Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krystyna Pyrzynska | -- | 3837 | 2023-05-19 20:48:21 | | | |
| 2 | Rita Xu | Meta information modification | 3837 | 2023-05-22 03:31:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pyrzynska, K. Carbon Nanotubes for Lead Ions. Encyclopedia. Available online: https://encyclopedia.pub/entry/44583 (accessed on 01 March 2026).
Pyrzynska K. Carbon Nanotubes for Lead Ions. Encyclopedia. Available at: https://encyclopedia.pub/entry/44583. Accessed March 01, 2026.
Pyrzynska, Krystyna. "Carbon Nanotubes for Lead Ions" Encyclopedia, https://encyclopedia.pub/entry/44583 (accessed March 01, 2026).
Pyrzynska, K. (2023, May 19). Carbon Nanotubes for Lead Ions. In Encyclopedia. https://encyclopedia.pub/entry/44583
Pyrzynska, Krystyna. "Carbon Nanotubes for Lead Ions." Encyclopedia. Web. 19 May, 2023.
Copy Citation
Lead is one of the most toxic heavy metals released into the environment through industrial sources. Its direct determination is often a problem due to the presence of relatively complex matrices as well as low content. Thus, the additional separation and preconcentration steps are necessary in the analytical procedures. Carbon nanotubes (CNTs) continue to attract significant interest for these purposes as they exhibit a high specific surface area, exceptional porosities, and numerous adsorption sites.
carbon nanotubes
Pb(II) ions
separation
1. Introduction
Carbon nanotubes (CNTs), ever since their discovery by Iijma, have gained considerable sustained attention from the scientific community [1][2][3][4]. Their structure consists of a tubular sheet of graphene with a honeycomb structure of carbon atoms. They can be divided into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) according to the number of graphene sheets. The properties and the type of CNT structure are significantly influenced by the synthesis techniques and subsequent parameters. Their high surface-to-volume ratio, geometry, and hollow structure make them widely applicable as sorbents, filters, or membranes for separation and enrichment purposes. MWCNTs provide a greater surface in comparison to SWCNTs due to the presence of concentric graphene sheets, resulting in enhanced interaction with the analytes. CNTs strongly adsorb several pollutants through various interactions, such as π-π and electrostatic interactions, hydrophobic effect, and covalent bonding [5][6]. Many studies have been also carried out on their applications in device modelling, coating materials, thin-film electronics, actuators and capacitors, sensors and biosensors, and energy storage [7][8][9][10][11][12]. CNTs have also found several applications in biomedical science for medical diagnosis and drug delivery [13][14]. Their antibacterial and antifungal properties have been used to prevent bacterial adhesion on medical devices [15].
There are many methods for the synthesis of CNTs, but carbon arc discharge, laser ablation, and chemical vapor deposition (CVD) techniques are commonly applied [7][16][17][18]. The arc discharge method provides the growth of CNTs with minimum structural defects in comparison with other techniques, but needs the use of a high temperature (>1700 °C), low pressure, and expensive gases. The laser ablation method has the advantage of producing a high yield of CNTs of good quality and purity in a short time; however, it can be performed only in a vacuum with controlled inert gases, which results in high costs. The main advantages of different CVD techniques include the ease of controlling the reaction course, a high yield, small amount of impurities, and relatively low operating temperature (<800 °C). Taguchi orthogonal array technique was applied to investigate the interdependent effect of various parameters during the synthesis of CNTs using a catalytic CVD method, such as temperature, acetylene, and argon flow rates as well as time on the diameter of the final product [19]. The obtained smaller nanotubes with a diameter range of 5–15 nm revealed a larger BET surface area of 1306 ± 5 m2/g and higher maximum sorption capacity at pH 5 for Pb(II), equal to 215.38 ± 0.03 mg/g (at 50 °C, 60 min sorption time), in comparison to MWCNTs with a bigger diameter range of 16–25 nm (qmax = 201.35 ± 0.02 mg/g and surface area of 1245 ± 4 m2/g). The microwave irradiation method with low cost and high efficiency has been also employed [20][21][22]. It operates at low temperatures and is easy to control. The synthesis methods are quickly improved year by year to produce a large amount of size-controlled CNTs for different applications. Purification of CNTs from amorphous carbon and catalyst particles is required after synthesis. It is usually achieved using hydrochloric acid treatment and sonication of nanotubes in different media [20].
Similar to graphene-based nanomaterials, pristine CNTs can be modified by covalent, non-covalent, defect-group and endohedral functionalization using small molecules as well as doping with heteroatoms [23][24][25][26][27]. The functionalization of pristine carbon nanotubes (for the example of SWCTs) is schematically depicted in Figure 1 [23]. The functional groups increase their hydrophilicity and may also act as a stabilizer against the aggregation of individual nanotubes, caused by the strong van der Waals interactions. Moreover, the functionalization with magnetic nanoparticles facilitates their separation from the solution, together with adsorbed analytes, by applying an external magnetic field without filtration or centrifugation [2][28]. Thus, the modified CNTs with active groups, reagents, or materials are widely explored using more mutual interactions that can significantly improve their sorption performance.
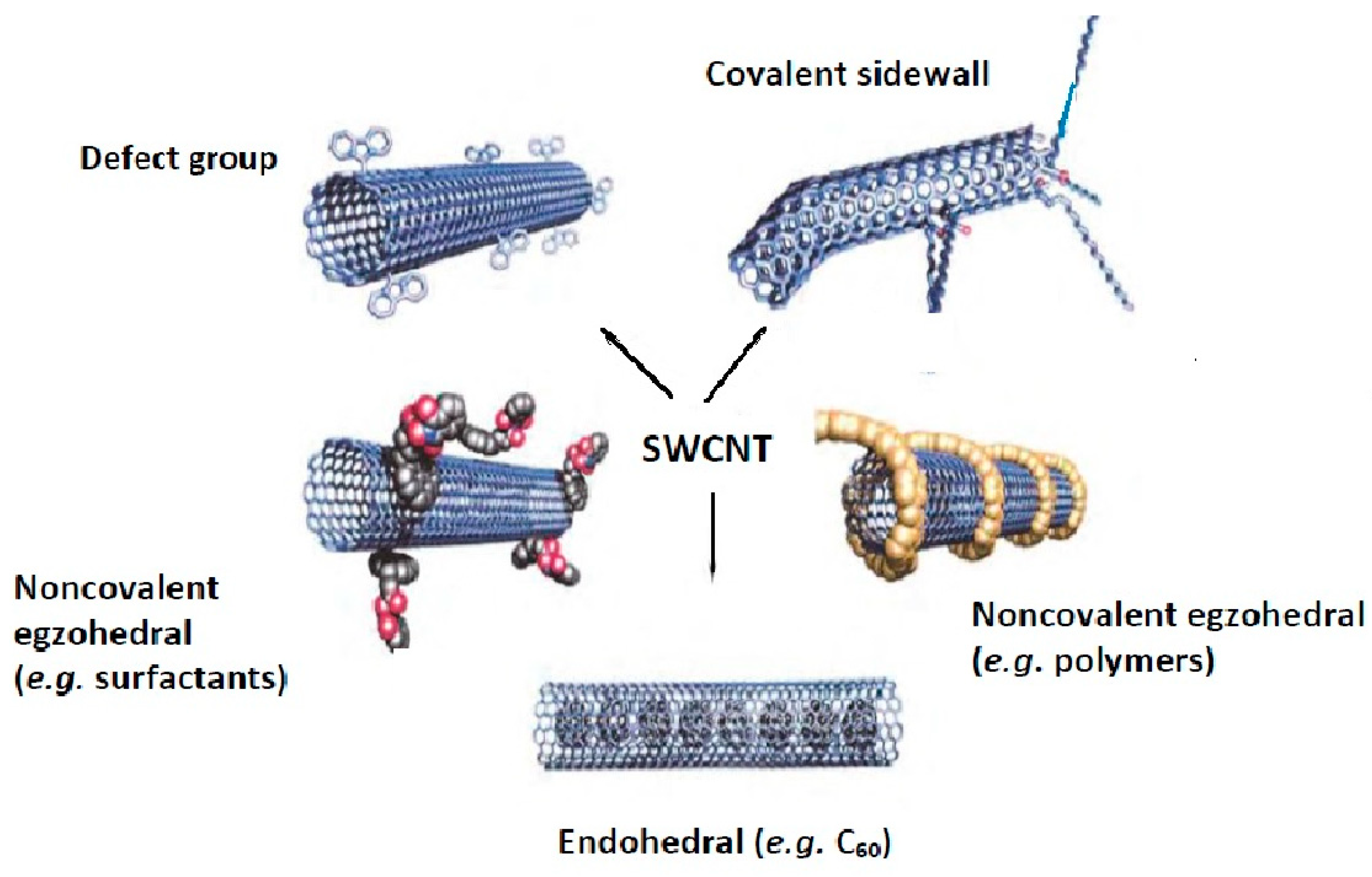
Figure 1. The methods for functionalization of carbon nanotubes.
The application of carbon nanotubes is very helpful for preconcentration, separation, and speciation analysis of metal ions as well as for their removal from wastewaters [28][29]. The analysis of environmental samples is often difficult due to intereferences from the complex matrices and low content of the analytes. Thus, a separation/preconcentration procedure is very often necessary to increase the sensitivity and selectivity of the applied analytical methods. Solid-phase extraction (SPE) and its alternatives are commonly used for this purpose owing to their rapid phase separation, high preconcentration factor, simple procedure, and the ability to combine them with different detection techniques [30][31][32].
2. Variations of SPE with CNTs
In the most proposed applications of CNTs for separation and preconcentration of Pb(II), conventional homemade packed columns or cartridges were used, where the sample loading step is performed by gravity flow or is pressure/vacuum-assisted. Dispersive solid phase extraction (DSPE) has been also evaluated as an alternative approach [33][34]. The sorbent is dispersed in a sample solution interacting directly with the target analytes. After the dispersion process is completed, the sorbent together with the retained analytes is separated by a mechanical process, such as centrifugation or filtration. Ultrasonic treatment was also used for the dispersion of sorbent, as it facilitates mass transfer [35][36]. The advantage of DSPE is the reduction in sample treatment time which allows more samples to be analyzed in a shorter period time. In magnetic dispersive solid phase extraction (MDSPE), the combination of CNTs with magnetic nanoparticles makes their separation much easier after the enrichment process by using an external magnet [19].
Recently, the miniaturization in dispersive microextraction using stir bar sorptive material with magnetic nanoparticles was presented [37]. The amount of sample is significantly reduced to a few microliters; therefore, it is very helpful for the analysis of low-availability samples, such as saline solutions.
Solid-phase microextraction (SPME) technique integrates a few operations in a simple step, such as sampling and analyte separation from sample matrices, its preconcentration, and sample collection [38]. The sample is exposed to fused silica fibre or stainless steel wires coated with a layer of carbon nanotubes that are directly exposed to the sample for a sufficiently long time. Several methods can be found in the literature to deposit CNTs in SPME fibres, such as physical attachment, chemical bonding, sol-gel method, electrochemical polymerization, or electrophoretic deposition [39][40].
In all SPE techniques, several factors influence the sorption process of metal ions on carbon nanotubes’ surfaces. The factors include pH, contact time, temperature, amount of CNTs, their surface charge, and the presence of other sample components. Sorption of Pb(II) is favoured when the pH of a sample solution is higher than a point of zero charge of the particle surface due to the electrostatic interaction. However, at a higher pH, precipitation of Pb(OH)2 may have significant participation in sorption. For this reason, the proposed pH for sorption of Pb(II) was mainly in the range of 5–7. The efficiency of sorption usually increases when the sorbent amount and extraction time are increasing, achieving equilibrium. The competitive effect from other matrix components may affect the sorption process of Pb(II), causing a decrease in its efficiency; thus, the high sorbent capacity as well as selectivity are favoured. In the separation/preconcentration procedures, a used eluent volume has also a significant effect on obtaining the highest analytical signal. Its low volume could not facilitate quantitative desorption, while a large volume dilutes the analyte and consequently the value of the enrichment factor (EF) is decreased. EF is one of the important parameters for evaluating the efficiency of a given SPE method.
3. CNTs for Separation and Enrichment of Pb(II)
3.1. Oxidized CNTs
Oxidative treatment of carbon nanotubes is a common way to introduce the oxygen functional groups, such as hydroxyl, carboxyl, and carbonyl, on the surface, and different oxidizing reagents, such as concentrated HNO3 and H2SO4, H2O2, and KMnO4 were used [41][42][43][44]. Thus, CNTs can adsorb heavy metal ions due to a partial negative charge on their surface. The amount of these groups depends on the used oxidant and increases in this order: HCl < NH4OH/H2O2 < H2SO4/H2O2 < refluxed HNO3 [43]. Double-oxidized multiwalled carbon nanotubes have also been proposed to increase the efficiency of heavy metals removal from wastewater [44]. First, MWCNTs are oxidized with concentrated HNO3 solution by refluxing at 120 °C for 4 h. In the second oxidation process, the mixture of HNO3 + H2SO4 (1:3, v/v) was used with sonication at 40–50 °C for 3 h. One of the main drawbacks of this procedure is the occurrence of nanotubes shortening by fragmentation and generation of defects in the graphitic network, particularly for oxidation with nitric acid. Bayazit and Inci reported that oxidation of SWCNTs with concentrated HNO3 under irradiation by UV-light increased their surface acidity more than ultrasonication [45]. The theoretical sorption capacity value of carbon nanotubes prepared by the UV-light method was 511.99 mg/g, while for ultrasonication it was 342.36 mg/g.
Wang et al. found that during the oxidation of CNTs with concentrated HNO3, the total amount of oxygen-containing functional groups increased quickly during 2 h of treatment to 1.6 and 2.7 mmol/g at 1 and 2 h treatment of acid, respectively [46]. Then, the increase was very slow from 6 h (2.8 mmol/g) to 10 h acid treatment (3.1 mmol/g). In addition, the amount of -OH and –C=O groups decreased after 2 h treatment, which was explained by the fact that these groups are further oxidized into carboxylic acid groups. Figure 2 shows the TEM images of pristine MWCNTs and MWCNTs acidified with nitric acid. Most of the contaminants present in pristine carbon nanotubes were removed after HNO3 treatment (Figure 2b) and Pb(II) adsorbed onto oxidized MWCNTs aggregated on their surface (Figure 2c), mostly on the caps and defective sites (Figure 2d).
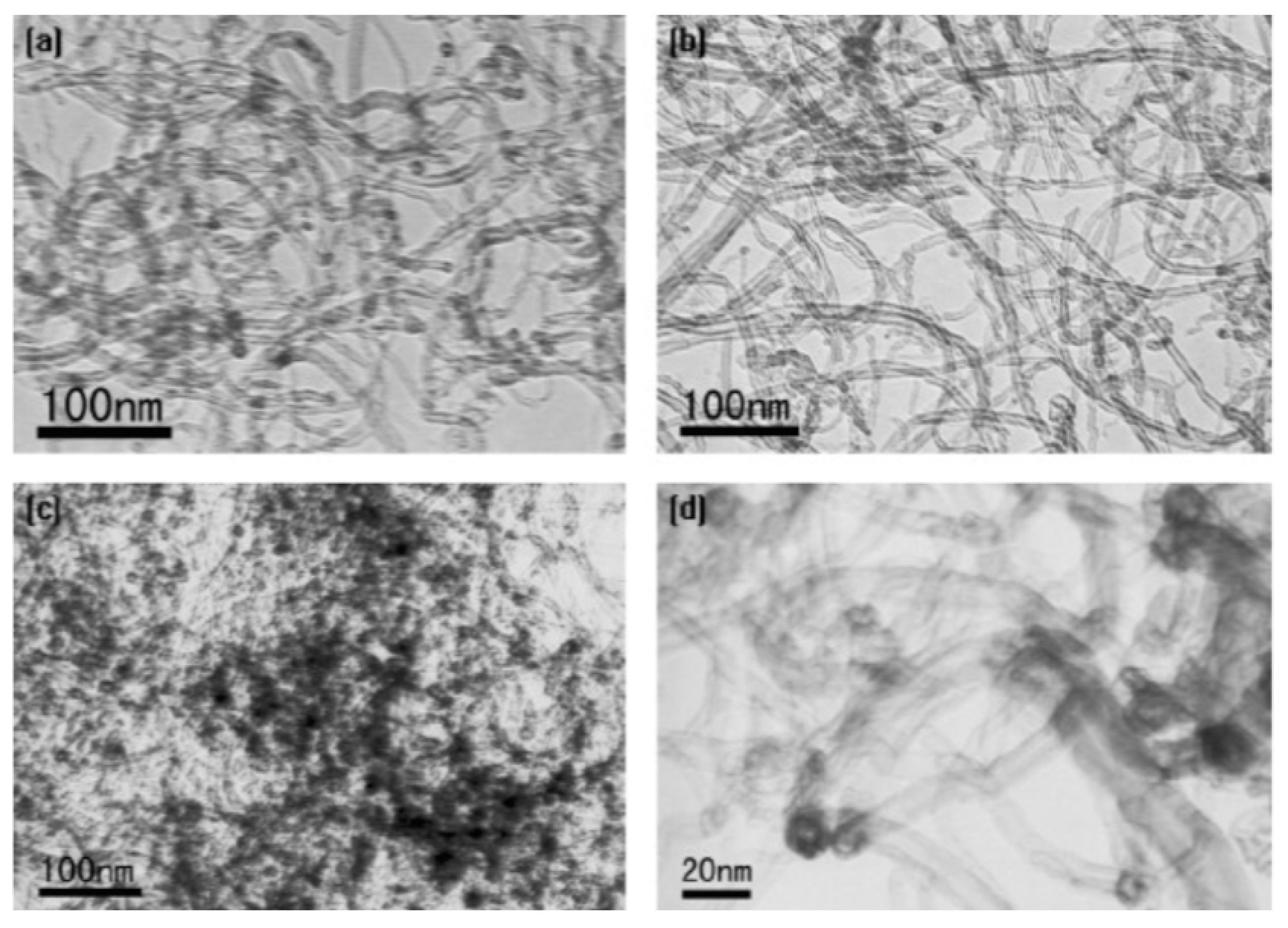
Figure 2. TEM image of (a) pristine MWCNTs; (b) oxidized with concentrated HNO3; (c,d) oxidized MWCNTs after sorption of Pb(II).
Oxygen plasma treatment, as an alternative method in relatively mild conditions to oxidation using strong chemical reagents, has been proposed [47][48][49]. The presence of -C=O and -COOH groups on carbon nanotubes was confirmed by data obtained from Fourier Transform Infrared spectroscopy. Compared to the acid treatment, fewer acidic groups was generated by plasma oxidation, but they had less damage and were generated in a much shorter treatment time. The sorption capacity of MWCNTs for lead(II) was greatly enhanced after plasma oxidation from 9.79 ± 0.46 mg/g (raw nanotubes) up to 54.11 ± 2.67 mg/g [47]. Hosseini et al. summarized the modification of carbon nanotubes through different types of plasma, notably plasmas that operate at an ambient temperature and atmospheric pressure [48]. It is worth mentioning that changing the gases used during plasma processing, different chemical groups, for example with nitrogen, can be generated on the CNT surface [49].
Sellaoui et al. analyzed the adsorption mechanism of Pb(II) ions onto carbon nanotubes via an experimental data set and physical models [50]. They used commercially available MWCNTs, which contained hydroxyl (content of 2.36–2.60% mass fraction) and carboxylic groups (content: 1.47–1.63% mass fraction). The experimentally quantified maximum metal adsorption capacity was about 240 mg/g at pH 5 and 300 min contact time. The obtained results were used to interpret the role of surface functionalities via statistical physics calculations. Two models (homogenous and heterogeneous) were applied for the analysis of adsorption data based on the model fitting results. The first model assumed that the adsorbent has a unique type of functional group responsible for the adsorption of Pb(II) and that a single adsorption energy generation can be hypothesized between the metal ions and the adsorbent surface. The second model hypothesizes the presence of two types of functional group binding lead ions, involving two different adsorption energies. The analysis of the model parameters showed that each functional group can adsorb several ions simultaneously [51]. The determined values of adsorption energies for both the functional groups were in the typical range of physical adsorption.
The adsorption mechanism of Pb2+ on a carbonaceous surface modified with oxygen functional groups was investigated by Xie et al. using the density functional theory method [52]. It was found that the adsorption of Pb(II) on the zigzag nanotube surface model (with seven benzene rings) was chemisorption with the adsorption energy in the range of −306.26 to −322.36 kJ/mol, while that on the armchair surface model with four benzene rings was physisorption (adsorption energy of −32.39 kJ/mol). The introduction of oxygen functional groups significantly enhanced the Pb(II) adsorption on the armchair surface. The physical sorption changed to chemisorption after introducing the oxygen functional groups, indicating the higher adsorption ability of the carbonaceous surfaces after modification. On the zigzag surface, however, the studied functional groups did not benefit the metal ions adsorption (Figure 3). Pb(II) tended to adsorb on the carbon atoms instead of moving to the oxygen atoms from the introduced functional groups for adsorption, which may suggest that the functional groups with oxygen promoted the Pb2+ adsorption by increasing the activity of their neighboring carbon atoms.
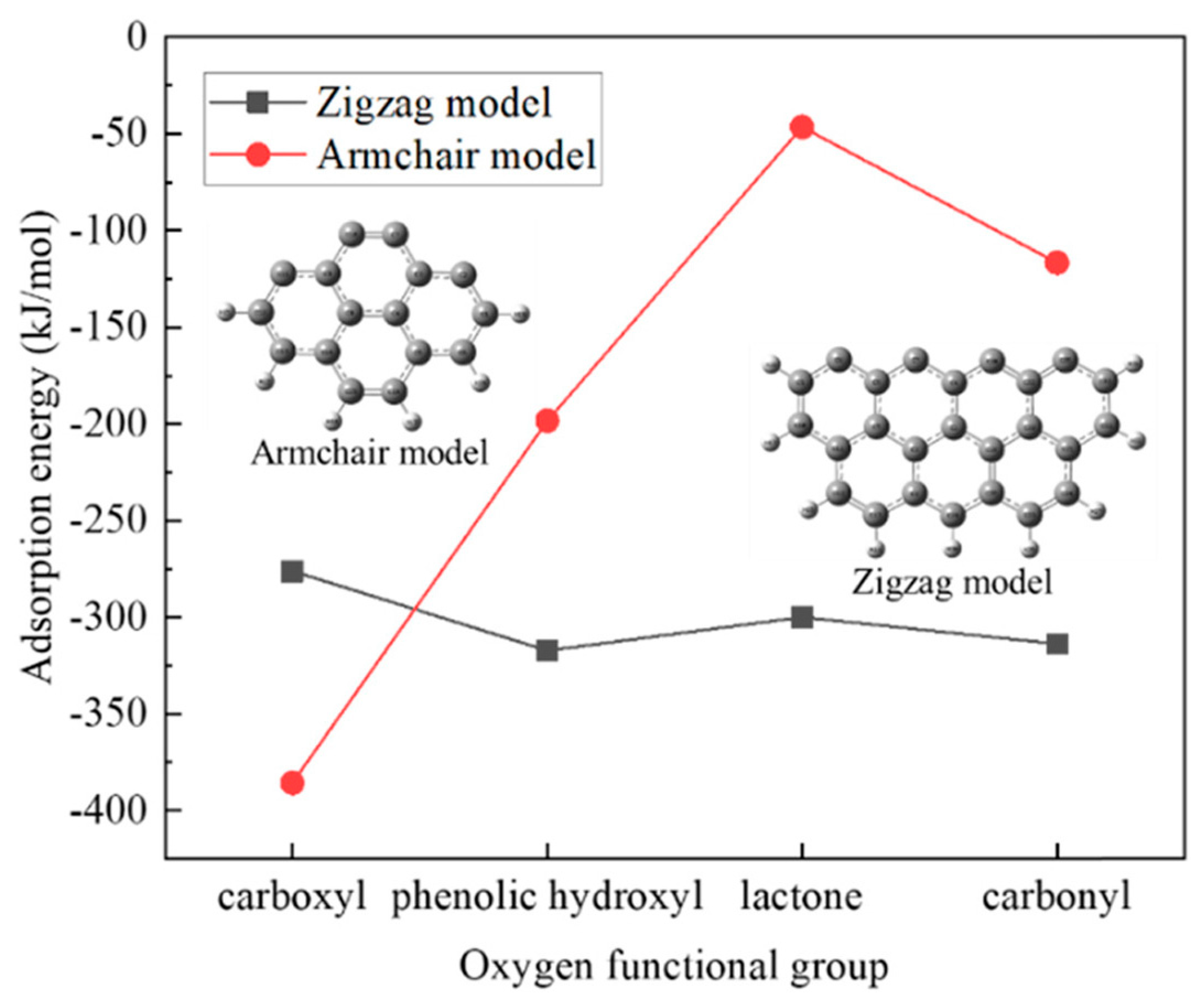
Figure 3. The effects of oxygen functional groups on the adsorption energy for Pb(II) on the zigzag and armchair nanotube surface models.
3.2. CNTs Modified with Organic Compounds
The modification of the surface of CNTs is one of the main research trends in the last years, as it is a way to improve their solubility as well as their sorption capacity and selectivity. Such modification increases the number of oxygen, nitrogen, sulfur, or other groups, and increases their dispersibility and the surface area [53][54]. Much research has been dedicated to the modification by forming covalent bonds between the carbon surface and the modifying reagent (covalent functionalization). The electrostatic and hydrophobic interactions, ion exchange, π–π electron binding, hydrogen bonding, and mesopore filling can be utilized for sorption of Pb(II) onto functionalized CNTs. In noncovalent functionalization, mainly hydrogen bonds, van der Waals, and hydrophobic interactions occur. The noncovalent functionalization of CNTs includes also coating with an appropriate ligand or surfactant, surface wrapping with polymer chains, and doping with metals or their oxides. The structure and original properties of CNTs are not changed after nonocovalent modification, but covalent functionalization is more stable and powerful.
The characterization of the presence of functional groups on the surface of CNTs and the efficiency of their functionalization can be carried out by various instruments, such as a transmission electron microscope (TEM), field emission scanning electron microscope (FESEM), energy dispersive X-ray (EDX), Raman spectroscope, thermogravimeter (TGA), Fourier transformed infrared spectroscope (FT-IR), and X-ray photoelectron spectroscope (XPS) [55]. The morphology and structure of carbon nanotubes are characterized by TEM and FESEM. The impurities and defects, such as amorphous carbon coatings and catalyst particles, could be observed as black dots on their surface inside the body. These techniques can allow the observing of the CNTs′ surface damage after chemical treatment. EDX measurements are used for quantitative measurements of the components on the CNTs′ surface. After the oxidation process, it is expected that EDX analysis shows more oxygen atoms as a result of grafting new oxygen-containing functional groups. FT-IR is used to analyze the chemical bonding and type of functional groups grafted onto the nanotubes.
Impregnation of magnetic MWCNTs with 1-(2-pyridazylo)2-naphtol (PAN) in vortex-assisted SPE was applied for preconcentration of Pb(II) at pH 5.5 from some herb and spice samples [56]. Elution was performed using 3 mL of 3 mol/L HNO3 in 10% acetone. The enrichment factor (EF), defined as the ratio of sample to eluent volume, was 10 in a very short time (1 min) [49]. The limit of detection was 16.6 µg/L with flame atomic absorption spectrometry (FAAS). The surface of oxidized carbon nanotubes was also modified with a cationic chelating agent, batophenanthroline [57]. Sorption was conducted at pH 9 in a borate buffer within 15 min. Due to the high preconcentration factor of 200, the proposed procedure for determination of Pb(II) in rice samples allowed researchers to obtain the limit of detection (LOD) as low as 0.25 µg/L using the simple FAAS detection in the range of 0.13 and 0.35 ng/mL.
Hanbali et al. evaluated the potential of MWCNTs grafted with various reagents for Pb(II) removal [58]. Carbon nanotubes were first oxidized with concentrated nitric acid and then converted to acid chloride. In the next step, MWCNTs were functionalized separately with hydroxylamine (HY), cysteine (CYS), and hydrazine (HYD). Finally, they were mixed with iron oxides and suspended for 2 h to obtain the appropriate nanocomposites with magnetic properties (Figure 4). The efficiency of these adsorbents toward Pb(II) was studied as a function of adsorbent dose, pH, metal ion initial concentration, temperature, and contact time. The optimum adsorption conditions were found to be at a pH of 8.0 and the maximum removal was attained after 30 min at room temperature. For MWCNT-CYS and MWCNT-HYD, these values were 1.15, 3.31, and 9.09 mg/g, respectively. Moreover, after the third regeneration step by treatment with 0.1 M HCl solution and reuse cycles, a small reduction in their efficiency was observed [58].
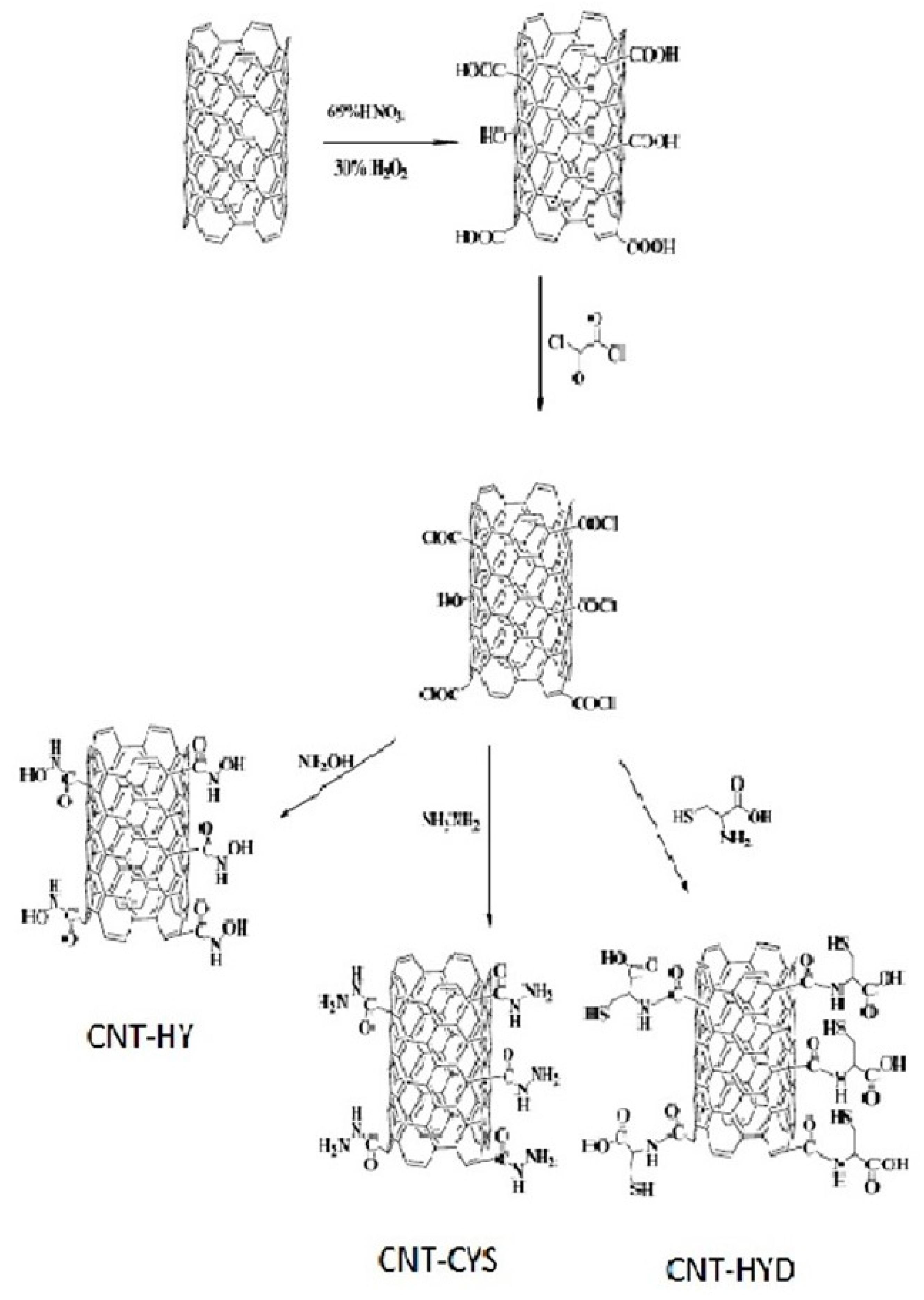
Figure 4. Scheme for preparation of magnetic MWCNTs functionalized with hydroxylamine (HY), cysteine (CYS), and hydrazine (HYD).
Other ligands which can form complexes with Pb(II) ions, such as 4′-(4-hydroxy-phenyl)-2,2′,6′,2′′-terpyridine [42], phenylenediamine [44], 5,7-dinitro-8-quinolinol [59], pyridine [60], sulfosalicylic acid [61], or hydroxamic acid derivatives [62], were covalently bonded on the carbon nanotube surface. According to the Langmuir adsorption model, the maximum adsorption capacity of Pb(II) after modification of MWCNTs with 5,7-dinitro-8- quinolinol was increased from 200 mg/g to 333.3 mg/g [59]. The adsorption rate of Pb(II) onto such modified carbon nanotubes reached equilibrium only after 1.5 h. The mean recovery of lead ions from the real wastewater samples collected from 4 different sites in the Suez Gulf in Egypt and spiked with 50 mg/L of Pb was 88.04%.
Bajaj et al. have proposed the flow injection system connected directly to the nebulizer of a FAAS spectrophotometer using MWCNTs modified with phenylenediamine for the preconcentration of lead ions before their determination in industrially contaminated water samples [63]. Such a methodology allows researchers to increase the sampling frequency up to 20 per hour. The preconcentration factors (PF), defined as the ratio of the slopes of the calibration curves with and without preconcentration step, were 94 and 73 for MWCNTs modified with phenylenediamine and for only oxidized MWCNTs, respectively. The linear relationship with the analyte concentration was obtained in the range of 3.80–260 μg/L with the LOD value equal to 1.2 μg/L.
MWCNTs functionalized with sulfosalicylic acid were obtained after oxidation and thiolation, followed by decoration with Fe3O4 nanoparticles [61]. Their high sorption capacity of 454.54 mg/g was used for lead preconcentration from water samples from electroplating industries.
Thiol-functionalized MWCNT was synthesised using 2-mercaptoethanol as a sulfur source [64]. The coordination between –SH groups and Pb(II) effectively enhances the adsorption performance of the obtained MWCNTs-SH nanoparticles. Their adsorption capacity at pH 5 was increased by approximately 30.5% in comparison to MWCNT-COOH. The maximum Pb(II) adsorption capacity derived from the Langmuir model was 144.9 mg/g. The surface of MWCNT-SH was negatively charged at pH > 1. This means that under these conditions the electrostatic repulsion increased, and the agglomeration phenomenon weakened. The chemical adsorption process was recognized as controlling the adsorption rate. To study the adsorption performance of MWCNT-SH in natural samples, various wastewaters with different concentrations of Pb(II) were used, such as copper smelting wastewater (lead concentration of 5.01 mg/L), tin tailings wastewater (54.07 mg/L), and antimony tailings wastewater (1.02 mg/L). The adsorption capacity of MWCNT-SH was 42.6, 239.7 and 9.4 mg/g, respectively, for these wastewaters.
The characteristics of Pb(II) adsorption from an aqueous solution onto multiwalled carbon nanotubes functionalised by di-t-butyl selenophosphoryl groups connected by a selenium atom were investigated by Kończyk et al. [65]. The structure of this newly proposed adsorbent is presented in Figure 5. The authors reported that adsorbent enabled the highest Pb(II) removal efficiency at pH 5.0 within 60 min. The adsorption capacity increased as the temperature increased, and the maximum value of 156.25 mg/g was obtained at 313 K. The obtained experimental data were well-matched with the pseudo-second-order kinetic model. For further analysis of the adsorption kinetics, the diffusion model proposed by Weber and Morris was considered. That model states that when the plot q(t) = f(t0.5) is linear and passes through the origin (0,0), the intraparticle diffusion process dominates. In a case where the linearity is ensured but the plot does not pass through the origin, the adsorption may be limited by film diffusion. However, deviation from the origin was observed, which indicated faster mass transfer through the boundary layer on the adsorbent surface at the beginning of adsorption; then, the process was controlled by slower Pb(II) diffusion inside the particles. The competitive adsorption studies from the solution containing also other metal ions indicated high selectivity for Pb(II) with the affinity decreasing in the following order: Pb(II) >> Cd(II) > Zn(II), Cu(II), Ni(II), Co(II).
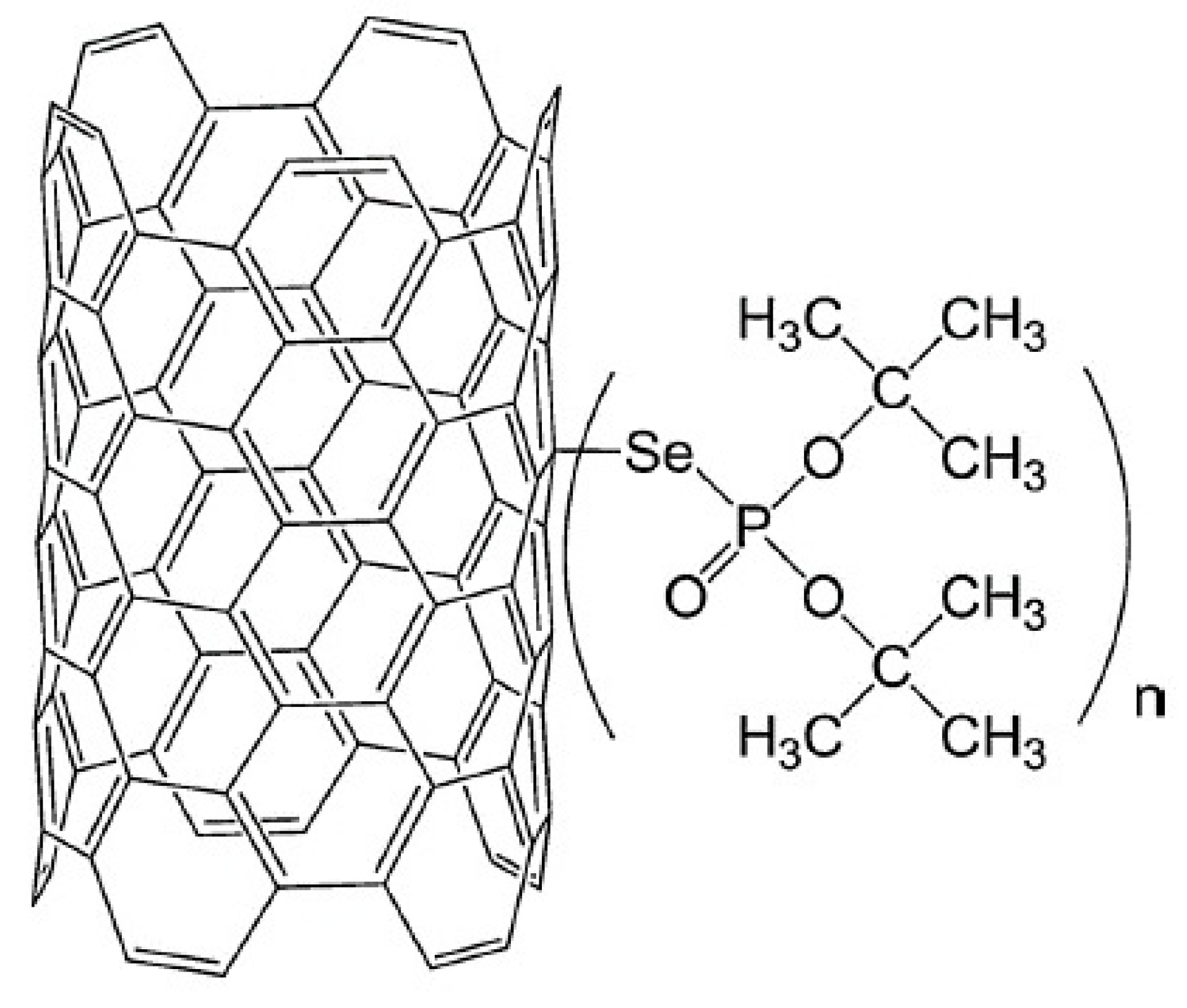
Figure 5. MWCNTS functionalised by di-t-butyl selenophosphoryl groups.
3.3. CNTs Decorated with Metal Oxides
CNTs decorated with metal oxide form a new class of hybrid nanomaterials that could potentially display not only the unique properties of nanoparticles and nanotubes but also additional novel physical and chemical properties due to their mutual interactions [59]. Thus, metal oxide nanoparticles supported on carbon nanotubes have been extensively studied and found to be effective adsorbents for the removal of heavy metal ions. The synthesis methodology for such materials has already been presented and discussed [66][67].
Apart from Fe2O3 or Fe3O4, present mainly in magnetic carbon nanotubes, various metal oxide nanoparticles have been decorated on the surface of CNTs to enhance their sorption properties and to broaden their applications [18][68]. These nanoparticles act also as a stabilizer against the aggregation of individual tubes, which is caused by strong van der Waals interactions. The addition of magnetic properties to CNTs, as was mentioned earlier, facilitates their collection after Pb(II) sorption from a sample solution. The silica structure was introduced to protect the magnetic cores against leaching, oxidation, and digestion in acidic media, as well as for improving their reusability [69].
For the preconcentration and separation of Pb(II), CNTs were successfully fitted with other metal oxides, such as NiO [70], MnO2 [71], or Al2O3 [72], as well as decorated with Au/Fe3O4 nanocomposites [73], thereby increasing their removal efficiencies. Egbosiuba et al. proposed nickel nanoparticles supported on MWCNTs, activated in an alkaline media, for the removal of Pb(II) from industrial wastewaters [74]. The proposed mechanism of Pb(II) sorption onto MWCNTs-KOH@NiNPs included electrostatic attraction, surface adsorption, ion exchange, and pore diffusion due to the incorporated nickel particles. The maximum adsorption capacity for this sorbent reached 480.95 mg/g. The obtained value of the maximum adsorption capacity for NiO/CNts (24.63 mg/g) is significantly lower than that reported earlier in the literature, e.g., for MnO2/CNTs (78.74 mg/g) [71] or Al2O3/CNTs (67.11 mg/g) [72]. In turn, the equilibrium in the studied NiO/CNts adsorption system is achieved relatively fast (10 min) in comparison to the other systems with metal oxides. Although Pb(II) adsorption by MnO2/CNTs occurred rapidly within the first 15 min of contact time, at least 2 h were needed to attain adsorption equilibrium [71].
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Li, W.K.; Shi, Y.P. Recent advances and applications of carbon nanotubes based composites in magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2019, 118, 652–665.
- Vesali-Naseh, M.; Naseh, M.R.V.; Ameri, P. Adsorption of Pb(II) ions from aqueous solutions using carbon nanotubes: A systematic review. J. Cleaner Prod. 2021, 291, 125917.
- Gusain, R.; Kumar, N.; Sinha Ray, S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111.
- Treviño, M.J.S.; Zarazùa, S.; Płotka-Wasylka, J. Nanosorbents as materials for extraction processes of environmental contaminants and others. Molecules 2022, 27, 1067.
- Trivedi, M.; Reecha, K. Recent development and applications of carbon nanotubes. Chem. Sci. Rev. Lett. 2020, 9, 502–510.
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192.
- Moghaddam, H.K.; Maraki, M.R.; Rajaei, A. Application of carbon nanotubes (CNT) on the computer science and electrical engineering: A review. Int. J. Reconfig. Embed. Syst. 2020, 9, 61–82.
- Rasheed, T.; Hassan, A.A.; Kausar, F.; Sher, F.; Bilal, M.; Iqbal, H.M.N. Carbon nanotubes assisted analytical detection-Sensing/delivery cues for environmental and biomedical monitoring. TrAC Trends Anal. Chem. 2020, 132, 116066.
- Garg, A.; Chalak, H.D.; Belarbi, M.O.; Zenkour, A.M.; Sahoo, R. Estimation of carbon nanotubes and their applications as reinforcing composite materials-An engineering review. Compos. Struct. 2021, 272, 114234.
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A.; Elshehy, E.A. Hierarchical inorganic–organic multi-shell nanospheres for intervention and treatment of lead-contaminated blond. Nanoscale 2013, 5, 7920–7927.
- El-Safty, S.A.; Khairy, M.; Shenashen, M.A.; Elshhehy, E.; Warkocki, W.; Sakai, M. Optical mesoscopic membrane sensor layouts for water-free and blood-free toxicants. Nano Res. 2015, 8, 3150–3163.
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube-A review on synthesis, properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003.
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: A critical review. Int. J. Nanomed. 2020, 15, 7063–7078.
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001.
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon nanotubes (CNTs) from synthesis to functionalized (CNTs) using conventional and new chemical approaches. J. Nanomater. 2021, 2021, 4972770.
- Gacem, A.; Modi, S.; Yadav, V.K.; Islam, S.; Patel, A.; Dawane, V.; Jameel, M.; Inwati, G.K.; Piplode, S.; Solanki, V.S.; et al. Recent advances in methods for synthesis of carbon nanotubes and carbon nanocomposite and their emerging applications: A descriptive review. J. Nanomater. 2022, 2022, 7238602.
- Pattanshetti, A.; Pradeep, N.; Chaitra, V.; Uma, V. Synthesis of multi-walled carbon nanotubes (MWCNTs) from plastic waste & analysis of garlic coated gelatin/MWCNTs nanocomposite films as food packaging material. Appl. Sci. 2020, 2, 730.
- Egbosiuba, T.C.; Abdulkareem, A.S.; Tijani, J.O.; Ani, J.I.; Krikstolaityte, V.; Srinivasan, M.; Veksha, A.; Lisak, G. Taguchi optimization design of diameter-controlled synthesis of multi walled carbon nanotubes for the adsorption of Pb(II) and Ni(II) from chemical industry wastewater. Chemosphere 2021, 266, 128937.
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi1, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393.
- Baghel, P.; Sakhiya, A.K.; Priyanka Kaushal, P. Ultrafast growth of carbon nanotubes using microwave irradiation: Characterization and its potential applications. Heliyon 2022, 8, e10943.
- Kure, N.; Hamidon, N.M.; Azhari, S.; Mamat, N.S.; Yusoff, H.M.; Isa, B.M.; Yunusa, Z. Simple microwave-assisted synthesis of carbon nanotubes using polyethylene as carbon precursor. J. Nanomater. 2017, 2017, 2474267.
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859.
- Dyachkova, T.P.; Rukhov, A.V.; Tkachev, A.G.; Tugolukov, E.N. Functionalization of carbon nanotubes: Methods, mechanisms and technological realization. Adv. Mater. Technol. 2018, 2, 18–41.
- Aslam, M.M.; Kuo, H.W.; Den, W.; Usman, M.; Sultan, H.; Ashraf, M. Functionalized carbon nanotubes (CNTs) for water and wastewater treatment: Preparation to application. Sustainability 2021, 13, 5717.
- Díez-Pascual, A.M. Chemical functionalization of carbon nanotubes with polymers: A brief overview. Macromol 2021, 1, 64–83.
- Guo, J.; Jiang, H.; Teng, Y.; Xiong, Y.; Chen, Z.; You, L.; Xiao, D. Recent advances in magnetic carbon nanotubes: Synthesis, challenges and highlighted applications. J. Mater. Chem. B 2021, 9, 9076–9099.
- Aigbe, U.O.; Osibore, O.A. Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578.
- Pyrzynska, K. Nanomaterials in speciation analysis of metals and metalloids. Talanta 2020, 212, 120784.
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal. Chem. 2018, 108, 347–369.
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487.
- Nouri, N.; Khorram, P.; Duman, O.; Sibel, T.; Hassan, S. Overview of nanosorbents used in solid phase extraction techniques for the monitoring of emerging organic contaminants in water and wastewater samples. Trends Environ. Anal. Chem. 2020, 25, e00081.
- Büyüktiryaki, S.; Keçili, R.; Hussain, C.M. Functionalized nanomaterials in dispersive solid phase extraction: Advances & prospects. TrAC Trends Anal. Chem. 2020, 127, 115893.
- Ghorbani, M.; Aghamohammadhassan, M.; Ghorbani, H.; Ali Zabihi, A. Trends in sorbent development for dispersive micro-solid phase extraction. Microchem. J. 2020, 158, 105250.
- Gugushe, A.S.; Mpupa, A.; Nomngongo, P.N. Ultrasound-assisted magnetic solid phase extraction of lead and thallium in complex environmental samples using magnetic multiwalled carbon nanotubes/zeolite nanocomposite. Microchem. J. 2019, 149, 103960.
- Krawczyk, M.; Jeszka-Skowron, M. Multiwalled carbon nanotubes as solid sorbent in dispersive micro solid-phase extraction for the sequential determination of cadmium and lead in water samples. Microchem. J. 2016, 126, 296–301.
- Azorín, C.; Benedé, J.L.; Alberto Chisvert, A. New challenges in sample preparation: Miniaturized stir bar sorptive dispersive microextraction as a high-throughput and feasible approach for low-availability sample preparation. Anal. Chim. Acta 2023, 1238, 340627.
- Song, X.Y.; Chen, J.; Shi, Y.P. Different configurations of carbon nanotubes reinforced solid-phase microextraction techniques and their applications in the environmental analysis. TrAC Trends Anal. Chem. 2017, 96, 263–275.
- ALOthman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651.
- Ghaemi, F.; Amiri, A.; Yunus, R. Methods for coating solid-phase microextraction fibers with carbon nanotubes. TrAC Trends Anal. Chem. 2014, 59, 133–143.
- Domagała, K.; Borlaf, M.; Traber, J.; Kata, D.; Graule, T. Purification and functionalisation of multi-walled carbon nanotubes. Mater Lett. 2019, 253, 272–275.
- Safo, I.A.; Liu, F.; Xie, K.; Wei Xia, W. Oxidation and stability of multi-walled carbon nanotubes in hydrogen peroxide solution. Mat. Chem. Phys. 2018, 214, 472–481.
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840.
- Rodríguez, C.; Leiva, E. Enhanced heavy metal removal from acid mine drainage wastewater using double-oxidized multiwalled carbon nanotubes. Molecules 2020, 25, 111.
- Bayazit, S.S.; Inci, I. Adsorption of Pb(II) ions from aqueous solutions by carbon nanotubes oxidized different methods. J. Ind. Eng. Chem. 2013, 19, 2064–2070.
- Wang, H.J.; Zhou, A.L.; Peng, F.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metalPb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466, 201–206.
- Yu, X.Y.; Luo, T.; Zhang, Y.X.; Jia, Y.; Zhu, B.J.; Fu, X.C.; Liu, J.H.; Huang, X.J. Adsorption of lead(II) on O2-plasma-oxidized multiwalled carbon nanotubes: Thermodynamics, kinetics, and desorption. ACS Appl. Mater. Interfaces 2011, 3, 2585–2593.
- Hosseini, H.; Mohammad Ghaffarzade, M. Surface functionalization of carbon nanotubes via plasma discharge: A review. Inorg. Chem. Commun. 2022, 138, 10927.
- Abdel-Fattah, E.; Ogawa, D.; Nakamura, K. Nitrogen functionalization of MWCNTs in Ar-N2 dielectric barrier discharge–Gas ratio effect. Mater. Sci Eng. B 2020, 61, 114680.
- Sellaoui, L.; Schnorr, C.E.; Dhaouadi, F.; Taamalli, S.; Louis, F.; El Bakali, A.; Dotto, G.L.; Silva, L.F.O.; Lamine, A.B.; Rtimi, S.; et al. Modeling the adsorption of divalent metallic cations onto multi-walled carbon nanotubes functionalized with COOH. J. Mol. Liq. 2022, 366, 120275.
- Gusain, R.; Kumar, N.; Fosso-Kankeu, E.; Ray, S.S. Efficient removal of Pb(II) and Cd(II) from industrial mine water by a hierarchical MoS2/SH-MWCNT nanocomposite. ACS Omega 2019, 4, 13922–13935.
- Xie, N.; Wang, H.; You, C. Role of oxygen functional groups in Pb2+ adsorption from aqueous solution on carbonaceous surface: A density functional theory study. J. Hazard. Mater. 2021, 405, 124221.
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid materials based on carbon nanotubes and nanofilters for environmental analysis. Front. Chem. 2020, 8, 546.
- Atif, M.; Afzaal, I.; Naseer, H.; Abrar, M.; Bongiovann, R. Review-Surface modification of carbon nanotubes: A tool to control electrochemical performance. ECS J. Solid State Sci. Technol. 2020, 9, 041009.
- Herrero-Latorre, C.; Álvarez-Méndez, J.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal. Chim. Acta 2015, 853, 77–94.
- Kamaş, D.; Karatepe, A.; Soylak, M. Vortex-assisted magnetic solid phase extraction of Pb and Cd in some herb samples on magnetic multiwalled carbon nanotubes. Turk. J. Chem. 2021, 45, 210–218.
- Feist, B.; Sitko, R. Method for the determination of Pb, Cd, Zn, Mn and Fe in rice samples using carbon nanotubes and cationic complexes of batophenanthroline. Food Chem. 2018, 249, 38–44.
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S.; Dagdag, O. Magnetic multiwalled carbon nanotube decorated with novel functionalities: Synthesis and application as adsorbents for lead removal from aqueous medium. Processes 2020, 8, 986.
- Abdel Salam, E.T.; Abou El-Nour, K.M.; Awad, A.A.; Orabi, A.S. Carbon nanotubes modified with 5,7,-dinitro-8-quinolinol as potentially applicable tool for efficient removal of industrial wastewater pollutants. Arab. J. Chem. 2020, 13, 109–119.
- Torkian, L.; Amini, M.M.; Gorji, T.; Sadeghi, O. A simple, rapid and sensitive method based on modified multiwalled carbon nanotubes for preconcentration and determination of lead ions in aqueous media in natural pHs. Arab. J. Chem. 2019, 12, 1315–1321.
- Islam, A.; Zaidi, N.; Ahmad, H.; Kumar, S. Functionalized carbon nanotubes for dispersive solid phase extraction and atomic absorption spectroscopic determination of toxic metals ions. Int. J. Environ. Sci. Technol. 2018, 16, 707–718.
- Al-Faiyz, Y.S.S.; Gouda, M. Multi-walled carbon nanotubes functionalized with hydroxamic acid derivatives for the removal of lead from wastewater: Kinetics, isotherm, and thermodynamic studiem. Polymers 2022, 14, 3870.
- Bajaj, S.; Jain, V.; Sharma, N.; Tiwari, S.; Saxena, R. Efficient lead preconcentration using two chemically functionalized carbon nanotubes in hyphenated injection-flame atomic absorption spectrometry system. J. Chromatogr. A 2021, 1638, 461888.
- Qu, G.; Zhou, J.; Liang, S.; Li, Y.; Ning, P.; Pan, K.; Ji, W.; Tang, H. Thiol-functionalized multi-walled carbon nanotubes for effective removal of Pb(II) from aqueous solutions. Mat. Chem. Phys. 2022, 278, 125688.
- Kończyk, J.; Żarska, S.; Ciesielski, W. Adsorptive removal of Pb(II) ions from aqueous solutions by multi-walled carbon nanotubes functionalised by selenophosphoryl groups: Kinetic, mechanism, and thermodynamic studies. Colloids Surf. A 2019, 575, 271–282.
- Tawfik, A.S. Insights into carbon nanotube-metal oxide composite: Embedding in membranes. Int. J. Sci. Res. Environ. Sci. Toxicol. 2017, 2, 1–4.
- Long, H.; Guo, C.; Wei, G.; Jiang, L.; Yu, Y. Facile synthesis of various carbon nanotube/metal oxide nanocomposites with high quality. Vacuum 2019, 166, 147–150.
- Mallakpour, S.; Elham Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367.
- Islam, A.; Chauhan, A.; Javed, H.; Rais, S.; Izhar Ahmad, I. Magnetic carbon nanotubes-silica binary composite for effective Pb(II) sequestration from industrial effluents: Multivariate process optimization. Clean-Soil Air Water 2021, 49, 2000401.
- Navaei Diva, T.N.; Zare, K.; Faleshi, F.; Yousefi, M. Synthesis, characterization, and application of nickel oxide/CNT nanocomposites to remove Pb2+ from aqueous solution. J. Nanostruct. Chem. 2017, 7, 273–281.
- Wang, S.G.; Gong, W.X.; Liu, X.W.; Yao, Y.W.; Gao, B.Y.; Yue, Q.Y. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 2007, 58, 17–23.
- Hsieh, S.H.; Horng, J.J. Adsorption behaviour of heavy metal ions by carbon nanotubes grown on microsize Al2O3 particles. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2007, 14, 77–84.
- Zondo, B.Z.; Sadare, O.O.; Simate, G.S.; Moothi, K. Removal of Pb2+ ions from synthetic wastewater using functionalized multiwalled carbon nanotubes decorated with green synthesized iron oxide-gold nanocomposite. Water SA 2022, 48, 304–316.
- Egbosiuba, T.C.; Egwunyenga, M.C.; Tijani, J.O.; Mustapha, S.; Abdulkareem, A.; Kovo, A.S.; Krikstolaityte, V.; Veksha, A.; Wagner, M.; Lisak, G. Activated multi-walled carbon nanotubes decorated with zero valent nickel nanoparticles for arsenic, cadmium and lead adsorption from wastewater in a batch and continuous flow modes. J. Hazard. Mater. 2022, 423, 126993.
More
Information
Subjects:
Chemistry, Analytical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
734
Revisions:
2 times
(View History)
Update Date:
22 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





