Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Inchingolo Riccardo | -- | 3209 | 2023-05-21 16:35:22 | | | |
| 2 | Jessie Wu | + 2 word(s) | 3211 | 2023-05-22 05:28:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rizzi, A.; Gammeri, L.; Cordiano, R.; Valentini, M.; Centrone, M.; Marrone, S.; Inchingolo, R.; Lohmeyer, F.M.; Cavaliere, C.; Ria, F.; et al. Molecules Used to Prevent Nasal Polyp Recurrences. Encyclopedia. Available online: https://encyclopedia.pub/entry/44610 (accessed on 07 February 2026).
Rizzi A, Gammeri L, Cordiano R, Valentini M, Centrone M, Marrone S, et al. Molecules Used to Prevent Nasal Polyp Recurrences. Encyclopedia. Available at: https://encyclopedia.pub/entry/44610. Accessed February 07, 2026.
Rizzi, Angela, Luca Gammeri, Raffaele Cordiano, Mariagrazia Valentini, Michele Centrone, Sabino Marrone, Riccardo Inchingolo, Franziska Michaela Lohmeyer, Carlo Cavaliere, Francesco Ria, et al. "Molecules Used to Prevent Nasal Polyp Recurrences" Encyclopedia, https://encyclopedia.pub/entry/44610 (accessed February 07, 2026).
Rizzi, A., Gammeri, L., Cordiano, R., Valentini, M., Centrone, M., Marrone, S., Inchingolo, R., Lohmeyer, F.M., Cavaliere, C., Ria, F., Cadoni, G., Gangemi, S., & Nucera, E. (2023, May 21). Molecules Used to Prevent Nasal Polyp Recurrences. In Encyclopedia. https://encyclopedia.pub/entry/44610
Rizzi, Angela, et al. "Molecules Used to Prevent Nasal Polyp Recurrences." Encyclopedia. Web. 21 May, 2023.
Copy Citation
Chronic rhinosinusitis with nasal polyps (CRSwNP) is the most bothersome phenotype of chronic rhinosinusitis, which is typically characterized by a Type 2 inflammatory reaction, comorbidities and high rates of nasal polyp recurrence, causing severe impact on quality of life. Nasal polyp recurrence rates, defined as the number of patients undergoing revision endoscopic sinus surgery, are 20% within a 5 year period after surgery. The cornerstone of CRSwNP management consists of anti-inflammatory treatment with local corticosteroids. The therapeutic strategies used to prevent nasal polyp recurrence (NPR) after surgical treatment are discussed.
chronic rhinosinusitis with nasal polyps
surgical polypectomy
intranasal corticosteroids
non-steroidal anti-inflammatory drugs
lysine-acetylsalicylate
diclofenac
ketoprofen
1. Intranasal Corticosteroids (INCs)
Intranasal corticosteroids (INCs) are the first-line therapeutical approach to CRSwNP. These molecules have an anti-congestion effect on the nasal mucosa and cause a reduction in polyp size, increasing nasal airflow [1]. The safety of INCs is well known. Many clinical trials have demonstrated the efficacy and safety profile of corticosteroids, such as mometasone furoate (MF), fluticasone furoate (FF), and fluticasone propionate (FP) [2]. INCs used in the postoperative phase allow good control of symptoms, and some authors described a reduction of polyp recurrence in the first year after functional endoscopic sinus surgery (FESS) [3]. In addition to anti-inflammatory and anti-edema effects, INCs could inhibit fibroblast proliferation (Figure 1).
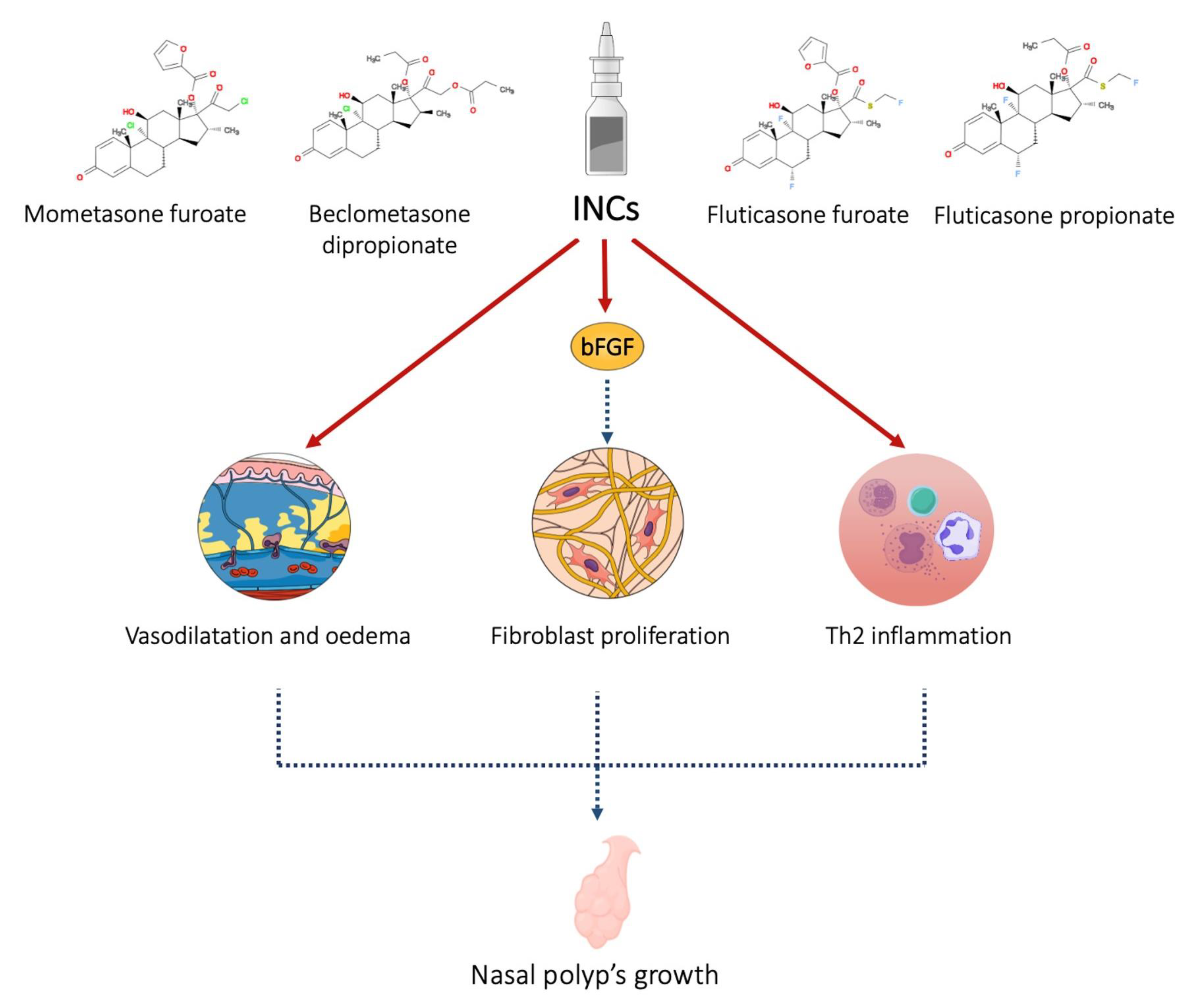
Figure 1. INCs act on the mucosa by reducing vasodilation and edema, inhibiting the production and release of pro-inflammatory cytokines. Furthermore, through the inhibition of basic fibroblast growth factor, they reduce the proliferation of fibroblasts and tissue remodeling.
Yariktas et al. demonstrated a reduction of basic fibroblast growth factor in the nasal mucosa of patients with nasal polyposis after INC treatment (mometasone furoate, 200 µg, once daily) [4]. Yu et al. demonstrated the role of glucocorticoids in inhibiting tissue remodeling in a clinical trial on 30 patients with CRSwNP after endoscopic surgery. The thickness of the basement membrane after three months of therapy was significantly reduced in the glucocorticoids group compared to the control group (p < 0.05) [5].
The first studies about INC use for polyp recurrence prevention were published in the 1980s. Virolainen and Puhakka demonstrated the efficacy of beclomethasone dipropionate in preventing the recurrence of nasal polyps. In their work, one year after surgery, polyps were absent in 54% of the patients treated with beclomethasone dipropionate. In the control group, polyps were absent in 13% of the patients [6]. These differences compared to the untreated subjects were observable after six months [7]. Treatment with flunisolide has been shown to reduce the risk of polyp recurrence in the first year after polypectomy [8][9]. Hartwig et al. demonstrated the safety and efficacy of budesonide (400 µg/daily) in preventing nasal polyps after sinus surgery [10]. A high dose of INC could be more effective as compared to a low dose of INC [11].
About that Kang et al. evaluated the recurrence of polyposis in 32 patients subjected to FESS. After 12 months, polyps recurred in 7.1% of patients treated with high-dose corticosteroids (triamcinolone acetonide–soaked gauze packing). In the group treated with low-dose corticosteroids, polyps recurred in 44% of patients [11].
However, in a prospective, double-blind, randomized study, triamcinolone acetonide had no significant efficacy compared to placebo, in patients with concomitant acetylsalicylic acid (ASA) intolerance [12].
Nevertheless, Seiberling and her group did not observe a substantial difference be-tween high-dose INC (dexamethasone) and low-dose INC such as FP [13].
Mometasone furoate has been shown to be effective in prolonging recurrence-free time [14]. In a randomized, double-blind, placebo-controlled, multicenter trial on 159 subjects, Stjärne et al. demonstrated that mometasone (200 µg, once daily) was superior to placebo.
Dijkstra et al. did not observe a reduction in the recurrence rate of nasal polyps in patients treated with fluticasone propionate after FESS. The recurrence rate of patients treated with intranasal FP after FESS was similar to the placebo group [15].
2. Acetylsalicylic Acid and Other Non-Steroidal Anti-Inflammatory Drugs
One of the main “alternative” pharmaceutical compounds used to reduce the recurrence of nasal polyps following surgical treatment is acetylsalicylic acid (ASA, aspirin). Since it is insoluble in liquids, a lysine molecule needs to be added to the acetylsalicylic acid to allow the use of lysine aspirin or lysine–acetylsalicylate (LAS) for inhalation or nasal challenge tests.
Lysine aspirin or lysine–acetylsalicylate can be used in patients with and without a history of aspirin sensitivity. Additionally, a condition known as the Samter triad, or aspirin illness, is defined as the coexistence of a nasal polyp, aspirin sensibility, and concurrent asthma. In aspirin-sensitive individuals, a repeated intake of ASA causes no adverse reaction during a “refractory period”. The presence of this period after a nasal challenge prompted researchers to study its topical effect on nasal polyp growth in ASA-sensitive patients [16].
From a pathophysiologic perspective, ASA affects inflammation by inhibiting cyclo-oxygenases (COX-1 and COX-2), which causes a shift in the metabolism of arachidonic acid toward the lipoxygenase pathway; this leads to an increase in leukotriene synthesis, and a decrease in prostaglandin E2. In ASA-sensitive patients, these traits potentially precipitate asthma attacks [17].
In an in vitro study, a non-specific, dose-dependent antiproliferative effect of aspirin on nasal polyps and normal skin fibroblast proliferation was revealed [18]. A report by Patriarca et al. [19] evaluated the effect of topical LAS (2000 µg weekly) on the recurrence of polyps after surgical therapy. In the 24 month study, 28 patients with ASA intolerance were enrolled (including 20 with ASA-triad), as well as 15 ASA-tolerant patients (control group). Compared to 81% of aspirin-sensitive controls, nasal polyp recurrences have been observed in 32% of treated patients. Additionally, in contrast to 67% of controls, no polyps recurred in the aspirin-tolerant patient group. Another work obtained similar results, although the results were less significant. A group of ASA-tolerant patients with nasal polyps (n = 20) was treated with topical LAS (2 mg weekly) unilaterally, using the other side of the nose as a control. The delayed recurrence of polyps was noted in all 18 patients, of which eight remained symptom-free after 15 months [20]. In further two long-term studies, Nucera et al. [21] assessed the effect of intranasal LAS in aspirin-sensitive and aspirin-tolerant patients with nasal polyps compared with controls. In the six-year follow-up study, 76 patients (38 with ASA sensitivity), who underwent surgical polypectomy, were treated with the equivalent of 4 mg of aspirin instilled six times a week. The recurrence rate of polyps compared with controls was 6.9% vs. 51.3% in the first year 1, 44.9% vs. 84.8% after three years, and 65% vs. 93.5% after six years. Moreover, no differences between the ASA-sensitive and ASA-tolerant patients were noted. In the second study 49 patients, who were previously treated with medical polypectomy, were enrolled, and they underwent topical applications of aspirin, as described above. At the three-year follow-up, the cumulative percentage of patients needing surgery was 32% for the LAS-treated group compared with 85% for the controls.
In contrast, a double-blinded, placebo-controlled, crossover trial [22] conducted in twenty ASA-sensitive patients did not show a significant clinical benefit on polyp growth or nasal inspiratory flow rate after topical treatment with the equivalent of 16 mg of aspirin every 48 h for six months.
Finally, in a research published by Ogata et al. [23], 13 patients with asthma and nasal polyps were included, all but one of whom previously underwent endoscopic polypectomy. LAS was applied topically for three months in doses equal to 37.8 mg of aspirin per day. When data were compared to measurements made at the start of the trial, it was found that nasal airflow, nasal nitric oxide levels, and polyp size all improved.
Other NSAIDs have been studied mainly in vitro. Ostwald et al. [24] used 12 molecules on fibroblasts from nasal polyp tissue at a concentration of 30 µg/mL. Corticosteroids achieved the strongest reduction in fibroblast growth, while NSAIDs, such as diclofenac and piroxicam, showed an intermediate-level effect. In another report [25], the selective COX-2 inhibitor, rofecoxib, at a concentration of 10,000 nM, strongly inhibited fibroblast proliferation, while nimesulide and ibuprofen showed no effect.
3. Furosemide
Other pharmacological molecules have been topically used to prevent polyp recurrence. Based on pathophysiological mechanisms, some clinical trials were performed using furosemide as an anti-edematous molecule. One of the key elements in polyp development is the edematous infiltrate [26], which grows due to inflammatory cells and their related cytokine (TNF, IL-1) and chemokine actions. These signaling molecules result in eosinophil persistence in the lamina propria of the nasal polyp, where its primary effectors, the major basic proteins, alter the ionic flux of the respiratory cell’s surface. In particular, the sodium net flux increases with a consequently augmented water absorption in the cells, resulting in edema formation [27].
The rationale for using topical furosemide in polyp recurrence is primarily based on the inhibition of a sodium–potassium–chloride symporter isoform (NKCC2) present on the basolateral membrane of nasal polyp epithelial cells [28][29]. This decreases sodium, chloride, and water absorption that leaves the interstice and reaches the surface of the mucosa.
In two different works, Passali et al. analyzed the effects of furosemide in preventing post-surgical relapses of nasal polyps. In an initial randomized clinical trial [30], 64 patients out of a cohort of 104 patients were treated with topical administrations of furosemide after surgical treatment. After a six-month endoscopic control, no relapse was reported in patients treated with furosemide (4 cases vs. 12 cases). In another work [27], the efficacy in preventing polyp formation of topical furosemide was compared with a topical corticosteroid (MF) and placebo. One hundred seventy patients were enrolled and divided into three groups (97 patients treated with furosemide, 33 treated with mometasone, and 40 treated with placebo). The results showed a comparative efficacy of furosemide and MF, with similar rates of polyp recurrence in the two groups (17.5% vs. 24.2%) compared with placebo (30%). In addition, regarding the severity of relapses, Stage III polyps were found in 11.8% of patients treated with furosemide and in 12.5% of patients treated with MF, compared with 66.7% of patients in the control group. In conclusion, in this study, furosemide was as effective as MF in preventing relapse. Moreover, patients who received no treatment experienced more severe degrees of relapse.
In a more recent, triple-blind clinical trial, Hashemian et al. [31] evaluated the severity of polyps, based on computed tomographic (CT) scans, using paranasal sinuses’ scoring methods, the Sino Nasal Outcome Test (SNOT-22), visual analog scale (VAS) and endoscopic grading using the Meltzer score, in 84 patients with CRSwNP who did not respond to medical treatment and were candidates for surgery. A substantial reduction in polyp grading was observed in the furosemide group based on endoscopic grading, SNOT-2, and VAS score [31].
4. Antibiotics and Antifungal Drugs
In the research, evidence about the use of antibiotics or antifungal molecules to prevent nasal polyp relapse can be found. The first studies on antibiotic use evaluated the efficacy of intranasal clarithromycin administration. In vitro studies demonstrated that this macrolide inhibits the production of pro-inflammatory cytokines, such as IL-5, IL-8, and GM-CSF [32].
The anti-inflammatory effects of macrolides could be due to their ability to inhibit the activation of the transcriptional factor NF-κB [33].
Piskunov and Bobacheva randomized 37 patients to two therapeutic strategies after endoscopic polyposinusotomy. The first group was treated with 250 mg of intranasal clarithromycin and INC for three months; the second group received INC for three months. The authors observed that clarithromycin at low doses caused significant stabilization of chronic polypoid rhinosinusitis remission and prevented the development of relapses 66% of patients. This study demonstrated the efficacy of the combined therapy compared to the INC alone and the safety of intranasal clarithromycin [34].
Subsequently, other works confirmed the usefulness of macrolides in preventing the early recurrence of nasal polyps after FESS. In patients with CRSwNP and surgery failure, low-dose clarithromycin treatment (250 mg once daily) for 3–6 months could prevent relapse of nasal polyps [35][36].
Furthermore, studies about the use of mitomycin C were conducted. Mitomycin C is an antineoplastic used in ophthalmologic and sinus surgery to prevent synechiae formation [37].
This antibiotic inhibits GM-CSF synthesis with a reduction of IL-5 secretion [38], and increases eosinophil apoptosis [39].
De Castro et al. studied the nasal mucosa of patients treated with topic mitomycin C after sinus surgery, evaluating the gene expression of IL-4, IL-5, IL-10, IL-13, chemokine (C-C motif) ligand 5 (CCL5), CCL24, colony-stimulating factor 2 (CSF2), transforming growth factor beta 1 (TGFB1), tumor necrosis factor-alpha (TNF-alpha), and beta-actin by quantitative PCR. They found that topical application of this drug induced a down-regulation of IL-5, CCL, CCL24, TNF-alpha, and CSF2. Therefore, De Castro et al. concluded that mitomycin C promotes the restoration of the normal microenvironment of the nasal mucosa; this could have applications in the prevention of recurrent eosinophilic nasal polyposis [40].
Some authors have also considered using antifungals to treat symptomatic CRS to prevent relapse of nasal polyps. In fact, in the late 1990s, evidence about the presence of mycotic microorganisms in a large percentage of subjects with CRS was found. Therefore, some authors started a series of studies about intranasal amphotericin B. Nevertheless, Lupa and Amedee reviewed studies in the literature and demonstrated the lack of utility of amphotericin B to treat patients with CRS [41].
In a prospective randomized placebo-controlled trial involving 33 patients, Gerlinger et al. [42] evaluated the effects of amphotericin B to prevent nasal polyp recurrence. Results have shown that administering intranasal amphotericin B after endoscopic surgery does not produce benefits compared to placebo [42].
5. Other Therapeutical Strategies
Many other molecules have been studied for the post-surgical treatment of nasal polyposis and the prevention of recurrences. Among these, antileukotrienes have been proposed for the post-surgical treatment of aspirin-exacerbated respiratory disease (AERD) patients. These patients’ mucosal tissue overexpresses cysteinyl leukotrienes (CysLTs) receptor and leukotriene C4 synthase (LTC4S) [43].
Dysregulation of LTC4 leads to an alteration in the arachidonic acid metabolisms and the overproduction of CysLTs. CysLTs have a role in eosinophilic inflammation, exerting pro-inflammatory and pro-fibrotic effects [44]. The use of antileukotrienes after endoscopic surgery showed similar results to INC therapy [45]. From a literature review published in June 2010 emerged a class IB recommendation for the use of antileukotrienes in patients undergoing sinus surgery [44].
Vuralkan et al. [45] compared montelukast 10 mg per day and a nasal spray of 400 mg MF, administered twice daily. Fifty patients were randomized into two groups after endoscopic sinus surgery and treated for six months. Montelukast and INC demonstrated similar results in symptom control, but MF showed a slightly lower recurrence rate [45].
The mechanisms of action of the main molecules used in the prevention of post-surgical recurrence of nasal polyposis are shown in Figure 2.
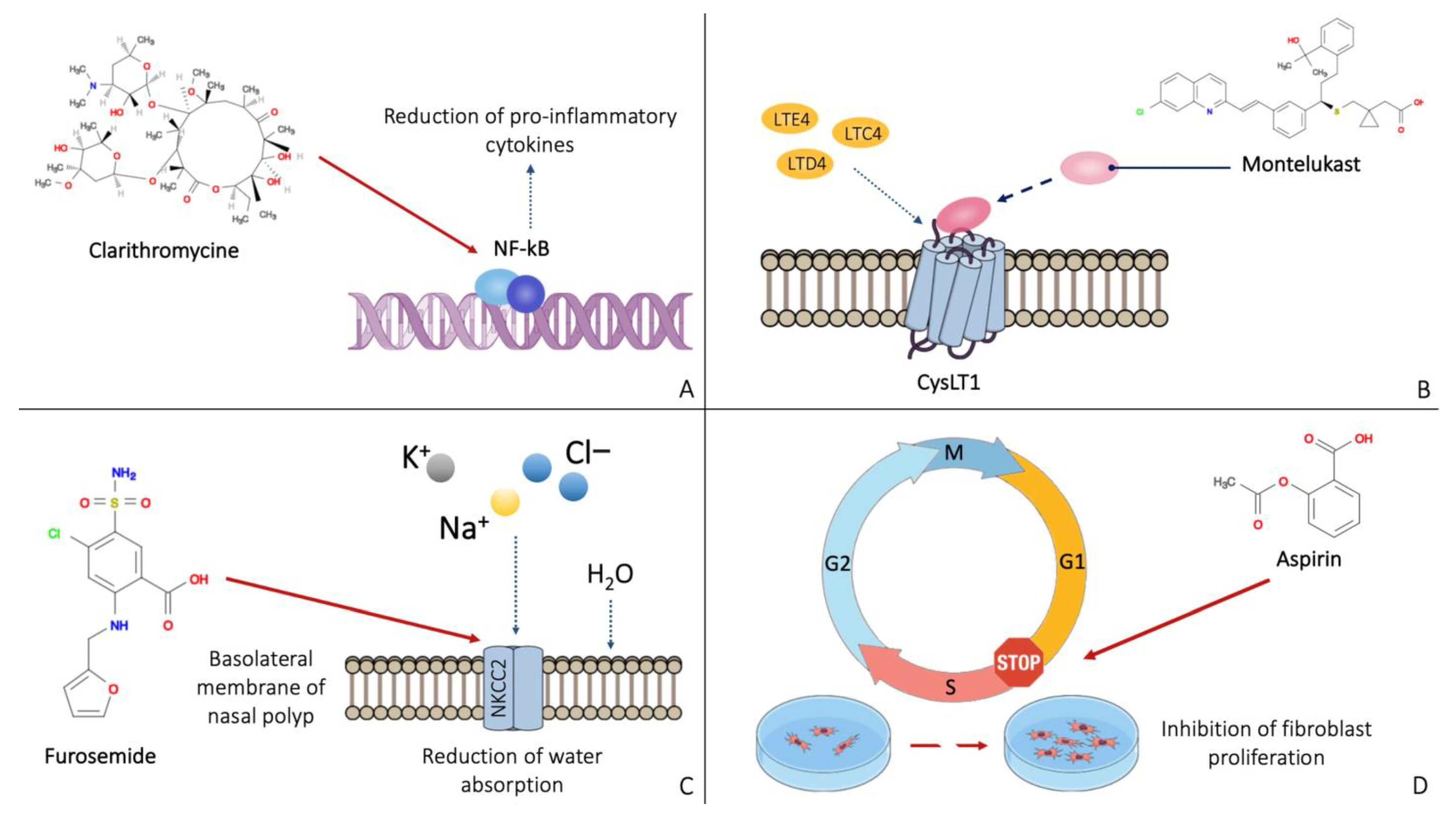
Figure 2. Mechanisms of action of the main molecules active on the nasal polyp: clarithromycin has an anti-inflammatory effect by inhibiting NF-kB (A). Montelukast binds the leukotriene receptor CysLT1, preventing its activation (B). Furosemide inhibits the NKCC2 receptor on the basolateral membrane of nasal polyp cells (C). Aspirin appears to block cells in the G1 phase, preventing the proliferation of fibroblasts (D).
Some authors evaluated the potential use of capsaicin. Sensory C-fibers in the nasal mucosa contain calcitonin gene-related peptide; this peptide has a vasodilator effect and may play a role in the vascular component of inflammation [46].
In nasal mucosa, increased levels of calcitonin gene-related peptide positively correlate with inflammatory cell infiltrate and symptom severity [47].
Zheng et al. [48] evaluated the efficacy of intranasal capsaicin application in preventing nasal polyp recurrence after sinus surgery. Patients treated with five intranasal capsaicin applications showed a significant reduction in the rate of polyp recurrences; capsaicin also demonstrated a good safety profile [48].
Isotonic and hypertonic solutions could play an important role. Saline douching in the post-surgery period could have an anti-inflammatory effect, reducing nasal discharge and edema [49]. The hypertonic solution was superior to the isotonic solution in symptom control [50].
Portenko and Dobrynin [51] studied the potential application of transcranial electrostimulation to reduce polyp recurrence in a small cohort of 23 patients with CRSwNP. They obtained a reduction in early recurrence (4.3%) in a follow-up period of 1.5–3 years [51].
Finally, an approach to the disease with herbal medicine could be considered. The anti-inflammatory effects of many herbal preparations are well known; there is various evidence on the use of decoctions to treat inflammatory skin diseases, such as urticaria and respiratory diseases (asthma, rhinitis) [52][53].
In literature, a case report about a patient with recurrent nasal polyposis treated with an herbal decoction and acupuncture is reported; this patient did not show polyp recurrence during three and half years of treatment [54].
Chang et al. used licorice (glycyrrhiza glabra) extract to treat CRSwNP through nasal irrigation and obtained a reduction of polyp size. In addition, through inhibition of the MAPK/ERK-1/2 signaling pathway, glycyrrhiza glabra could attenuate fibroblast differentiation, extracellular matrix production, and cell migration [55].
Nevertheless, studies on the use of herbal medicine to prevent nasal polyp recurrence require further insights.
6. Biological Therapies
According to the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA), patients with severe CRSwNP and comorbid asthma or those whose blood eosinophil count is higher than 300 cells/mL are more likely to exhibit Type 2 inflammation and may, therefore, benefit from Type 2 biologic therapies, such as anti–IL-4/receptor alpha and anti–IL-5/receptor alpha molecules [56].
Dupilumab is a humanized monoclonal antibody targeting the IL-4 receptor alpha chain (IL-4Rα). The alpha chain is a subunit of the IL-4R complex, a heterodimer that mediates the action of Il-4 and IL-13. The axis between IL-4/IL-13 and IL-4R promotes Th2 inflammation [57].
Dupilumab is the first monoclonal antibody to be approved in the US and Europe for the treatment of not-well-controlled CRSwNP [58].
Two phase III multicenter, randomized, double-blind, placebo-controlled trials, LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52, demonstrated the efficacy of this treatment in adult patients with severe CRSwNP. Dupilumab, in addition to standard daily therapy, was well tolerated and led to a reduction of polyp size and severity of symptoms [59]. The efficacy and safety were evaluated and also demonstrated in real-world studies [60].
A humanized monoclonal antibody called mepolizumab binds to and deactivates IL-5, the main cytokine in charge of eosinophil proliferation, activation, and survival [61]. Mepolizumab has received approval for the treatment of various eosinophilic disorders in numerous countries across the world. These include CRSwNP, eosinophilic granulomatosis with polyangiitis, hyper eosinophilic syndrome, and severe eosinophilic asthma [62]. Mepolizumab reduced nasal polyp size, improved nasal obstruction symptoms, decreased the need for actual nasal surgery, decreased the use of systemic corticosteroids, improved sinonasal symptoms in patients with severe CRSwNP, and had an acceptable safety profile, based on the phase III SYNAPSE trial [63]. Furthermore, the same study also demonstrated its effectiveness in treating nasal obstruction among patients with high basal eosinophil counts [63]. A recent evaluation of SYNAPSE patients (grouped by comorbid asthma, AERD, and baseline blood eosinophil count) showed that patients with baseline blood eosinophils count >150 cells/mL versus <150 cells/mL, and those with ≥300 cells/mL versus <300 cells/mL tended to have better therapeutic benefits; this confirms that eosinophils are an appropriate and effective target in severe CRSwNP [64].
A recent multicentric real-life study on severe asthmatic patients treated with mepolizumab, with comorbid CRSwNP, showed an improvement of CRSwNP outcomes (SNOT-22 score, nasal polyp score and blood eosinophil count) in an Italian cohort of forty-three patients [65].
Finally, biological therapy for nasal polyps can also act on IgE. IgE may play a role in the inflammatory microenvironment of the nasal polyp, which is confirmed by the elevated polyclonal local IgE production in some patients with nasal polyposis [66].
Omalizumab is a humanized anti-IgE antibody that binds to the IgE and prevents IgE binding to the high-affinity receptors (FcεRI) [67]. This monoclonal antibody was found to be effective and safe in the treatment of nasal polyposis [68].
In two Phase III trials, POLYP-1 and POLYP-2, omalizumab was superior to placebo in patients with or without previous FESS. In addition, efficacy was demonstrated regardless of eosinophil levels, as well as the concomitant presence of asthma or aspirin sensitivity [69].
References
- Kwah, J.H.; Peters, A.T. Nasal Polyps and Rhinosinusitis. Allergy Asthma Proc. 2019, 40, 380–384.
- Sastre, J.; Mosges, R. Local and Systemic Safety of Intranasal Corticosteroids. J. Investig. Allergol. Clin. Immunol. 2012, 22, 1–12.
- Fandiño, M.; Macdonald, K.I.; Lee, J.; Witterick, I.J. The Use of Postoperative Topical Corticosteroids in Chronic Rhinosinusitis with Nasal Polyps: A Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2013, 27, e146–e157.
- The Effect of Topical Corticosteroid on Basic Fibroblast Growth Factor in Nasal Polyp Tissue-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16011129/ (accessed on 15 February 2023).
- Yu, R.; Zhu, D.; Dong, Z. Effects of glucocorticoid on tissue remodeling of nasal mucosa of chronic rhinosinusitis with nasal polyposis after endoscopic surgery. Chin. J. Otorhinolaryngol. Head Neck Surg. 2006, 41, 773–776.
- The Effect of Intranasal Beclomethasone Dipropionate on the Recurrence of Nasal Polyps after Ethmoidectomy-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/6988929/ (accessed on 15 February 2023).
- Karlsson, G.; Rundcrantz, H. A Randomized Trial of Intranasal Beclomethasone Dipropionate after Polypectomy. Rhinology 1982, 20, 144–148.
- Drettner, B.; Ebbesen, A.; Nilsson, M. Prophylactive Treatment with Flunisolide after Polypectomy. Rhinology 1982, 20, 149–158.
- Flunisolide Nasal Spray 0.025% in the Prophylactic Treatment of Nasal Polyposis after Polypectomy. A Randomized, Double Blind, Parallel, Placebo Controlled Study-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/4001759/ (accessed on 15 February 2023).
- Budesonide Nasal Spray as Prophylactic Treatment after Polypectomy (a Double Blind Clinical Trial)-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/3279143/ (accessed on 15 February 2023).
- Kang, I.G.; Yoon, B.K.; Jung, J.H.; Cha, H.E.; Kim, S.T. The Effect of High-Dose Topical Corticosteroid Therapy on Prevention of Recurrent Nasal Polyps after Revision Endoscopic Sinus Surgery. Am. J. Rhinol. 2008, 22, 497–501.
- Vento, S.I.; Blomgren, K.; Hytönen, M.; Simola, M.; Malmberg, H. Prevention of Relapses of Nasal Polyposis with Intranasal Triamcinolone Acetonide after Polyp Surgery: A Prospective Double-Blind, Placebo-Controlled, Randomised Study with a 9-Month Follow-Up. Clin. Otolaryngol. 2012, 37, 117–123.
- Seiberling, K.A.; Kidd, S.C.; Kim, G.H.; Church, C.A. Efficacy of Dexamethasone Versus Fluticasone Nasal Sprays in Postoperative Patients With Chronic Rhinosinusitis With Nasal Polyps. Am. J. Rhinol. Allergy 2019, 33, 478–482.
- Stjärne, P.; Olsson, P.; Alenius, M. Use of Mometasone Furoate to Prevent Polyp Relapse after Endoscopic Sinus Surgery. Arch. Otolaryngol. Head. Neck Surg. 2009, 135, 296–302.
- Dijkstra, M.D.; Ebbens, F.A.; Poublon, R.M.L.; Fokkens, W.J. Fluticasone Propionate Aqueous Nasal Spray Does Not Influence the Recurrence Rate of Chronic Rhinosinusitis and Nasal Polyps 1 Year after Functional Endoscopic Sinus Surgery. Clin. Exp. Allergy 2004, 34, 1395–1400.
- Patriarca, G.; Nucera, E.; DiRienzo, V.; Schiavino, D.; Pellegrino, S.; Fais, G. Nasal Provocation Test with Lysine Acetylsalicylate in Aspirin-Sensitive Patients. Ann. Allergy 1991, 67, 60–62.
- Szczeklik, A.; Gryglewski, R.J.; Czerniawska-Mysik, G. Relationship of Inhibition of Prostaglandin Biosynthesis by Analgesics to Asthma Attacks in Aspirin-Sensitive Patients. Br. Med. J. 1975, 1, 67–69.
- Bruzzese, N.; Sica, G.; Iacopino, F.; Paludetti, G.; Schiavino, D.; Nucera, E.; Scarano, E.; Patriarca, G. Growth Inhibition of Fibroblasts from Nasal Polyps and Normal Skin by Lysine Acetylsalicylate. Allergy 1998, 53, 431–434.
- Patriarca, G.; Bellioni, P.; Nucera, E.; Schiavino, D.; Papa, G.; Schinco, G.; Fais, G.; Pirotta, L.R. Intranasal Treatment with Lysine Acetylsalicylate in Patients with Nasal Polyposis. Ann. Allergy 1991, 67, 588–592.
- Scadding, G.K.; Hassab, M.; Darby, Y.C.; Lund, V.J.; Freedman, A. Intranasal Lysine Aspirin in Recurrent Nasal Polyposis. Clin. Otolaryngol. Allied Sci. 1995, 20, 561–563.
- Nucera, E.; Schiavino, D.; Milani, A.; Del Ninno, M.; Misuraca, C.; Buonomo, A.; D’Ambrosio, C.; Paludetti, G.; Patriarca, G. Effects of Lysine-Acetylsalicylate (LAS) Treatment in Nasal Polyposis: Two Controlled Long Term Prospective Follow up Studies. Thorax 2000, 55 (Suppl. S2), S75–S78.
- Parikh, A.A.; Scadding, G.K. Intranasal Lysine-Aspirin in Aspirin-Sensitive Nasal Polyposis: A Controlled Trial. Laryngoscope 2005, 115, 1385–1390.
- Ogata, N.; Darby, Y.; Scadding, G. Intranasal Lysine-Aspirin Administration Decreases Polyp Volume in Patients with Aspirin-Intolerant Asthma. J. Laryngol. Otol. 2007, 121, 1156–1160.
- Ostwald, J.; Graumüller, S.; Dommerich, S.; Hoff, M. Influence of rhinologic usual and unusual drugs on fibroblasts from nasal polyps in cell culture. Laryngorhinootologie 2003, 82, 408–415.
- Beata, R.-N.; Wojciech, F.; Stanisław, S.; Ryszard, M. The Influence of Anti-Inflammatory Drugs on the Proliferation of Fibroblast Derived from Nasal Polyps. Auris Nasus Larynx 2005, 32, 225–229.
- Bernstein, J.M. The Molecular Biology of Nasal Polyposis. Curr. Allergy Asthma Rep. 2001, 1, 262–267.
- Passàli, D.; Bernstein, J.M.; Passali, F.M.; Damiani, V.; Passàli, G.C.; Bellussi, L. Treatment of Recurrent Chronic Hyperplastic Sinusitis with Nasal Polyposis. Arch. Otolaryngol. Head. Neck Surg. 2003, 129, 656–659.
- Liedtke, C.M.; Cole, T.S. PKC Signaling in CF/T43 Cell Line: Regulation of NKCC1 by PKC-Delta Isotype. Biochim. Biophys. Acta 2000, 1495, 24–33.
- Das, S. Topical Furosemide Possibly Beneficial for Reducing Recurrence Rates After Nasal Polyp Surgery. JAMA Otolaryngol. Head. Neck Surg. 2016, 142, 1049–1050.
- Passàli, D.; Mezzedimi, C.; Passàli, G.C.; Bellussi, L. Efficacy of Inhalation Form of Furosemide to Prevent Postsurgical Relapses of Rhinosinusal Polyposis. ORL J. Otorhinolaryngol. Relat. Spec. 2000, 62, 307–310.
- Hashemian, F.; Ghorbanian, M.A.; Hashemian, F.; Mortazavi, S.A.; Sheikhi, M.; Jahanshahi, J.; Poorolajal, J. Effect of Topical Furosemide on Rhinosinusal Polyposis Relapse After Endoscopic Sinus Surgery: A Randomized Clinical Trial. JAMA Otolaryngol. Head. Neck Surg. 2016, 142, 1045–1049.
- Wallwork, B.; Coman, W.; Feron, F.; Mackay-Sim, A.; Cervin, A. Clarithromycin and Prednisolone Inhibit Cytokine Production in Chronic Rhinosinusitis. Laryngoscope 2002, 112, 1827–1830.
- Cervin, A.; Wallwork, B. Macrolide Therapy of Chronic Rhinosinusitis. Rhinology 2007, 45, 259–267.
- Piskunov, G.Z.; Bobacheva, T.I. Experience with treatment of chronic polypoid rhinosinusitis using low doses of clarithromycin in the postoperative period. Vestn. Otorinolaringol. 2012, 1, 47–51.
- Varvyanskaya, A.; Lopatin, A. Efficacy of Long-Term Low-Dose Macrolide Therapy in Preventing Early Recurrence of Nasal Polyps after Endoscopic Sinus Surgery. Int. Forum Allergy Rhinol. 2014, 4, 533–541.
- Varvianskaia, A.V.; Lopatin, A.S. The effectiveness of long-term treatment of polypous rhinosinusitis with low doses of macrolides. Vestn. Otorinolaringol. 2013, 5, 22–27.
- Chung, J.H.; Cosenza, M.J.; Rahbar, R.; Metson, R.B. Mitomycin C for the Prevention of Adhesion Formation after Endoscopic Sinus Surgery: A Randomized, Controlled Study. Otolaryngol. Head. Neck Surg. 2002, 126, 468–474.
- Crosara, P.F.T.B.; Vasconcelos, A.C.; Guimarães, R.E.S.; Becker, H.M.G.; Becker, C.G.; Crosara, S.L.R.; Nascimento, E. Effect of Mitomycin C on the Secretion of Granulocyte Macrophages Colonies Stimulating Factor and Interleukin-5 in Eosinophilic Nasal Polyps Stromal Culture. Braz. J. Otorhinolaryngol. 2005, 71, 459–463.
- Anjos, C.P.G. dos; Vasconcelos, A.C.; Crosara, P.F.T.B.; Anjos, G.C. dos; Becker, C.G.; Guimarães, R.E.S. Apoptosis in Eosinophilic Nasal Polyps Treated in Vitro with Mitomycin C. Braz. J. Otorhinolaryngol. 2012, 78, 32–37.
- de Castro, M.C.M.; Rocha-Silva, F.; Gomes, L.I.; Zauli, D.A.G.; de Moraes Mourão, M.; de Castro, M.M.; Guimarães, R.E.S.; Teixeira-Carvalho, A.; Martins-Filho, O.A. Impact of Mitomycin C on the MRNA Expression Signatures of Immunological Biomarkers in Eosinophilic Nasal Polyposis. Am. J. Rhinol. Allergy 2013, 27, 32–41.
- Lupa, M.; Amedee, R. Is Topical Amphotericin B Efficacious in the Treatment of Chronic Rhinosinusitis? Laryngoscope 2010, 120, 1080–1081.
- Gerlinger, I.; Fittler, A.; Mayer, A.; Patzkó, A.; Fónay, F.; Pytel, J.; Botz, L. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis. Can recidive polyposis be prevented? Orv. Hetil. 2008, 149, 1737–1746.
- Rodríguez-Jiménez, J.C.; Moreno-Paz, F.J.; Terán, L.M.; Guaní-Guerra, E. Aspirin Exacerbated Respiratory Disease: Current Topics and Trends. Respir. Med. 2018, 135, 62–75.
- Rasp, G. Is There a Role for Leukotriene Antagonists in the Prevention of Recurrent Nasal Polyps? Curr. Opin. Allergy Clin. Immunol. 2010, 10, 200–205.
- Vuralkan, E.; Saka, C.; Akin, I.; Hucumenoglu, S.; Unal, B.U.; Kuran, G.; Ocal, B. Comparison of Montelukast and Mometasone Furoate in the Prevention of Recurrent Nasal Polyps. Ther. Adv. Respir. Dis. 2012, 6, 5–10.
- Lacroix, J.S.; Buvelot, J.M.; Polla, B.S.; Lundberg, J.M. Improvement of Symptoms of Non-Allergic Chronic Rhinitis by Local Treatment with Capsaicin. Clin. Exp. Allergy 1991, 21, 595–600.
- Lacroix, J.S.; Kurt, A.M.; Pochon, N.; Bretton, C.; Lundberg, J.M.; Deshusses, J. Neutral Endopeptidase Activity and Concentration of Sensory Neuropeptide in the Human Nasal Mucosa. Eur. Arch. Otorhinolaryngol. 1995, 252, 465–468.
- Zheng, C.; Wang, Z.; Lacroix, J.S. Effect of Intranasal Treatment with Capsaicin on the Recurrence of Polyps after Polypectomy and Ethmoidectomy. Acta Otolaryngol. 2000, 120, 62–66.
- Freeman, S.R.M.; Sivayoham, E.S.G.; Jepson, K.; de Carpentier, J. A Preliminary Randomised Controlled Trial Evaluating the Efficacy of Saline Douching Following Endoscopic Sinus Surgery. Clin. Otolaryngol. 2008, 33, 462–465.
- Perić, A.; Kovačević, S.V.; Barać, A.; Gaćeša, D.; Perić, A.V.; Jožin, S.M. Efficacy of Hypertonic (2.3%) Sea Water in Patients with Aspirin-Induced Chronic Rhinosinusitis Following Endoscopic Sinus Surgery. Acta Otolaryngol. 2019, 139, 529–535.
- Portenko, G.M.; Dobrynin, K.V. Anti-recurrence treatment of patients with polyposis rhinosinusitis in initial vagotonic type of the vegetative tonus. Vestn. Otorinolaringol. 2001, 5, 36–39.
- Gammeri, L.; Panzera, C.; Calapai, F.; Cicero, N.; Gangemi, S. Asian Herbal Medicine and Chronic Urticaria: Which Are the Therapeutic Perspectives? Nat. Prod. Res. 2022, 12, 1–18.
- Guo, R.; Pittler, M.H.; Ernst, E. Herbal Medicines for the Treatment of Allergic Rhinitis: A Systematic Review. Ann. Allergy Asthma Immunol. 2007, 99, 483–495.
- Miao, E.Y. Recurrent Nasal Polyps Treated by Chinese Herbal Decoction and Acupuncture: A Case Report. J. Altern. Complement. Med. 2010, 16, 691–695.
- Chang, G.-H.; Yang, P.-R.; Cheng, Y.-C.; Hsu, K.-H.; Wu, C.-Y.; Yang, Y.-H.; Lin, Y.-S.; Hsu, C.-M.; Tsai, M.-S.; Tsai, Y.-T.; et al. Nasal Irrigation with Licorice Extract (Glycyrrhiza Glabra) in Treating Nasal Polyps by Reducing Fibroblast Differentiation and Extracellular Matrix Production in TGF-Β1-Stimulated Nasal Polyp-Derived Fibroblasts by Inhibiting the MAPK/ERK-1/2 Pathway-An in Vitro and in Clinic Study. BMC Complement. Med. Ther. 2022, 22, 313.
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and Safety of Omalizumab in Nasal Polyposis: 2 Randomized Phase 3 Trials. J. Allergy Clin. Immunol. 2020, 146, 595–605.
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2020, 50, 5–14.
- Hoy, S.M. Dupilumab: A Review in Chronic Rhinosinusitis with Nasal Polyps. Drugs 2020, 80, 711–717.
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and Safety of Dupilumab in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from Two Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Phase 3 Trials. Lancet 2019, 394, 1638–1650.
- Haxel, B.R.; Hummel, T.; Fruth, K.; Lorenz, K.; Gunder, N.; Nahrath, P.; Cuevas, M. Real-World-Effectiveness of Biological Treatment for Severe Chronic Rhinosinusitis with Nasal Polyps. Rhinology 2022, 60, 435–443.
- Mepolizumab in the Management of Severe Eosinophilic Asthma in Adults: Current Evidence and Practical Experience-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30354852/ (accessed on 15 February 2023).
- Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_it.pdf (accessed on 10 April 2023).
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for Chronic Rhinosinusitis with Nasal Polyps (SYNAPSE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2021, 9, 1141–1153.
- Bachert, C.; Sousa, A.R.; Han, J.K.; Schlosser, R.J.; Sowerby, L.J.; Hopkins, C.; Maspero, J.F.; Smith, S.G.; Kante, O.; Karidi-Andrioti, D.E.; et al. Mepolizumab for Chronic Rhinosinusitis with Nasal Polyps: Treatment Efficacy by Comorbidity and Blood Eosinophil Count. J. Allergy Clin. Immunol. 2022, 149, 1711–1721.e6.
- Gallo, S.; Castelnuovo, P.; Spirito, L.; Feduzi, M.; Seccia, V.; Visca, D.; Spanevello, A.; Statuti, E.; Latorre, M.; Montuori, C.; et al. Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study. J. Pers. Med. 2022, 12, 1304.
- Bachert, C.; Maurer, M.; Palomares, O.; Busse, W.W. What Is the Contribution of IgE to Nasal Polyposis? J. Allergy Clin. Immunol. 2021, 147, 1997–2008.
- Eschenbacher, W.; Straesser, M.; Knoeddler, A.; Li, R.-C.; Borish, L. Biologics for the Treatment of Allergic Rhinitis, Chronic Rhinosinusitis, and Nasal Polyposis. Immunol. Allergy Clin. North. Am. 2020, 40, 539–547.
- Bakakos, A.; Schleich, F.; Bakakos, P. Biological Therapy of Severe Asthma and Nasal Polyps. J. Pers. Med. 2022, 12, 976.
- Damask, C.; Chen, M.; Holweg, C.T.J.; Yoo, B.; Millette, L.A.; Franzese, C. Defining the Efficacy of Omalizumab in Nasal Polyposis: A POLYP 1 and POLYP 2 Subgroup Analysis. Am. J. Rhinol. Allergy 2022, 36, 135–141.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
937
Revisions:
2 times
(View History)
Update Date:
22 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




