You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raquel Leão Orfali | -- | 2120 | 2023-05-17 19:46:07 | | | |
| 2 | Sirius Huang | Meta information modification | 2120 | 2023-05-18 11:10:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Soares, G.B.; Orfali, R.L.; Averbach, B.L.; Yosipovitch, G.; Aoki, V. Atopic Dermatitis in Latin America. Encyclopedia. Available online: https://encyclopedia.pub/entry/44455 (accessed on 26 December 2025).
Soares GB, Orfali RL, Averbach BL, Yosipovitch G, Aoki V. Atopic Dermatitis in Latin America. Encyclopedia. Available at: https://encyclopedia.pub/entry/44455. Accessed December 26, 2025.
Soares, Georgia Biazus, Raquel Leao Orfali, Beatriz Lacerda Averbach, Gil Yosipovitch, Valeria Aoki. "Atopic Dermatitis in Latin America" Encyclopedia, https://encyclopedia.pub/entry/44455 (accessed December 26, 2025).
Soares, G.B., Orfali, R.L., Averbach, B.L., Yosipovitch, G., & Aoki, V. (2023, May 17). Atopic Dermatitis in Latin America. In Encyclopedia. https://encyclopedia.pub/entry/44455
Soares, Georgia Biazus, et al. "Atopic Dermatitis in Latin America." Encyclopedia. Web. 17 May, 2023.
Copy Citation
Atopic dermatitis (AD) is a prevalent condition in Latin America that can have a substantial impact on quality of life. Diagnosing AD is challenging due to broad clinical features and lack of universal diagnostic criteria. Furthermore, lack of physician training, barriers to access, and socioeconomic inequalities hinder effective disease management. Ethnoracial disparities in AD need to be addressed, as they may impact not only in the diagnosis, but also in severity scores which are relevant parameters for evaluating the efficacy of therapeutic agents.
atopic dermatitis
Latin America
epidemiology
ethnic/racial
clinical features
1. Introduction
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease that often presents in childhood and is estimated to affect 15–20% of children and 1–3% of adults worldwide [1]. The pathophysiology of AD is multifactorial, and clinical characteristics are varied. AD is associated with a high disease burden that has a profound impact on quality of life [2][3]. Latin America (LA) encompasses about 8.5% of the world’s population and is one of the regions with the most social inequality [4]. This inequality, combined with diverse racial/ethnic, geographical, and social elements in the region contributes to healthcare disparities that make understanding and treating conditions such as AD challenging.
2. Epidemiology of AD
Two large multicenter studies have provided important data on the prevalence of AD in Latin America. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase 1 showed that the prevalence varied in different Latin American countries, ranging from around 4% in Mexico to as high as 10.9% in Chile in 6 to 7-year-olds and 10.8% in Paraguay in 13 to 14-year-olds [5]. Phase 3 of ISAAC was completed seven years later and included significantly more centers than Phase 1, spanning 14 Latin American countries. For children ages 6 to 7, the Latin American centers with the highest AD prevalence included Quito, Ecuador (22.5%) and Barranquilla, Colombia (20.9%). For adolescents, prevalence ranged from 2.8% in the Mexicali Valley, Mexico to 24.6% in Barranquilla, Colombia [5]. Another international, multicenter study found that Brazil had the highest prevalence of AD in all age groups among Latin American countries (20.1%) [6]. Smaller studies have also been conducted to examine the prevalence of AD within Latin American countries. For example, Garcia et al. reported that in Bogota, Colombia, 42.3% of children in their study had dermatological conditions, and 6.5% had AD [7]. Another study in Brazil found mean AD prevalence rates of 8.2% in schoolchildren and 5.0% among adolescents [8]. A recent study evaluating one-year prevalence of AD in Brazil through a population-based telephone survey, revealed that the age-adjusted prevalence of AD was 2.27% [9]. Data on the epidemiology of adult AD in Latin America are extremely limited, with one multicenter retrospective study in Brazil named ADAPT SA, a multicentric, non-interventional study to describe clinical features and disease management of adult patients with atopic dermatitis followed at tertiary hospitals [10].
Studies in the US and Europe have shown a higher AD prevalence in Black children, but the mean prevalence in LA regions with a predominantly Black population varied significantly, ranging from 4.4% in Northern Brazil to 10.1% in Cuba [5][11]. This supports the notion that there is genetic variation among African subgroups. Furthermore, certain genes involved in immune regulation and epithelial barrier function are involved in the pathogenesis of AD in specific ethnic groups. Filaggrin loss-of-function mutations, for example, play a key role in the development of AD in European populations [11]. The population in some of the LA countries is predominantly of European origin. A study in Chile showed that filaggrin variants commonly seen in European patients with AD were observed in 9.3% of Chilean patients [12]. There was an impaired expression of filaggrin and claudin-1 (a tight junction protein), in the skin of Brazilian patients. Interestingly, these proteins were found to be increased in conjunctival epithelial cells of patients with AD when compared to healthy controls, which could reflect a reactive response to AD-induced inflammation [13]. Differences in climate, humidity, and UV exposure can also all contribute to disease prevalence. A positive correlation between prevalence of AD and latitude was found in ISAAC Phase 1, and ISAAC Phase 3 further showed that the prevalence and severity of AD were higher in centers near the Equator [5][14]. The association between latitude and the prevalence of AD symptoms has been further demonstrated in other Latin American studies [8].
3. Immunological Studies in AD
One Brazilian analysis found increased expression of IL-22 in AD dermal lesions, emphasizing a possible Th22 deviation in these patients [15]. In another study, Orfali et al. reported increased IL-17 in both the serum and skin lesions of AD patients when compared to controls, as well as increased IL-22-expressing CD4/CD8 T cells in AD lesions and an impaired CD4 cytokine response after staphylococcal enterotoxin administration [16].
An ongoing pilot study from a Brazilian referral university hospital on atopic dermatitis in adults evaluated the clinical presentation, the expression of skin barrier proteins in cutaneous and ocular epithelia and the in situ immunological profile of Th17 and Th22 axes. The initial findings showed distinct patterns regarding the expression of skin barrier proteins in the cutaneous and ocular epithelia of AD patients, with variations in ethnic/racial profile (Figure 1—Unpublished data).
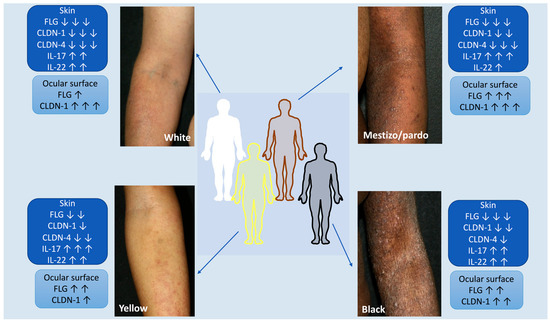
Figure 1. Atopic dermatitis and ethnic/racial variations in a Brazilian pilot ongoing cohort study: clinical presentation, expression of skin barrier proteins in cutaneous and ocular epithelia and in situ immunological profile of Th17 and Th22 axes. The initial findings show differences in the expression of skin barrier proteins present in the cutaneous and ocular epithelia of AD patients, with variations in the ethnoracial profiles (Unpublished data). The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
4. AD: Diagnosis and Clinical Practice Guidelines
A study in Mexico found that 42% of physicians used these criteria to achieve a diagnosis of AD, whereas a survey completed by AD Therapeutic Area Experts throughout Brazil showed that 82% of AD experts use it [17][18]. However, there are other diagnostic criteria available. Countries in Latin America such as Colombia, Argentina, and Brazil have their own clinical practice guidelines for AD diagnosis and management [19]. Adhering to diagnostic criteria is extremely important to objectively evaluate patients and avoid misdiagnoses. The diagnosis of AD may be delayed in LA due to the presence of other tropical cutaneous diseases that present with pruritus and lichenification such as scabies, papular urticaria, and miliaria. Studies in tropical countries showed that up to 80% of infectious dermatoses such as scabies were initially misdiagnosed as AD [20]. The use of ancillary tests to assist in the diagnosis of AD has also been discussed. AD has been characterized as extrinsic (IgE mediated) and intrinsic (non-allergic), and levels of IgE may correlate with disease severity [21]. Therefore, measuring levels of this immunoglobulin may be helpful when diagnosing AD. In one epidemiologic study of 80 Brazilian patients, the mean circulating IgE level was 18,340 UI/mL [21]. However, this must be interpreted carefully in Latin America, for a large part of the non-allergic population may have increased IgE levels due to the high incidence of helminth infections, which could cause sensitization [22]. A study conducted in a poor urban area of Brazil found positive associations between the number of helminth infections and the production of allergic inflammatory markers including peripheral eosinophilia, increased serum IgE levels, and helminth antigen-stimulated Th2 cytokine production [23].
5. Clinical Characteristics, Impact on Quality of Life, and AD Assessment
The Brazilian ADAPT study analyzed the clinical characteristics of AD and found that most patients presented with flexural lesions in the popliteal and antecubital regions, and lesions distributed on extensor surfaces such as the extremities and trunk [10]. At the first visit, the main AD reported features were erythema (54.5%), pruritus (55.1%), and dry skin (48.7%), and lesions had the characteristic lichenified or eczematous morphology [10]. In a cohort of 1,650 Argentinian patients with AD, 40% experienced high itch intensity and frequency, and 96% reported bleeding and suppuration [24]. A cross-sectional study in Colombia found that disease distribution was mostly flexural and combined with either eyelid dermatitis, hand eczema, or cheilitis, and that most patients suffered from moderate disease when evaluated using body surface area (BSA) and eczema area and severity index (EASI) score [25]. In a recently published abstract, results from a study evaluating the burden of AD in Brazil, Mexico, and Argentina found that severe pruritus (Worst Pruritus NRS > 7) was reported by 54.4% of patients and with effect on the quality of life close to 50% of the 180 evaluated patients [26].
A Brazilian study evaluated AD patients using the Beck depression inventory, the inventory of stress symptoms for adults, and the dermatology life quality index (DLQI) and found that 38.7% of patients had moderate to severe depressive symptoms and 22.6% had severe depressive symptoms. Furthermore, 73.3% of patients experienced symptoms of psychological stress, and 45.2% reported a significant QoL impairment. Pruritus was one of the main symptoms that contributed to these findings [27]. In Argentina, one survey-based study reported that AD impacted quality of life in 85.6% of participants [24]. Sleep disorders are also a common comorbidity and contribute to reduced QoL, as well as emotional and functional impairment. Latin American children with AD have higher values on the Children’s Sleep Habits Questionnaire (CSHQ) than controls, meaning that they experienced more sleep disorders including sleep anxiety, night awakening, parasomnias, and daytime sleepiness. High CSHQ scores were also found to correlate with increased disease severity [28]. Unfortunately, these psychological factors are not usually adequately addressed in many LA countries, with only 11.8% of AD patients reporting they received therapy or psychological support in the ADAPT study [10].
Clinical assessment of atopic dermatitis utilizes a variety of validated tools. Sanchez et al. found that using international assessment guidelines such as SCOring Atopic Dermatitis (SCORAD) and DLQI significantly reduced the severity of symptoms and improved the quality of life in a cohort of Colombian AD patients [29]. Brazilian studies have shown that 65.6% of patients were classified as having severe disease, and 56% of AD patients had one or more hospitalizations during their lifetime, indicating a need for better disease control [10][21]. Other Latin American studies suggest that comorbidities are linked to increased disease severity, further highlighting the need to accurately assess the disease burden in these patients [19].
6. Therapeutic Management of AD
First-line therapy in most Latin American clinical practice guidelines consists of emollients, baths, and irritant avoidance [17][19]. Medications such as topical steroids and topical calcineurin inhibitors (TCI) are also used as first- and second-line therapy [17][19][30]. Although topical steroids are considered to be easily accessible, efficacy and costs may vary, and they can be associated with undesirable side effects such as cutaneous atrophy and adrenal suppression [17]. TCIs are recommended for use in more sensitive areas such as the face and genitals, although they are not widely used due to cost [17][30].
Phototherapy, oral corticosteroids, and systemic immunosuppressants such as methotrexate (MTX) and cyclosporine are used for more severe cases that do not respond to topical therapy, but each comes with its unique set of challenges [17]. Although phototherapy is an effective and safe method, it is not widely used for dermatitis in Latin America and is not easily accessible for patients who live far away from phototherapy facilities [22][31]. Oral corticosteroids are associated with significant side effects and are not meant for chronic use, although they are often used in this manner in Latin America [22]. For example, a study in Brazil found that corticosteroids were the most frequently used systemic AD treatment, with 32.6% of patients taking oral steroids for a mean duration of 65.4 days [10]. Most systemic immunosuppressants are not approved for AD treatment in Latin American countries and are used off-label [17]. These therapies also require frequent monitoring and may be costly. Guidelines developed by the Latin American Society of Allergy, Asthma, and Immunology AD Committee provided strong recommendations for the use of cyclosporine A and weak recommendations for the use of methotrexate, stating that further studies are needed to evaluate these treatments in Latin America [22]. However, both therapies are still commonly used, with 24.1% of patients on cyclosporine and 13.4% of participants on MTX in a Brazilian cohort of AD patients [10]. Furthermore, a recent Brazilian study reported significant reduction of EASI, SCORAD, and pruritus after 24 weeks of MTX therapy, thus recommending the use of this drug in refractory moderate to severe AD [32]. Biologic agents such as dupilumab are becoming more widely used in Latin America as countries continue to approve its use, with a few countries such as Brazil even approving it for children ages 6 to 11 years [19]. JAK inhibitors such as upadacitinib and baricitinib have also been approved in various Latin American countries [31]. Unfortunately, accessibility is limited due to cost, public vs. private coverage, and lack of objective measures assessing disease severity (which are required for drug approval) [31]. Furthermore, patients may opt to use alternative or complementary therapies, even though randomized controlled trials are needed to prove their efficacy in AD. One study from Brasilia, Brazil showed that 63.5% of children with AD had used alternative therapies such as homeopathy or phytotherapy to manage their disease [33]. Another Colombian study reported that 37% of patients used alternative medicine as a treatment option [25].
References
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; Group, I.P.T.S. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743.
- Yon, J.-A.-L.; Lee, S.-K.; Keng, J.-W.; Chow, S.-C.; Liew, K.-B.; Teo, S.-S.; Shaik Mossadeq, W.M.; Marriott, P.J.; Akowuah, G.A.; Ming, L.C.; et al. Cassia alata (Linnaeus) Roxburgh for Skin: Natural Remedies for Atopic Dermatitis in Asia and Their Pharmacological Activities. Cosmetics 2022, 10, 5.
- Beattie, P.E.; Lewis-Jones, M.S. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br. J. Dermatol. 2006, 155, 145–151.
- Barreto, S.M.; Miranda, J.J.; Figueroa, J.P.; Schmidt, M.I.; Munoz, S.; Kuri-Morales, P.P.; Silva, J.B., Jr. Epidemiology in Latin America and the Caribbean: Current situation and challenges. Int. J. Epidemiol. 2012, 41, 557–571.
- Sole, D.; Mallol, J.; Wandalsen, G.F.; Aguirre, V.; Latin American, I.P.S.G. Prevalence of symptoms of eczema in Latin America: Results of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3. J. Investig. Allergol. Clin. Immunol. 2010, 20, 311–323.
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e412.
- Garcia, E.; Halpert, E.; Borrero, E.; Ibanez, M.; Chaparro, P.; Molina, J.; Torres, M. Prevalence of skin diseases in children 1 to 6 years old in the city of Bogota, Colombia. World Allergy Organ. J. 2020, 13, 100484.
- Sole, D.; Wandalsen, G.F.; Camelo-Nunes, I.C.; Naspitz, C.K.; Group, I.B. Prevalence of symptoms of asthma, rhinitis, and atopic eczema among Brazilian children and adolescents identified by the International Study of Asthma and Allergies in Childhood (ISAAC)—Phase 3. J. Pediatr. Rio J. 2006, 82, 341–346.
- Miot, H.A.; Aoki, V.; Orfali, R.L.; Sole, D.; Mallozi, M.C.; Rodrigues, T.C.; Silverberg, J.I. The (one-year) prevalence of atopic dermatitis in Brazil: A population-based telephone survey. J. Eur. Acad. Dermatol. Venereol. 2023.
- Arruda, L.K.; Yang, A.C.; Aoki, V.; Criado, R.F.; Pires, M.C.; Lupi, O.; Fabricio, L.H.; Richman, D.; Silvi, S. Clinical Features and Disease Management in Adult Patients with Atopic Dermatitis Receiving Care at Reference Hospitals in Brazil: The ADAPT Study. J. Investig. Allergol. Clin. Immunol. 2021, 31, 236–245.
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357.
- Cardenas, G.V.; Iturriaga, C.; Hernandez, C.D.; Tejos-Bravo, M.; Perez-Mateluna, G.; Cabalin, C.; Urzua, M.; Venegas-Salas, L.F.; Fraga, J.P.; Rebolledo, B.; et al. Prevalence of filaggrin loss-of-function variants in Chilean population with and without atopic dermatitis. Int. J. Dermatol. 2022, 61, 310–315.
- Callou, T.M.P.; Orfali, R.L.; Sotto, M.N.; Pereira, N.V.; Zaniboni, M.C.; Aoki, V.; Brito, M.P.; Matsuda, M.; Santo, R.M. Increased expression of Filaggrin and Claudin-1 in the ocular surface of patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 247–254.
- Weiland, S.K.; Husing, A.; Strachan, D.P.; Rzehak, P.; Pearce, N.; Group, I.P.O.S. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup. Environ. Med. 2004, 61, 609–615.
- Orfali, R.L.; da Silva Oliveira, L.M.; de Lima, J.F.; de Carvalho, G.C.; Ramos, Y.A.L.; Pereira, N.Z.; Pereira, N.V.; Zaniboni, M.C.; Sotto, M.N.; da Silva Duarte, A.J.; et al. Staphylococcus aureus enterotoxins modulate IL-22-secreting cells in adults with atopic dermatitis. Sci. Rep. 2018, 8, 6665.
- Orfali, R.L.; Yoshikawa, F.S.Y.; Oliveira, L.; Pereira, N.Z.; de Lima, J.F.; Ramos, Y.A.L.; Duarte, A.; Sato, M.N.; Aoki, V. Staphylococcal enterotoxins modulate the effector CD4(+) T cell response by reshaping the gene expression profile in adults with atopic dermatitis. Sci. Rep. 2019, 9, 13082.
- Borzutzky, A.; Larco, J.I.; Luna, P.C.; McElwee, E.; Pires, M.C.; Rico Restrepo, M.; Saez-de-Ocariz, M.; Sanchez, J. Atopic Dermatitis in Latin America: A Roadmap to Address Data Collection, Knowledge Gaps, and Challenges. Dermatitis 2022, 33, S83–S91.
- Mesquita, K.; Colombini, M.; Duarte, G.; Ferreira, S.B.; Yang, A.; Mallozi, M.; Lupi, O.; Guidacci, M.; Abreu, D.; Paiva, H. Unveiling atopic dermatitis burden in Brazil: A report from clinical assistance perspective. JBES Braz. J. Health Econ./J. Bras. De Econ. Da Saúde 2019, 11, 153–160.
- Sanchez, J.; Cherrez-Ojeda, I.; Galvan, C.; Garcia, E.; Hernandez-Mantilla, N.; Londono Garcia, A.; McElwee, E.; Rico Restrepo, M.; Rivas, E.; Hidalgo, B. The Unmet Needs in Atopic Dermatitis Control in Latin America: A Multidisciplinary Expert Perspective. Dermatol. Ther. 2021, 11, 1521–1540.
- Caraballo, L.; Zakzuk, J.; Lee, B.W.; Acevedo, N.; Soh, J.Y.; Sanchez-Borges, M.; Hossny, E.; Garcia, E.; Rosario, N.; Ansotegui, I.; et al. Particularities of allergy in the Tropics. World Allergy Organ. J. 2016, 9, 20.
- Orfali, R.L.; Shimizua, M.M.; Takaoka, R.; Zaniboni, M.C.; Ishizaki, A.S.; Costa, A.A.; Tiba, A.P.L.; Sato, M.N.; Aoki, V. Atopic dermatitis in adults: Clinical and epidemiological considerations. Rev. Da Assoc. Med. Bras. 2013, 59, 270–275.
- Sanchez, J.; Paez, B.; Macias, A.; Olmos, C.; de Falco, A. Atopic dermatitis guideline. Position paper from the Latin American Society of Allergy, Asthma and Immunology. Rev. Alerg. Mex. 2014, 61, 178–211.
- Alcantara-Neves, N.M.; de SG Britto, G.; Veiga, R.V.; Figueiredo, C.A.; Fiaccone, R.L.; da Conceicao, J.S.; Cruz, A.A.; Rodrigues, L.C.; Cooper, P.J.; Pontes-de-Carvalho, L.C.; et al. Effects of helminth co-infections on atopy, asthma and cytokine production in children living in a poor urban area in Latin America. BMC Res. Notes 2014, 7, 817.
- Echeverria, C.; Angles, M.V.; Larralde, M.; Luna, P.C.; Mazzuoccolo, L.D.; Moreno, P. Impact of atopic dermatitis on quality of life: A large web-based survey from Argentina. Rev. Fac. Cien Med. Univ. Nac. Cordoba 2022, 79, 369–373.
- Sanclemente, G.; Hernandez, N.; Chaparro, D.; Tamayo, L.; Lopez, A.; Colombian Atopic Dermatitis Research, G. Epidemiologic features and burden of atopic dermatitis in adolescent and adult patients: A cross-sectional multicenter study. World Allergy Organ. J. 2021, 14, 100611.
- Jardim Criado, R.F.; Rodrigues, T.; de Campos, L.; Cestari, T.; Maspero, J.; Luna, P.C.; Angles, M.V.; Antila, M. 347 The real-world burden of atopic dermatitis: MEASURE-AD multicountry study results from Brazil, Mexico and Argentina. Br. J. Dermatol. 2023, 188, ljac140-040.
- Castro, C.R.; Andrade, M.E.B.; Pires, R.M.G.; Pires, M.C. Evaluation of depression, stress and quality of life indexes in patients with atopic dermatitis. An. Bras. Dermatol. 2021, 96, 627–629.
- Urrutia-Pereira, M.; Sole, D.; Rosario, N.A.; Neto, H.J.C.; Acosta, V.; Almendarez, C.F.; Avalos, M.M.; Badellino, H.; Berroa, F.; Alvarez-Castello, M.; et al. Sleep-related disorders in Latin-American children with atopic dermatitis: A case control study. Allergol. Immunopathol. 2017, 45, 276–282.
- Sanchez, J.; Toro, Y.; Cardona, R. Clinical impact in the real life of guidelines recommendations for atopic dermatitis in a tropical population (TECCEMA cohort). Rev. Alerg. Mex. 2017, 64, 260–269.
- Aoki, V.; Lorenzini, D.; Orfali, R.L.; Zaniboni, M.C.; Oliveira, Z.N.P.; Rivitti-Machado, M.C.; Takaoka, R.; Weber, M.B.; Cestari, T.; Gontijo, B.; et al. Consensus on the therapeutic management of atopic dermatitis—Brazilian Society of Dermatology. An. Bras. Dermatol. 2019, 94, 67–75.
- Sanchez, J.; Ale, I.S.; Angles, M.V.; Fogelbach, G.G.; Jansen, A.M.; Takaoka, R.; Borzutzky, A. Healthcare Disparities in Atopic Dermatitis in Latin America: A Narrative Review. Dermatol. Ther. 2023, 13, 399–416.
- Samorano, L.P.; Takaoka, R.; Zaniboni, M.C.; Aoki, V. Methotrexate for atopic dermatitis in adults: A prospective study from a reference center in Brazil. J. Dtsch. Dermatol. Ges. 2021, 19, 294–296.
- Aguiar Junior Ndos, R.; Costa, I.M. The use of alternative or complementary medicine for children with atopic dermatitis. An. Bras. Dermatol. 2011, 86, 167–168.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
771
Revisions:
2 times
(View History)
Update Date:
18 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




