Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md. Mahbubur Rahman | -- | 2990 | 2023-05-22 12:14:25 | | | |
| 2 | Beatrix Zheng | + 152 word(s) | 3142 | 2023-05-23 04:24:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rahman, M.M.; Lee, J.Y.; Kim, Y.H.; Park, C. Procedures and Applications of Epidural and Intrathecal Injection. Encyclopedia. Available online: https://encyclopedia.pub/entry/44656 (accessed on 02 March 2026).
Rahman MM, Lee JY, Kim YH, Park C. Procedures and Applications of Epidural and Intrathecal Injection. Encyclopedia. Available at: https://encyclopedia.pub/entry/44656. Accessed March 02, 2026.
Rahman, Md. Mahbubur, Ji Yeon Lee, Yong Ho Kim, Chul-Kyu Park. "Procedures and Applications of Epidural and Intrathecal Injection" Encyclopedia, https://encyclopedia.pub/entry/44656 (accessed March 02, 2026).
Rahman, M.M., Lee, J.Y., Kim, Y.H., & Park, C. (2023, May 22). Procedures and Applications of Epidural and Intrathecal Injection. In Encyclopedia. https://encyclopedia.pub/entry/44656
Rahman, Md. Mahbubur, et al. "Procedures and Applications of Epidural and Intrathecal Injection." Encyclopedia. Web. 22 May, 2023.
Copy Citation
Epidural and intrathecal routes are the most effective drug administration methods for pain management in clinical and experimental medicine to achieve quick results, reduce required drug dosages, and overcome the adverse effects associated with the oral and parenteral routes. Beyond pain management with analgesics, the intrathecal route is more widely used for stem cell therapy, gene therapy, insulin delivery, protein therapy, and drug therapy with agonist, antagonist, or antibiotic drugs in experimental medicine
epidural route
intrathecal route
drug delivery
rats
mice
1. Epidural and Intrathecal Injection Procedures
Epidural and intrathecal injections can be performed via acute needle puncture or catheterization. Acute needle puncture is usually used for single drug administration but can also be used for multiple doses [1], and intervals of 24 h, 2 days, and 7 days have been reported (Table 1). Acute needle puncture can be performed in anesthetized [2] or unanesthetized animals [1][3]; however, intrathecal injections in unanesthetized animals require greater skills. The intervertebral space of the spinal column is commonly used, but the lumbar region [2][4], especially L5–L6, is most commonly used for single acute injection [5][6]. However, multiple injections were performed through a single location, e.g., L5-L6 [1][7], L4-L5 [8], and top of the foramen [9] (Table 1). Proper needle insertion to the appropriate location and an accurate volume and concentration of the injectate, based on the species, are critical for the success and the recovery of animals; these are briefly discussed in Table 1, Table 2, Table 3 and Table 4. For an effective IT injection via acute puncture, only one try is required; however, if the first effort fails, the needle should be removed, and a second attempt may be made. If the second effort fails, a different intervertebral space should be chosen [10]. In addition, shaving and aseptic preparation of the skin, needles, and administering agents are also important for success.
The exact positioning of a needle to the epidural space is difficult noninvasively and requires high skill because the distance between the epidural and intrathecal spaces is very small in rats and mice. Therefore, the applications of direct epidural injections using needle puncture in mice and rats are limited because the needle can easily penetrate the dura mater and enter the intrathecal space. Therefore, intrathecal injection by acute needle puncture [1][7][11], intrathecal catheterization [12][13][14], and epidural catheterization [15][16][17] but not epidural injection by needle puncture is frequently used in mice and rats.
1.1. Procedure of Intrathecal Injection by Acute Needle Puncturing
After administering anesthesia (e.g., volatile isoflurane), the thoracolumbar region is shaved. Needles are inserted through the intervertebral space where bony parts are absent, which is covered by the ligamentum flavum [18]. The angle of needle insertion varies but has been reported at over 20° [3][19][20][21], 45° [22][23], at 45° shown in videos but reported to be 70–80° in the discussion section [5], and at 70–80° shown in videos [11] in mice; an angle of 15–30° has been reported in rats [23]. Therefore, it is difficult to conclude what the optimal injection angle is (Figure 3 and Figure 4). However, the authors of the current research also insert needles at angles of 70–80° and then reduce the angle to 30–45° during drug injection to easily spread out the injected drug from a narrow needle and to prevent CSF leakage during needle withdrawal (Figure 4). During insertion, when the tip of the needle reaches the bottom of the spinal canal (upper part of the vertebral body), the needle should not be pushed further as it may penetrate the intervertebral disc into the abdominal cavity [24] (Figure 1 and Figure 3). The depth of needle insertion has been reported to be approximately 0.30 cm in rats after skin and muscle incision and 0.30–0.40 cm without skin and muscle incision in mice [22].
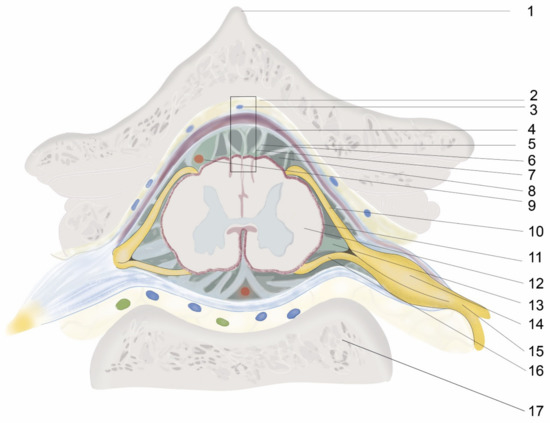
Figure 1. Illustration of cross-sectional anatomy of the spinal colum. 1. Spinous process, 2. Epidural space, 3. Intervertebral venous plexus, 4. Duramater, 5. Arachnoid mater, 6. Arachnoid trabeculae, 7. Figure 2, 8. Subarachnoid space and CSF, 9. Blood vessels, 10. Dorsal nerve root, 11. Pia mater, 12. Spinal cord, 13. Ligamentum denticulatum, 14. Dorsal nerve root ganglion, 15. Dorsal nerve root, 16. Ventral nerve root, 17. Vertebral body.
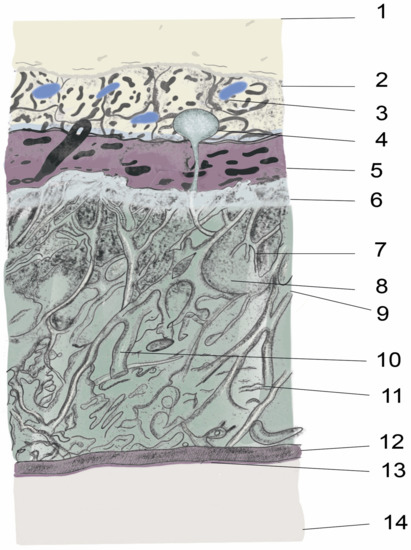
Figure 2. Illustration of anatomy of the spinal meninges and adjacent parts. 1. Ligament flavum, 2. Epidural space, 3. Intervertebral venous plexus, epidural fat 4. Arachnoid villi, 5. Duramater, 6. Arachnoid mater, 7. Arachnoid trabeculae, 8. CSF, 9. Subarachnoid space, 10. Major blood vessel, 11. Collagen fibrils, 12. Pia mater, 13. Glia limitans, 14. Spinal cord.
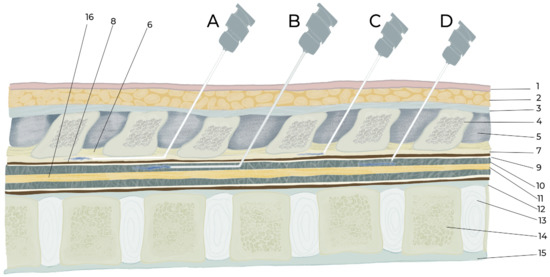
Figure 3. Illustration of intraspinal epidural and intrathecal drug delivery by catheterization and injection. A. Epidural catheterization, B. Intrathecal catheterization, C. Epidural injection, D. Intrathecal injection. 1. Skin, 2. Subcutaneous fat and muscle, 3. Supraspinal ligament, 4. Supraspinal process of vertebra, 5. Interspinous ligament, 6. Ligament flavum, 7. Epidural space, 8. Duramater, 9. Arachnoid mater, 10. Subarachnoid space, 11. Spinal cord, 12. Posterior longitudinal ligament, 13. Inter vertebral disc, 14. Vertebral body, 15. Anterior longitudinal ligament, 16. Spinal cord.
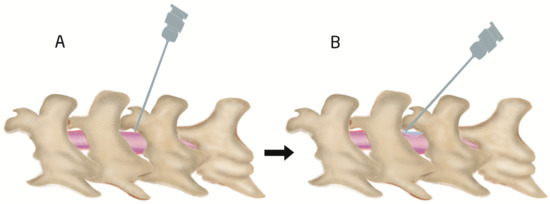
Figure 4. Illustration of intraspinal intrathecal injection site and angle. First insertion of needle at 70–80° angle (A) and then reduction until 30–45° (B) during drug injection to spread the injected drug easily and to prevent CSF leak out during withdrawing of needle.
The iliac crest and the L5–L6 intervertebral space are located by detection of the supraspinous process (Figure 1) of these two vertebrae (Figure 5). The pelvic girdle is then softly held by one hand to fix the dorsoventral position, and, on the other hand, a sterile needle is inserted at the appropriate angle to penetrate the ligamentum flavum and dura mater to reach the arachnoid space. However, some researchers use 1–3 cm longitudinal skin incisions over the spinous process of the desired intervertebral space in rats for better confirmation of the location during a single acute intrathecal needle puncture [23][25][26]. The drug is then delivered slowly to allow for spreading. The delivery time varies (Table 1) and should be selected considering the characteristics of the injected solution and the species. In addition, after drug delivery, care should be taken to wait 15 s to 1 min before withdrawing the syringe (Table 1); otherwise, drugs may be pulled back into the syringe. Appropriate intrathecal positioning can typically be confirmed via the tail-flick test, but this response does not occur every time. Dura mater puncture can also be confirmed by other characteristics, such as the formation of an “S” shape by the tail, by hind paw retraction, and occasionally by backflow of the CSF [10][14][18]. After injecting a drug, temporary motor paralysis also occurs, which is a sign of successful drug administration.
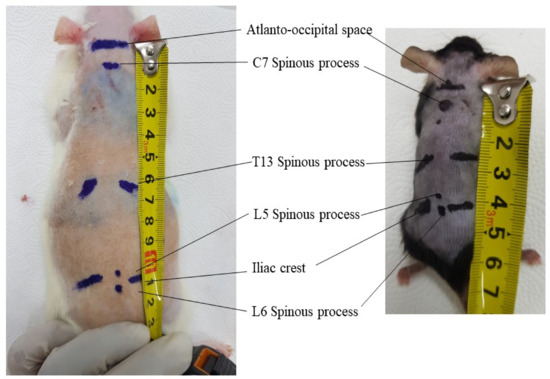
Figure 5. External location of antero-occipital space, C7, T13, L5, L6 spinous process, and iliac crest in 8 weeks old male Sprague Dawley rat and 8 weeks old male C57bl/6 mice. The average distance from antero-occipital space to L6 spinous process is 11 cm in rats and 4.2 cm in mice.
The injection volume is usually 5–10 μL in mice and 10–50 μL in rats, but different volumes have also been used (Table 1, Table 2, Table 3 and Table 4). An intrathecal injection of 30–50% of the volume of the total CSF volume is defined as a larger volume [27]. After the thoracolumbar intrathecal administration of a larger volume, it is immediately transferred to the cere-bro-cervical region. Thirty-three and forty-two percent of the total CSF volume in a thoraco-lumbar intrathecal administration was reported as tolerable in humans and non-human primates, respectively [27]. However, the limits of safe injection volumes have not been characterized in mice and rats, and further experimental studies are needed. The total volume of CSF in mice and rats is 35–40 μL and 150 μL, respectively [28]. The needle size for intrathecal injection by needle puncture in rats and mice varies between articles, but a 30-G needle is commonly used for mice [1][29], and 25-G is used for rats [10][11] (Table 1 and Table 2).
Furthermore, the dead space is sometimes ignored, but it has a significant impact on the accurate dosing of a drug because a very small total drug volume is injected via these routes. Dead space is the internal volume of the catheter or needle through which a drug passes from the syringe to the targeted region (e.g., epidural or intrathecal space). If the dead space is 10 µL and the injected volume is 10 µL, then the entire amount of the drug will remain in the catheter. To solve this problem, the dead space can first be filled with the injected agent, and the targeted volume should then be withdrawn in the syringe and then injected. Another method is by flushing with an equal volume of saline to replace the dead space occupied by the drugs [14][16][30]. However, care should be taken to prevent air bubbles when changing the syringe, which may cause adverse effects such as the alteration of subarachnoid pressure and injury to the nerves or meninges [31].
1.2. Epidural and Intrathecal Catheterization
Epidural and intrathecal catheterization are convenient and less stressful for animals when applying multiple doses for long-term medication. Intrathecal catheterization is administered in the atlanto-occipital and thoracolumbar regions [4][30], but epidural catheterization is only administered in the thoracolumbar region (Table 2 and Table 3). Catheters are inserted into the epidural and intrathecal space either through the intraspinal space by penetrating/cutting the ligament flavum [4][18] or by laminectomy (e.g., dorsal laminectomy, lateral laminectomy) [18]. In addition, atlanto-occipital catheterization is conducted by penetrating the anterior atlanto-occipital membrane that joins the upper cranial border of the anterior arch of the atlas (C1) to the anterior inferior surface of the foramen magnum [13][30]. The catheter is usually extended from L4 to L6 until the lumbar enlargement region [12][32] because the spinal nerves of these regions have pain and motor function-related clinical significance for supplying the hind legs. However, in a spinal cord injury model, the target is to reach the injured area (e.g., from the atlanto-occipital ar-ea to the T10–T11 injured area) [33]. The length of the spinal column of rats and mice should be known before catheterization, and the length from the atlanto-occipital space to the L6 vertebra is approximately 11 cm in rats and 4.2 cm in mice (Figure 5). One study [34] showed that the length was consistently 11 cm in rats. Therefore, in atlanto-occipital catheterization, a catheter should be extended 8–11 cm in rats and 3–4 cm in mice (Table 2 and Table 3). Therefore, care should be taken to avoid dura mater puncture in epidural catheterization and to avoid spinal cord injury in intrathecal catheterization. Usually, the length of catheter insertion for thoraco-lumbar catheterization in mice and rats is 1–2 cm but varies, with a maximum reported length of 4 cm [35]. The other end of the catheter is tunneled subcutaneously, and the opening is at the skin of the neck region, and the opening of the catheter is sealed or joined to a pump. Dura mater puncture can be confirmed based on behavioral responses such as those that occur with intrathecal injection. If animals exhibit any neurological deficits arising from the surgical procedure, they should be excluded from further experiments [12]. Additionally, 3 days after surgery, 10 μL of 2% lidocaine is often added to observe temporary motor paralysis and sensory loss for 20–40 min. If this does not occur, the animal should be excluded [14][21][21][30]. For epidural catheterization, animals should be excluded if CSF is aspirated or behaviors associated with dura mater puncture are observed [15] because epidural catheters should remain in the epidural space and above the dura mater where CSF is absent. Invasive epidural and intrathecal catheterization can be performed by direct observation of the dura mater by placing the catheter above the dura mater for epidural catheterization and by puncturing the dura and maintaining the catheter below the dura mater. Dead space should also be cautiously considered because of long catheters. PE-10 tubing is commonly used for intrathecal and epidural catheterization in rats and mice (Table 2 and Table 3). There are no studies showing how long catheters can be maintained after implantation. However, accurate placement of catheters was confirmed after 45 days in mice [36] and 9 months in rats [34]. Nonetheless, after experiments, the proper placement of catheters must be confirmed [21][36][37]. The injection site, injectate volume, concentration, frequency, duration, and purpose of epidural and intrathecal injections by catheterization are discussed in Table 1, Table 2, Table 3 and Table 4.
Table 1. Intrathecal injection by acute needle puncture in mice and rats.
| Species | Injection Site | Volume | Syringe Size | Time of Injection | Time of Syringe Withdrawal |
Number of Injections |
Reference |
|---|---|---|---|---|---|---|---|
| C57BL/6 mice | L4–5 | 10 μL | 30-G needle to 50-μL Hamilton | 3 μL/min | The needle was removed 1 min after completion and was kept in Trendelenburg position 5 min more | Single | [11] |
| C57BL/6 mice | L5–6 | 10 μL | 30-G 0.5-in needle | - | - | Three injections, 24-h intervals | [29] |
| Kunming mice | L5–L6 | 10 μL | Delivered for more than 30 s | Syringe maintained for an additional 15 s to ensure diffusion before removal | Single | [7] | |
| C57BL/6J mice | L5–6 | 5 μL | 30-G in 10-μL Hamilton | - | Three injections at two-day intervals | [1] | |
| FVB/NJ mice | L5–L6 | 8 μL | 27-G needle 25-μL Hamilton syringe |
1 μL/4 s | 1 min after finishing delivery | Single | [5] |
| Mice | L5–L6 | 5 μL | 30 G | 1 μL/6 s | 15 s | Single | [19] |
| C57BL/6 mice | top of the foramen magnum | 20 μL | 25-G, 1-mL syringe | Slowly | After 2 min | Three injections at 7 days intervals | [9] |
| CDI mice | L5–6 | 10 μL | 30-G needle | - | - | Single | [38] |
| Mice | 20 μL | 30-G 1/2 in 50 μL Hamilton | Injections were delivered as a bolus within 5 s |
Single | [39] | ||
| SD rat | L2–3 | 0.2 mL or 2 mL | 1-mL syringe | 1-mL syringe | After injection, rats placed upside-down at a 45° angle for 15 min | Single | [26] |
| SD rat | L5–6 | 30 μL | 31 G | - | Single | [6] | |
| Wistar rats | L4–5 | 15 μL | 26 G | 3 μL/min | - | Two injections, 24-h intervals | [8] |
| Wistar rats | L6–S1 | 0.02 mL/kg, average of 0.05 mL per rat | 25 G | 1 mL/min, average: 3 s/injection | 1 mL/min, average: 3 s /injection | Single | [10] |
| Wistar rats | L3–4 or L4–5 | 25 G | 1 min | Single | [25] |
Table 2. Intrathecal injection by catheterization in rat and in mouse.
| Species | Site of Insertion | Catheter Size and Total Length | Inserted Length | Dead Space and Filling Agent | Injected Volume | Reference |
|---|---|---|---|---|---|---|
| Lumbar | ||||||
| SD rat | L4–5 | PE-10 (0.6 mm diameter) | 1–2 cm | 20 µL, saline | 10 μL | [12] |
| SD rats | L4–5 | PE-10 tube, 12 cm | 2 cm | - | - | [4] |
| SD rats | L5–6 | PE-10 (0.6 mm diameter), 10 cm | 4 cm | - | 10 μL | [35] |
| SD rats | L5–6 | PE-10 (0.6 mm diameter), 15 cm | 3 cm | 4.5 µL, saline (7 µL) | 10 μL | [14] |
| SD rat | L2 laminectomy, tip located between L3 and L5 | SUBL-14 | L3–L5 | 10 µL | 25 or 50 μL | [40] |
| Rats | T13–L1 | PE-5 catheters (outside diameter: 0.36 mm) | L2–L5 | 6 µL, PBS | 20 µL | [36] |
| Atlanto-occipital | ||||||
| SD rat | Atlanto-occipital | ALZET catheter (PU-10 28G |
8 cm caudally to reach lumbar enlargement | 10 μL, sterile saline | 20 μL | [30] |
| SD rat | Atlanto-occipital | PE-10 | 8.5 cm caudally to reach lumbar enlargement | 10 μL, sterile saline | 10 μL | [41] |
| Mice | Atlanto-occipital | -ALZET IT mice catheter -O’Buckley IT catheter |
2.5 cm | [13] |
Table 3. Epidural catheterization in rat and in mouse.
| Species | Site of Insertion | Catheter Size and Total Length | Inserted Length | Dead Space and Filling Agent | Injected Volume | Reference |
|---|---|---|---|---|---|---|
| SD rat | L4–5 | PE-10 (0.6 mm diameter) | 1–2 cm | 20 µL, saline | 10 μL | [12] |
| SD rat | T13–L1 | PE-10 | ~3.0 cm until L5–6 | 100 µL of hyaluronic acid, 0.9% saline | 100 µL of hyaluronic acid, 0.9% saline | [15] |
| SD art | T13–L1 | PE-10 catheter | ~3.0 cm until L5–6 | 10 µL of sa | 160 µL | [16] |
| Mice | T11–T12 | PU-10catheter | 1 cm | - | 50 µL | [17] |
2. Uses and Application of Epidural and Intrathecal Injection
The epidural route is widely used for the induction of anesthesia in large animals and humans [42]. However, this route is widely used for analgesic purposes and not for anesthesia. In rats and mice, the intraperitoneal, intravenous, and intrarespiratory routes (i.e., for volatile anesthetics) are used to induce anesthesia. These routes are most commonly used for pain management with analgesics [41]. However, beyond pain management, the intrathecal route is widely used for stem cell therapy [8][9][38][43][44], gene therapy [11][29][44][45], delivery of immune cells [39], sedation [2], protein therapy, insulin delivery [46], mineral delivery [47], chemical delivery [15][47][48][49], and drug therapy using agonists [4][49][50], antagonists [4][40], antibiotics [47][51], and antiparasitic drugs [52] (Table 4). Intraspinal injection used in pain models can be divided into cancer pain models [44], including chemotherapy, induced pain models [1][52], and non-cancer pain models, such as models of arthritis [53], rheumatoid arthritis [49], diabetes-induced neuropathic pain [46][50], chronic pancreatitis-induced pain [54], spinal injury-induced pain [33][36], post-herpetic neuralgia [4], foraminal stenosis-induced pain [15][16], chronic DRG compression-induced pain [8], spared nerve injury [55], intrathecal capsaicin-induced spontaneous pain [48], chronic post-ischemia neuropathic pain [56], spinal nerve ligation-induced pain [6][12], and the acetic acid-induced writhing test [47] (Table 4). Furthermore, the intraspinal route is also used for the evaluation of safety and analgesic effects in normal healthy animals [41][57]. Beyond pain management, the intrathecal route is also used for drug administration for the amelioration of spinal injury-induced spasticity [36] and the induction of itching and scratching in behavioral models [58] and a pruritis model [3] (Table 4).
Table 4. Different uses and applications of epidural and intrathecal injections.
| Species | Method of Drugs Administration | Disease Model | Types of Agents Injected | Purpose of Injection | Concentration | Injected Volume and Vehicle | Reference |
|---|---|---|---|---|---|---|---|
| SD rat | ITc | Resiniferatoxin-induced postherpetic neuralgia | -Amiloride, a potent ASIC3 inhibitor -7,8-DHF, TrkB agonist, 3 mg/kg |
-To evaluate involvement of ASIC3 and TrkB signaling in pain in dorsal root ganglia | 100-μg amiloride daily for 7 days -3-mg/kg, TrkB agonist for 7 days |
10 μL | [4] |
| SD rat | ITc | Spinal nerve ligation-induced pain model | Phosphodiesterase 4B-specific siRNA | -To reduce neuroinflammation | 2 μg | [12] | |
| SD rat | ITinj | Chronic pancreatis model | Cognate receptor C–X–C chemokine receptor type 4 (CXCR4) inhibitor | -to reduce pancreatic pain |
5 μg/10 μL daily for one week | 10 μL | [54] |
| SD rat | ITc | Freund’s complete adjuvant-induced rheumatoid arthritis | Crocin | -To reduce rheumatoid arthritis-induced pain | 100 mg/kg | 20 μL | [49] |
| SD rat | ITc | Bone cancer pain model | Genetically engineered human bone marrow stem cells | -To reduce bone cancer pain | 6 × 106 cells |
10 μL | [44] |
| SD rat | ITinj | Neuropathic pain | Adipose tissue-derived stem cells (ASCs) | -To relieve neuropathic pain | 1 × 106 cells | 30 μL DMEM | [6] |
| SD rat | EDc | Foraminal stenosis-induced pain | Hyaluronic acid (HA) | -To relieve neuropathic pain | 100 µL of HA | 100 µL of HA | [15] |
| SD rat | EDc | Healthy rats | Gabapentin | -To evaluate safety and toxicity | 30 mg | 300 μL | [57] |
| SD rat | EDc | Lumbar foraminal stenosis-induced pain | Polydeoxyribonucleotide | -To evaluate analgesic effect | 0.1 mg/kg | 160 µL | [16] |
| Wistar Rat | ITc | Spinal cord ischemia | human umbilical cord blood stem cells | To improve spinal cord function | 1 × 104 HUCBSCs |
10 μL | [43] |
| SD rat | ITc | Spinal cord injury model | Embryonic Stem Cell-Derived Spinal GABAergic Neural Precursor Cells |
To reduce central neuropathic pain and motor function | 1 × 106 cells | - | [33] |
| Wistar rat | ITinj | Chronic DRG compression-induced pain model | Bone marrow stromal cell | -To reduce neuropathic Pain |
1 × 106 cells | 15 μL | [8] |
| CD1 mice | ITinj | CCI-induced neuropathic pain model | Bone marrow stromal cell | -To reduce neuropathic pain | 1 or 2.5 × 105 cells | 10 μL | [38] |
| Rat | ITc | Spinal cord injury-induced spasticity | -Potassium-chloride cotransporter KCC2 - BDNF |
-To evaluate the involvement of KCC2 and BDNF in spasticity | 20 μg 10 μg |
20 μL | [36] |
| Rat | ITc | Phasic andincisional pain | Gentamycin, Streptomycin, Neomycin | -To evaluate Antinociceptive potency of aminoglycoside antibiotics | 5 μg, 15 μg, 15 μg, respectively | 10 μL | [51] |
| Rat | ITc | Diabetes-induced neuropathic pain | Insulin | -To evaluate Antinociceptive potency of insulin | 0.2 U | 10 μL | [46] |
| Rats, Mice | ITc | Diabetes-induced neuropathic pain | Sirtuin 1 agonist, SRT1720 | -To reduce neuropathic pain |
0.8 μg in rats, 1.4 μg in mice | 10 μL | [50] |
| Rat | ITc | Spared nerve injury (SNI) | TMEM16A, U0126 inhibitors | -To find out the mechanisms of neuropathic pain | 10 μg, 10 μg | 10 μL | [55] |
| Mice | ITinj | Chemotherapy (Paclitaxel)-induced neuropathic pain | -Artesunate | -To reduce chemotherapy-induced neuropathic pain | 100 μg | 5 μL | [52] |
| Mice | Neuropathic Pain | Decursin | -To reduce pain | [1] | |||
| Mice | ITinj | Spontaneous pain | Capsaicin | To induce spontaneous pain | 0.5 µg in 10 μL | [48] | |
| Mice | ITinj | PAR-2 activator trypsin-induced scratching behavior | -gastrin-releasing peptide (GRP) -Opioids |
-To reduce scratching behavior | 1 nmol/5 μL | 5 μL | [58] |
| Mice | ITinj | Morphine-induced pruritis | Morphine | -To induce scratching behavior | 0.3 nmol | 5 μL | [3] |
| Mice | ITinj | Chronic post-ischemia neuropathic pain model | Human mesenchymal stem cells | -To reduce pain behavior | 2 × 105 cells | 5 μL | [56] |
| Mice | ITinj | Collagen-induced arthritis | ERK1/2 inhibitor (U0126), Tramadol, and NMDAR antagonist D-2-amino-5-phosphonovaleric acid | -To reduce pain behavior | 1.6 µg, 250 µg, 0.5 µg, respectively | 5 μL | [53] |
| Mice | ITc | Acetic acid-induced writhing test | Neomycin, gentamicin |
-To evaluate antinociceptive effects | 0.5–20.0 µg, 5–40 µg, respectively | 10 μL | [47] |
ITc, intrathecal catheterization; ITInj, intrathecal injection; EDc epidural catheterization, TMEM16A, transmembrane protein 16A.
References
- Son, D.B.; Choi, W.; Kim, M.; Go, E.J.; Jeong, D.; Park, C.K.; Kim, Y.H.; Lee, H.; Suh, J.W. Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells 2021, 10, 547.
- Li, X.; Zhang, W. Influence of intrathecal injection with dexmedetomidine on the behavioral ability and analgesic effects on rats with neuropathic pain and expression of protein kinase C in the spinal dorsal horn. Exp. Ther. Med. 2018, 16, 3835–3840.
- Ye, Y.S.; Pan, A.Z.; Zhen, Y.; Kang, M.R.; Zhang, B.; Yi, W.M. Antipruritic effects of electroacupuncture on morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-kappaB pathway. Neuroreport 2019, 30, 331–337.
- Wei, X.; Wang, L.; Hua, J.; Jin, X.H.; Ji, F.; Peng, K.; Zhou, B.; Yang, J.; Meng, X.W. Inhibiting BDNF/TrkB.T1 receptor improves resiniferatoxin-induced postherpetic neuralgia through decreasing ASIC3 signaling in dorsal root ganglia. J. Neuroinflamm. 2021, 18, 96.
- Li, D.; Li, Y.; Tian, Y.; Xu, Z.; Guo, Y. Direct Intrathecal Injection of Recombinant Adeno-associated Viruses in Adult Mice. J. Vis. Exp. 2019, 144, e58565.
- Jwa, H.S.; Kim, Y.H.; Lee, J.; Back, S.K.; Park, C.K. Adipose Tissue-Derived Stem Cells Alleviate Cold Allodynia in a Rat Spinal Nerve Ligation Model of Neuropathic Pain. Stem Cells Int. 2020, 2020, 8845262.
- Qian, Y.; Wang, Q.; Jiao, J.; Wang, G.; Gu, Z.; Huang, D.; Wang, Z. Intrathecal injection of dexmedetomidine ameliorates chronic neuropathic pain via the modulation of MPK3/ERK1/2 in a mouse model of chronic neuropathic pain. Neurol. Res. 2019, 41, 1059–1068.
- Teng, Y.; Zhang, Y.; Yue, S.; Chen, H.; Qu, Y.; Wei, H.; Jia, X. Intrathecal injection of bone marrow stromal cells attenuates neuropathic pain via inhibition of P2X4R in spinal cord microglia. J. Neuroinflamm. 2019, 16, 271.
- Harris, V.K.; Yan, Q.J.; Vyshkina, T.; Sahabi, S.; Liu, X.; Sadiq, S.A. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J. Neurol. Sci. 2012, 313, 167–177.
- Thomas, A.A.; Detilleux, J.; Sandersen, C.F.; Flecknell, P.A. Minimally invasive technique for intrathecal administration of morphine in rats: Practicality and antinociceptive properties. Lab Anim. 2017, 51, 479–489.
- Bey, K.; Ciron, C.; Dubreil, L.; Deniaud, J.; Ledevin, M.; Cristini, J.; Blouin, V.; Aubourg, P.; Colle, M.A. Efficient CNS targeting in adult mice by intrathecal infusion of single-stranded AAV9-GFP for gene therapy of neurological disorders. Gene Ther. 2017, 24, 325–332.
- Ji, Q.; Di, Y.; He, X.; Liu, Q.; Liu, J.; Li, W.; Zhang, L. Intrathecal injection of phosphodiesterase 4B-specific siRNA attenuates neuropathic pain in rats with L5 spinal nerve ligation. Mol. Med. Rep. 2016, 13, 1914–1922.
- Oladosu, F.A.; Ciszek, B.P.; O’Buckley, S.C.; Nackley, A.G. Novel intrathecal and subcutaneous catheter delivery systems in the mouse. J. Neurosci. Methods 2016, 264, 119–128.
- Storkson, R.V.; Kjorsvik, A.; Tjolsen, A.; Hole, K. Lumbar catheterization of the spinal subarachnoid space in the rat. J. Neurosci. Methods 1996, 65, 167–172.
- Nahm, F.S.; Lee, P.B.; Choe, G.Y.; Lim, Y.J.; Kim, Y.C. Therapeutic effect of epidural hyaluronic acid in a rat model of foraminal stenosis. J. Pain Res. 2017, 10, 241–248.
- Lee, H.J.; Ju, J.; Choi, E.; Nahm, F.S.; Choe, G.Y.; Lee, P.B. Effect of epidural polydeoxyribonucleotide in a rat model of lumbar foraminal stenosis. Korean J. Pain 2021, 34, 394–404.
- Xu, Z.; Agbigbe, O.; Nigro, N.; Yakobi, G.; Shapiro, J.; Ginosar, Y. Use of high-resolution thermography as a validation measure to confirm epidural anesthesia in mice: A cross-over study. Int. J. Obstet. Anesth. 2021, 46, 102981.
- Chen, L.; Jiang, M.; Pei, L. Comparison of three methods of drug delivery in the rat lumbar spinal subarachnoid space. Anat. Rec. 2012, 295, 1212–1220.
- Shao, H.; Xue, Q.; Zhang, F.; Luo, Y.; Zhu, H.; Zhang, X.; Zhang, H.; Ding, W.; Yu, B. Spinal SIRT1 activation attenuates neuropathic pain in mice. PLoS ONE 2014, 9, e100938.
- Crowley, S.T.; Fukushima, Y.; Uchida, S.; Kataoka, K.; Itaka, K. Enhancement of Motor Function Recovery after Spinal Cord Injury in Mice by Delivery of Brain-Derived Neurotrophic Factor mRNA. Mol. Ther. Nucleic Acids 2019, 17, 465–476.
- Hou, Y.; Wang, L.; Gao, J.; Jin, X.; Ji, F.; Yang, J. A modified procedure for lumbar intrathecal catheterization in rats. Neurol. Res. 2016, 38, 725–732.
- Chen, Y.; Mazur, C.; Luo, Y.; Sun, L.; Zhang, M.; McCampbell, A.; Tomassy, G.S. Intrathecal Delivery of Antisense Oligonucleotides in the Rat Central Nervous System. J. Vis. Exp. 2019, 152, e60274.
- Wang, H.C.; Cheng, K.I.; Chen, P.R.; Tseng, K.Y.; Kwan, A.L.; Chang, L.L. Glycine receptors expression in rat spinal cord and dorsal root ganglion in prostaglandin E2 intrathecal injection models. BMC Neurosci. 2018, 19, 72.
- Mazur, C.; Fitzsimmons, B.; Kamme, F.; Nichols, B.; Powers, B.; Wancewicz, E. Development of a simple, rapid, and robust intrathecal catheterization method in the rat. J. Neurosci. Methods 2017, 280, 36–46.
- Quintana, E.; Judas; Kallenbach, K. Intrathecal injection of human umbilical cord blood stem cells attenuates spinal cord ischaemic compromise in rats CONFERENCE DISCUSSION. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 762.
- Kim, H.; Na, D.L.; Lee, N.K.; Kim, A.R.; Lee, S.; Jang, H. Intrathecal Injection in A Rat Model: A Potential Route to Deliver Human Wharton’s Jelly-Derived Mesenchymal Stem Cells into the Brain. Int. J. Mol. Sci. 2020, 21, 1272.
- Belov, V.; Appleton, J.; Levin, S.; Giffenig, P.; Durcanova, B.; Papisov, M. Large-Volume Intrathecal Administrations: Impact on CSF Pressure and Safety Implications. Front. Neurosci. 2021, 15, 604197.
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95.
- Njoo, C.; Heinl, C.; Kuner, R. In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J. Vis. Exp. 2014, 85, e51229.
- Kaya, C.; Atalay, Y.O.; Meydan, B.C.; Ustun, Y.B.; Koksal, E.; Caliskan, S. Evaluation of the neurotoxic effects of intrathecal administration of (S)-(+)-Ketoprofen on rat spinal cords: Randomized controlled experimental study. Braz. J. Anesthesiol. 2019, 69, 403–412.
- Hubler, M.; Litz, R.J.; von Kummer, R.; Albrecht, D.M. Intrathecal air following spinal anaesthesia. Anaesthesia 2002, 57, 307–308.
- Kawamata, T.; Omote, K.; Kawamata, M.; Iwasaki, H.; Namiki, A. Antinociceptive interaction of intrathecal alpha2-adrenergic agonists, tizanidine and clonidine, with lidocaine in rats. Anesthesiology 1997, 87, 436–448.
- Hwang, I.; Hahm, S.C.; Choi, K.A.; Park, S.H.; Jeong, H.; Yea, J.H.; Kim, J.; Hong, S. Intrathecal Transplantation of Embryonic Stem Cell-Derived Spinal GABAergic Neural Precursor Cells Attenuates Neuropathic Pain in a Spinal Cord Injury Rat Model. Cell Transpl. 2016, 25, 593–607.
- Ouchi, K.; Sekine, J.; Koga, Y.; Nakao, S.; Sugiyama, K. Establishment of an animal model of sedation using epidural anesthesia that uses the tail-flick test for evaluating local anesthetic effects in rats. Exp. Anim. 2013, 62, 137–144.
- Hou, J.; Xia, Z.; Xiao, X.; Wan, X.; Zhao, B. Neurotoxicity of intrathecal injections of dexmedetomidine into the rat spinal dorsal horn. Neural Regen. Res. 2012, 7, 1765–1770.
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307.
- Jasmin, L.; Ohara, P.T. Long-term intrathecal catheterization in the rat. J. Neurosci. Methods 2001, 110, 81–89.
- Chen, G.; Park, C.K.; Xie, R.G.; Ji, R.R. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J. Clin. Investig. 2015, 125, 3226–3240.
- Taiwo, O.B.; Kovacs, K.J.; Larson, A.A. Chronic daily intrathecal injections of a large volume of fluid increase mast cells in the thalamus of mice. Brain Res. 2005, 1056, 76–84.
- Estrada, J.A.; Ducrocq, G.P.; Kim, J.S.; Kaufman, M.P. Intrathecal injection of brilliant blue G, a P2X7 antagonist, attenuates the exercise pressor reflex in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R223–R232.
- Yoon, M.H.; Park, K.D.; Lee, H.G.; Kim, W.M.; An, T.H.; Kim, Y.O.; Huang, L.J.; Hua, C.J. Additive antinociception between intrathecal sildenafil and morphine in the rat formalin test. J. Korean Med. Sci. 2008, 23, 1033–1038.
- Schaffer, D.P.H.; de Araujo, N.; Otero, A.R.; Dorea Neto, F.A.; Barbosa, V.F.; Martins Filho, E.F.; Oria, A.P. Cardiorespiratory effects of epidural anesthesia using lidocaine with morphine or dexmedetomidine in capuchin monkeys (Sapajus sp.) undergoing bilateral tubal ligation surgery, anesthetized with isoflurane. J. Med. Primatol. 2017, 46, 311–319.
- Judas, G.I.; Ferreira, S.G.; Simas, R.; Sannomiya, P.; Benicio, A.; da Silva, L.F.; Moreira, L.F. Intrathecal injection of human umbilical cord blood stem cells attenuates spinal cord ischaemic compromise in rats. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 757–762.
- Sun, Y.; Tian, Y.; Li, H.; Zhang, D.; Sun, Q. Antinociceptive Effect of Intrathecal Injection of Genetically Engineered Human Bone Marrow Stem Cells Expressing the Human Proenkephalin Gene in a Rat Model of Bone Cancer Pain. Pain Res. Manag. 2017, 2017, 7346103.
- Chang, M.F.; Hsieh, J.H.; Chiang, H.; Kan, H.W.; Huang, C.M.; Chellis, L.; Lin, B.S.; Miaw, S.C.; Pan, C.L.; Chao, C.C.; et al. Effective gene expression in the rat dorsal root ganglia with a non-viral vector delivered via spinal nerve injection. Sci. Rep. 2016, 6, 35612.
- Kou, Z.Z.; Li, C.Y.; Hu, J.C.; Yin, J.B.; Zhang, D.L.; Liao, Y.H.; Wu, Z.Y.; Ding, T.; Qu, J.; Li, H.; et al. Alterations in the neural circuits from peripheral afferents to the spinal cord: Possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front. Neural Circuits 2014, 8, 6.
- Dogrul, A.; Yesilyurt, O. Effects of intrathecally administered aminoglycoside antibiotics, calcium-channel blockers, nickel and calcium on acetic acid-induced writhing test in mice. Gen. Pharmacol. 1998, 30, 613–616.
- Han, Q.; Kim, Y.H.; Wang, X.; Liu, D.; Zhang, Z.J.; Bey, A.L.; Lay, M.; Chang, W.; Berta, T.; Zhang, Y.; et al. SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons. Neuron 2016, 92, 1279–1293.
- Wang, J.F.; Xu, H.J.; He, Z.L.; Yin, Q.; Cheng, W. Crocin Alleviates Pain Hyperalgesia in AIA Rats by Inhibiting the Spinal Wnt5a/beta-Catenin Signaling Pathway and Glial Activation. Neural Plast. 2020, 2020, 4297483.
- Zhang, Z.; Ding, X.; Zhou, Z.; Qiu, Z.; Shi, N.; Zhou, S.; Du, L.; Zhu, X.; Wu, Y.; Yin, X.; et al. Sirtuin 1 alleviates diabetic neuropathic pain by regulating synaptic plasticity of spinal dorsal horn neurons. Pain 2019, 160, 1082–1092.
- Prado, W.A.; Machado Filho, E.B. Antinociceptive potency of aminoglycoside antibiotics and magnesium chloride: A comparative study on models of phasic and incisional pain in rats. Braz. J. Med. Biol. Res. 2002, 35, 395–403.
- Li, Y.; Kang, J.; Xu, Y.; Li, N.; Jiao, Y.; Wang, C.; Wang, C.; Wang, G.; Yu, Y.; Yuan, J.; et al. Artesunate Alleviates Paclitaxel-Induced Neuropathic Pain in Mice by Decreasing Metabotropic Glutamate Receptor 5 Activity and Neuroinflammation in Primary Sensory Neurons. Front. Mol. Neurosci. 2022, 15, 902572.
- Xu, Y.; Zhang, K.; Miao, J.; Zhao, P.; Lv, M.; Li, J.; Fu, X.; Luo, X.; Zhu, P. The spinal NR2BR/ERK2 pathway as a target for the central sensitization of collagen-induced arthritis pain. PLoS ONE 2018, 13, e0201021.
- Zhu, H.Y.; Liu, X.; Miao, X.; Li, D.; Wang, S.; Xu, G.Y. Up-regulation of CXCR4 expression contributes to persistent abdominal pain in rats with chronic pancreatitis. Mol. Pain 2017, 13, 1744806917697979.
- Chen, Q.; Kong, L.; Xu, Z.; Cao, N.; Tang, X.; Gao, R.; Zhang, J.; Deng, S.; Tan, C.; Zhang, M.; et al. The Role of TMEM16A/ERK/NK-1 Signaling in Dorsal Root Ganglia Neurons in the Development of Neuropathic Pain Induced by Spared Nerve Injury (SNI). Mol. Neurobiol. 2021, 58, 5772–5789.
- Yoo, S.H.; Lee, S.H.; Lee, S.; Park, J.H.; Lee, S.; Jin, H.; Park, H.J. The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice. Korean J. Pain 2020, 33, 23–29.
- Choi, S.S.; Kim, Y.C.; Lim, Y.J.; Lee, C.J.; Lee, P.B.; Lee, S.C.; Sim, W.S.; Choi, Y.L. The neurological safety of epidural gabapentin in rats: A light microscopic examination. Anesth. Analg. 2005, 101, 1422–1426.
- Maciel, I.S.; Azevedo, V.M.; Pereira, T.C.; Bogo, M.R.; Souza, A.H.; Gomez, M.V.; Campos, M.M. The spinal inhibition of N-type voltage-gated calcium channels selectively prevents scratching behavior in mice. Neuroscience 2014, 277, 794–805.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
23 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





