophyllin—A Food Colorant and Its Application in Pharmacokinetic Study. Chem. Biodivers. 2020, 17, e2000223.
- Suzuki, I.; Kubota, H.; Terami, S.; Hara, T.; Hirakawa, Y.; Iizuka, T.; Tatebe, C.; Ohtsuki, T.; Yano, T.; Sato, K.; et al. Development of an analytical method for copper chlorophyll and sodium copper chlorophyllin in processed foods. Jpn. J. Food Chem. Saf. 2016, 23, 55–62.
- Chong, H.S.; Park, H.I.; Cho, S.R.; Yamaguchi, T.; Lee, O.K.; Park, J.-H.; Lee, C.; Lee, G.-Y.; Yun, S.S.; Lim, H.-S.; et al. Establishment and Validation of an Optimized Analytical Method for Sodium Copper Chlorophyllin in Food Using HPLC and LC/MS. J. Korean Soc. Food Sci. Nutr. 2018, 47, 550–558.
- Chong, H.S.; Park, Y.J.; Kim, E.G.; Park, Y.L.; Kim, J.M.; Yamaguchi, T.; Lee, C.; Suh, H.-J. The Optimization and Verification of an Analytical Method for Sodium Iron Chlorophyllin in Foods Using HPLC and LC/MS. J. Food Hyg. Saf. 2019, 34, 148–157.
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Detection of the color adulteration of green table olives with copper chlorophyllin complexes (E-141ii colorant). LWT Food Sci. Technol. 2012, 1, 311–318.
- Mendes-Pinto, M.M.; Ferreira, A.N.C.S.S.; Caris-Veyrat, C.; Pinto, P.G.D. Carotenoid, Chlorophyll, and Chlorophyll-Derived Compounds in Grapes and Port Wines. J. Agric. Food Chem. 2005, 53, 10034–10041.
- Mortensen, A.; Geppel, A. HPLC-MS analysis of the green food colorant sodium copper chlorophyllin. IFSET 2007, 8, 419–425.
- Mínguez-Mosquera, M.I.; Garrido-Fernández, J. Chlorophyll and carotenoid presence in olive fruit (Olea europaea, L.). J. Agri. Food Chem. 1989, 37, 1–7.
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413.
- Huang, S.; Hung, C.; Wu, W.; Chen, B. Determination of chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 105–112.
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal Degradation Kinetics of Chlorophyll Pigments in Virgin Olive Oils. 1. Compounds of Series a. J. Agric. Food. Chem. 2010, 58, 6200–6208.
- Kao, T.H.; Chen, C.J.; Chen, B.H. An improved high performance liquid chromatography–photodiode array detection–atmospheric pressure chemical ionization–mass spectrometry method for determination of chlorophylls and their derivatives in freeze-dried and hot-air-dried Rhinacanthus nasutus (L.) Kurz. Talanta 2011, 8, 349–355.
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.Q.; Paglia, G. UPLC-UV-MSE analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Bioanal. Chem. 2012, 404, 3145–3154.
- Isakau, H.A.; Trukhacheva, T.V.; Petrov, P.T. Isolation and identification of impurities in chlorin e6. J. Pharm. Biomed. Anal. 2007, 45, 20–29.
- Loh, C.H.; Inbaraj, B.S.; Liu, M.H.; Chen, B.H. Determination of Chlorophylls in Taraxacum formosanum by High-Performance Liquid Chromatography–Diode Array Detection–Mass Spectrometry and Preparation by Column Chromatography. J. Agric. Food. Chem. 2012, 60, 6108–6115.
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Development of an accurate and direct method for the green food colorants detection. Food Res. Int. 2020, 136, 109484.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
4 times
(View History)
Update Date:
02 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




