Chronic heart failure (CHF) is one of principal health problems in industrialized countries. Despite therapeutical improvement, based on drugs and exercise training, it is still characterized by elevated mortality and morbidity. Data show that protein energy malnutrition, clinically evident primarily with sarcopenia, is present in more than 50% of CHF patients and is an independent factor of CHF prognosis. Several pathophysiological mechanisms, primarily due to the increase in blood hypercatabolic molecules, have been proposed to explain this phenomenon. Nutritional supplementation with proteins, amino acids, vitamins and antioxidants have all been used to treat malnutrition. However, the success and efficacy of these procedures are often contradictory and not conclusive. Exercise reduces mortality and increases functional capacity, although it also increases the catabolic state with energy expenditure and nitrogen-providing substrate needs.
1. The Clinical Problem
Chronic heart failure (CHF) is one of principal health problems of the industrialized countries. It is a complex syndrome where, although the “
primum movens” is heart disease, it also affects many organs and systems of the human body
[1]. Despite recent therapeutical improvements based on a combination of specific medical approaches/therapies, CHF is still characterized by elevated mortality and morbidity
[2].
Interestingly, recently the benefits of cardiac rehabilitation and exercise training have been shown in patients with heart failure, including a reduction in morbidity and mortality. However, data also show that even light exercise causes a significant catabolic demolition of muscular proteins
[3]. This catabolic effect of exercise is more prominent in patients with malnutrition. It must therefore specify that malnutrition is a generic term that includes two pathophysiological and clinical conditions: overnutrition and undernutrition.
It can say that surplus nutrients intake causes overnutrition, which in turn causes obesity. However, the presence of obesity does not necessarily suggest that there is no alteration of protein metabolism. Indeed, we should remember that there is a clinical condition, namely sarcopenic obesity, in which the patient is obese, but has all the characteristics of sarcopenia, including reduced muscle mass and muscle strength. Therefore, the presence of protein metabolism disarrangements should also be sought in obese patients with CHF.
On the contrary, undernutrition is due to a lack of nutrients. It can result in reduced growth or weight loss according to age and/or concomitant diseases. Undernutrition can occur either due to protein energy wasting or as a result of micronutrient deficiencies. It can be a major health problem both in children, where it is responsible for incomplete physical and mental development, as well as in adults. Interestingly, undernutrition is particularly present in elderly people and women. It should be pointed out that undernutrition is an increasing health problem in people aged over 65 years in developed countries, mainly due to physical, psychological and social factors. Indeed, aged people reduce dietary intake because they may have both physical and/or social problems, such as chewing and swallowing difficulties, depression, intestinal-related diseases, and/or poverty and loneliness.
The signs and symptoms of micronutrient deficiencies depend on which micronutrients are lacking. Basically, an inadequate intake of micronutrients includes iodine, vitamins and iron. Clinically, these can cause, respectively, hypothyroidism, hypovitaminosis and hypo-hemoglobinemia (named anemia). Interestingly, anemia is often present in patients with CHF, and it is commonly caused by iron deficiency, although other micronutrients are also needed for hemoglobin synthesis
[4].
2. Malnutrition
Food intake can be evaluated through questionnaires or, more accurately, day–food diaries. Patients’ signs of protein energy malnutrition could be identified looking at a loss of muscle mass and strength within different settings. Indeed, a simple measurement of anthropometric variables, such as tricipital skin-fold thickness (index of fat mass) and arm muscle area (index of lean mass), and a hand grip test can be measured in outpatients. More sophisticated and demanding techniques, such as ultrasound and/or dual X-ray absorptiometry (DXA), can also be used in hospitalized patients. Additionally, specific blood markers such as albumin, hemoglobin, lymphocytes count, red cells, transferrin and retinol-binding proteins could be measured to confirm the presence of metabolic malnutrition
[5].
There are no single tests which optimally diagnose protein energy malnutrition. However, the scholars’ advice is that evaluating albumin in plasma is mandatory. Indeed, this test is cheap, available in any laboratory, and if the levels fall under 3.5 g/dL, malnutrition should be suspected and great attention should be paid to those patients. Albumin has a long half-life (about 3 weeks), thus it takes time to normalize its values. However, on the contrary, if found to be low, long-term malnutrition and any other cause of hypo-albuminemia should be carefully excluded (i.e., proteinuria, blood loss, liver insufficiency, acute inflammation, etc.). In any case, there is much research showing that hypoalbuminemia strongly correlates to mortality, independent of the illness
[6]. There is growing evidence to show that malnutrition, and thus hypoalbuminemia, should be treated primarily with essential amino acids (EAAs), since non-essential ones contribute to urea, but not albumin synthesis
[7][8].
To monitor the short-term adequacy of therapy in very demanding conditions, the normalization of proteins marked by shorter-term syntheses would be used. If these are not successfully influenced by nutritional therapy, it indicates that the therapy is inadequate to match the needs, so the prognosis could worsen.
If there are any signs of protein energy malnutrition, it would be also helpful to identify specific nutritional deficiencies by measuring blood vitamins and ions, which are the indispensable co-factors of the enzymes which govern anabolic pathways
[9][10].
The causes of protein energy malnutrition in patients with CHF, especially when elderly, is probably multifactorial. Reduced food intake due to loss of appetite, change in smell sensory function, impaired chewing due to pathologies and/or dental prostheses, social isolation, as well as intestinal malabsorption from altered mesenteric circulation and/or dysbiosis, could all be responsible for CHF-induced malnutrition
[11][12].
However, the signs of protein energy malnutrition are present not only in CHF, but also in many different chronic and acute diseases such as cancer, infectious and collagen vascular disorders. All these diseases have a common finding: the increase in catabolic molecules such as hormones, and pro-inflammatory cytokines such as TNFα and IL1-6
[13].
Nowadays, it is well known that both catabolic neurohormones and inflammatory cytokines play a significant role in the progression of heart failure, inducing severe metabolic perturbations, such as insulin resistance syndrome (IRS), responsible for muscular wasting, cachexia and global metabolic disorders
[14]. Indeed, IRS significantly influences the biochemistry of peripheral muscle, globular proteins and adipose tissue already compromising other causes of malnutrition, such as reduced food intake. The lack of anabolic stimulation due to IRS causes protein degradation and amino acid (AA) release firstly in the muscles, and then an alteration in globular circulating proteins. AAs are deaminated and their carbon skeleton is used: (a) in the liver, to produce glucose by gluconeogenesis and (b) in the whole body, to support the increased global energy and metabolic needs. This condition is also called hypermetabolic syndrome (HS) and causes protein disarrangements, which worsen clinical conditions of chronic diseases, including CHF
[1][15].
3. Exercise Training and Nutritional Supplementation
3.1. Exercise Training
It is well established that aerobic resistant exercise training stimulates anabolic stimuli, thus reducing muscular wasting, mortality and hospitalization, and improving metabolism in CHF patients.
It has been demonstrated that both light exercise and aerobic exercises, and/or overall fitness maintain telomere length. This is a very interesting phenomenon because telomere length controls the terminal regions of chromosomal DNA from progressive degradation, and ensures the integrity of linear chromosomes, correlating with cellular damage including senescence-induced damage
[16].
Other studies show that physical activity is strongly correlated with epigenetic changes. Indeed, exercise influences DNA methylation and consequently transcription or the repression of genes, which influence cell proliferation
[16]. In addition, metabolomic studies show that physical exercise modifies thousands of blood metabolites, which are in turn able to influence, global metabolism and inflammation
[16]. However, one question remains unresolved: what type of physical activity/exercise has the greatest health promoting effects? Clinical data show that physical activities have pharmacological-like effects, called “gymno-mimetics”, which is why aerobic exercise for CHF patients is recommended by the current European Society of Cardiology (ESC) guidelines (class I, level of evidence A)
[17][18], as well as regular excise and physical activities being inserted into the guidelines for the maintenance of general health by the World Health Organization
[19].
3.2. Nutritional Supplementation
As mentioned earlier, data suggest that CHF-induced hypercatabolic syndrome causes AA metabolism alterations and the protein disarrangement of both globular and muscular proteins. As a result, the exogenous supplementation of food preproteins and/or free AAs could be a valid therapeutical strategy to use with CHF patients. However, data also show that improvement of the metabolic and nutritional status of muscle-depleted CHF patients occurs only when adequate energy protein intake is combined with a specific mixture of all free forms of essential AAs (EAAs) in a stoichiometric ratio, and not with a simple increase in food protein intake
[20].
Firstly, food proteins must be digested by pancreatic enzymes and the resulting AAs would then be absorbed by the intestine and introduced into the bloodstream to be transported to cells. As recently shown in aged and/or diseased patients’ pancreas, exocrine efficiency and intestinal metabolism are progressively reduced with consequently altered digestion and the absorption of food components. On the contrary, free AAs do not need to be digested, but are rapidly absorbed and immediately available in the blood for protein syntheses
[21][22].
Secondly, from a nutritional point of view, AAs are classified as EAAs, which cannot be synthetized in the body and are therefore needed in the human diet, and non-essential (NEAAs) which can be produced in the body according to the metabolic need, so that their presence in the diet is not strictly necessary.
Indeed, EAAs and their metabolites have recently been defined as “Metabokine” because they are able to influence local metabolism and systemic physiology
[23], not only through direct metabolic influence, but also modifying the gene expression via epigenetic action
[24]. Indeed, it has been shown that monocarboxylic acid, branched-chain essential amino acid (BCEAA) derivatives, 3-methyl-2-oxovaleric acid (MOVA), B-hydroxy-isobutyric acid (BHIBA) and amino acid 5-oxoproline (5OP) regulate adipocyte and myocyte metabolic gene expression of the enzymes involved in fatty acid oxidation.
4. Molecular Hypothesis of Exercise Training and Nutritional Supplementation Alliance
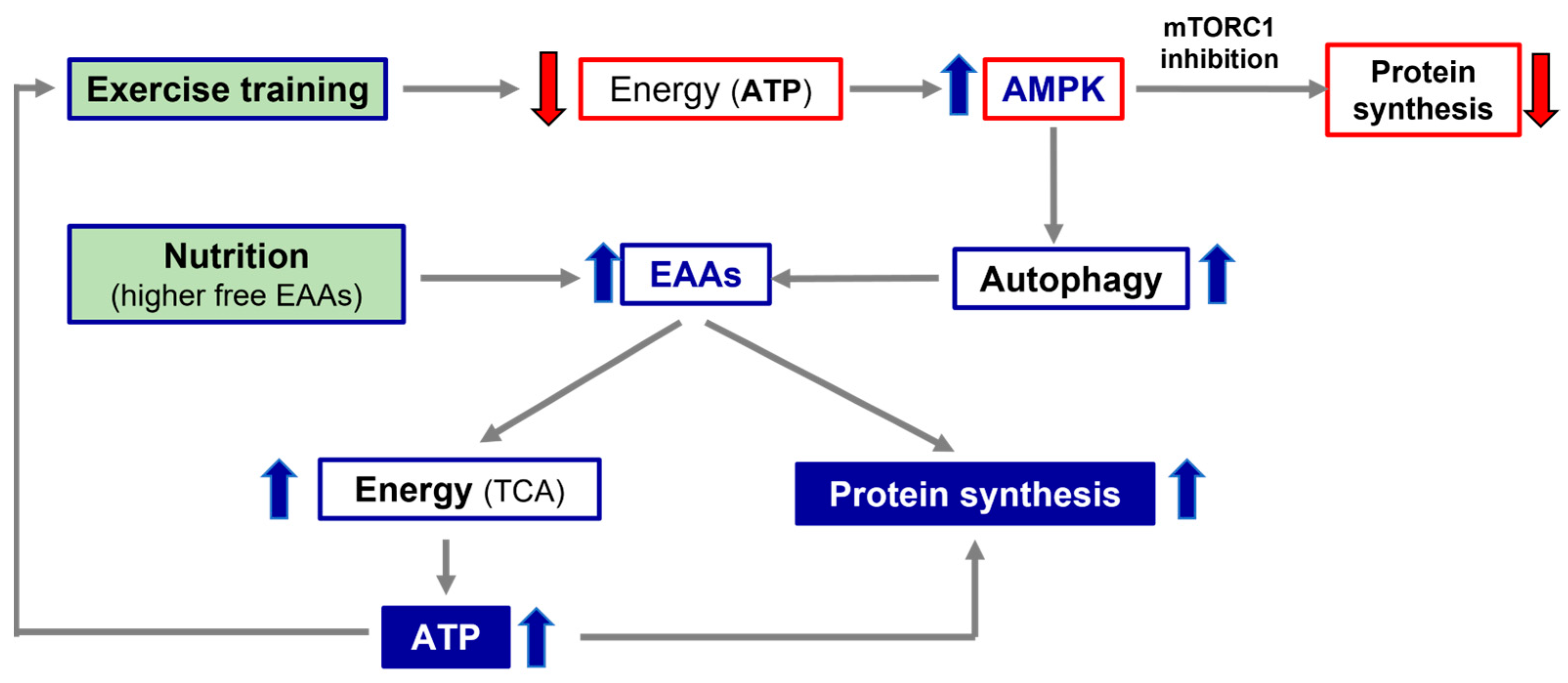
During exercise, cells consume energy-splicing adenosine tri-phosphate (ATP), which is split off from one of its three phosphates, becoming ADP (adenosine di-phosphate) and phosphates. Likewise, energy is also released when a phosphate is removed from ADP to form adenosine monophosphate (AMP). Interestingly, increasing AMP concentrations stimulates AMP-activated protein kinase (AMPK), an enzyme which acts as an energy sensor and activates a process called autophagy (A). A is characterized by the breakdown and recycling of the components of aged macromolecules, including proteins and lipids, promoting substrate availability for energy production
[25].
It is well known that physical exercise induces A. However, exercise-induced A is not totally negative because it allows for the energy production fundamental for the preservation of energy production and mitochondrial functions during muscular contraction. Therefore, it has been postulated that A induced by exercise is an adaptive response that avoids mitochondrial damage in turn, induced by physical activity due to energy deficiency caused by the reduced availability of energetic substrates during exercise
[26]. To modulate energy expenditure/production, AMPK also spares energy by blunting the multi-enzymatic system, mammalian or mechanistic target of rapamycin (mTOR)
[25].
mTOR is a multi-enzymatic complex which regulates many fundamental processes of cell life (i.e., protein synthesis, autophagy and others), integrating external (i.e., nutrients such as EAAs, growth factors and others) and internal stimuli (energy reduction). It is intuitive that the mTOR system is a central regulator of mammalian metabolism and physiology, with important roles in the regulation of cell functions such as energy production and protein synthesis, cell survival and duplication, integrating stimuli through complex molecular signaling, which is not yet fully understand.
mTOR is formed mainly of two distinct complexes, mTORC1 and TORC2. These two sub-units contain other regulatory proteins (Raptor, Protor PRAs40, Rictor), most of which act as the active repressors of mTORC activity. Both complexes may be inactivated by a subunit called DEP domain-containing mTOR-interacting protein, named Deptor
[27]. Indeed, an elevated cell concentration of AMPK inhibits mTORC1-dependent protein synthesis and activates A to make energetic molecules available. On the contrary, A is blocked when AMPK decreases and cellular ATP increases.
5. Conclusions
Nutritional supplementation with a mix of all free EAAs and co-factors of anabolic pathways (if found reduced and/or altered), concomitantly with physical exercise, could be allies in the treatment of CHF and reinforce the effects of standard medical therapy. According to the molecular and pathophysiological evidence, a road map to turn nutritional supplementation and physical activity into an effective alliance was proposed.
Firstly, the presence of protein energy malnutrition with signs of sarcopenia and globular proteins disarrangement (i.e., through anthropometric measurements and blood albumin quantification, etc.) in CHF patients should be looked for and evaluated.
Secondly, the alteration of the processes interfering with a patient’s optimal nutrition, such as intestinal dysbiosis or leaky gut syndrome, as well as possible deficiencies of the molecules involved in the conservation of anabolic pathways (i.e., vitamins, ions), should all be evaluated. If nutrition and/or metabolic molecules are altered and/or reduced, specific therapies should be initiated and continued until their functions and/or concentrations reach normal values. The pivotal role of the alliance of exercise and nutrition with higher free EAA availability, in promoting protein synthesis and energy production, is illustrated in Figure 1.
Figure 1. Simplistic representation of the importance of exercise and nutrition in promoting protein synthesis and energy production. TCA, tricarbossilic acid cycle; ATP, adenosine tri-phosphate; AMPK, AMP-activated protein kinase; EAAs, essential amino acids.