Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicole Sparks | -- | 1866 | 2023-05-17 16:43:21 | | | |
| 2 | Conner Chen | Meta information modification | 1866 | 2023-05-18 03:25:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iwobi, N.; Sparks, N.R. Osteogenesis and Its Hormone Regulation. Encyclopedia. Available online: https://encyclopedia.pub/entry/44447 (accessed on 08 February 2026).
Iwobi N, Sparks NR. Osteogenesis and Its Hormone Regulation. Encyclopedia. Available at: https://encyclopedia.pub/entry/44447. Accessed February 08, 2026.
Iwobi, Nneamaka, Nicole R. Sparks. "Osteogenesis and Its Hormone Regulation" Encyclopedia, https://encyclopedia.pub/entry/44447 (accessed February 08, 2026).
Iwobi, N., & Sparks, N.R. (2023, May 17). Osteogenesis and Its Hormone Regulation. In Encyclopedia. https://encyclopedia.pub/entry/44447
Iwobi, Nneamaka and Nicole R. Sparks. "Osteogenesis and Its Hormone Regulation." Encyclopedia. Web. 17 May, 2023.
Copy Citation
Hormones are major contributors to osteogenesis and deviations in hormone expression can lead to an undesired bone formation outcome. Bone development is hormone dependent, with each hormone having its own receptor in bone tissue and controlled by several endocrine glands. Therefore, these pathways are susceptible to endocrine disruption by environmental insults, including EDCs, that can cause osteogenic defects.
osteoblasts
osteogenesis
hormones
1. Introduction
Congenital bone defects are a major public health concern. According to the World Health Organization (WHO) [1], birth defects are the second leading cause of deaths for infants (in 28 days) and children under 5 years of age, resulting in nearly 3.3 million deaths globally [1]. In the United States alone, 1 in every 33 babies are born with a birth defect each year, and accounts for 20% of infant and child mortality. This is a highly concerning issue that needs to be addressed. Congenital malformations can be attributed to genetic and non-genetic factors. Non-genetic factors, particularly environmental factors, are among the most concerning leading to an increased risk of birth defects [2][3]. Therefore, it is imperative to uncover the mechanisms by which environmental toxicants affect bone development and prompt the risk of skeletal defects. Osteogenesis is the process of bone formation, whereby osteoblasts, the bone forming cells, produce a mineralized extracellular matrix during early development, adult bone homeostasis, and bone remodeling after an injury [4]. Osteoblast lineage commitment is tightly controlled by mechanisms including epigenetic, transcription factors, and signaling pathways. Elucidating such genetic processes is key to understanding normal and abnormal bone development. Specifically, understanding how environmental factors contribute to the dysregulation of hormone signaling pathways during osteogenesis will help provide insights into the molecular mechanisms of bone disorders and diseases and the development of diagnostic tools and treatments.
2. Osteogenesis
Bone formation is the result of two processes: intramembranous ossification, which is the formation of flat bone, i.e., thin layers of connective tissue and top of the skull; and endochondral ossification, which is the process by which bone tissue, cartilage, is formed in early fetal development and then replaced with bone later [5][6][7]. Osteoblasts are derived from progenitor neural crest (NC) and mesodermal cells, where NC cells typically go through intramembranous ossification and endochondral ossification for mesoderm derived osteoblasts. A shared precursor between NC and mesoderm cells are mesenchymal stem cells (MSCs), which have the capacity to differentiate into osteoblast, chondrocytes, myoblasts, and adipocytes [8]. Proliferation, matrix maturation, and mineralization are the key stages of osteoblast development which require the expression of distinct osteoblast markers. The most common markers of osteoblast development are alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), type I collagen (COL1A1), osteopontin (OPN), bone sialoprotein (BSP), and osteocalcin (OCN). ALP, RUNX2, and COL1A1, which are early osteoblast markers, and OPN, BSP, and OCN represent later stages of osteoblast differentiation [9][10][11][12]. Exposure to environmental toxicants, such as air pollution, flame retardants, or tobacco products, during these susceptible periods of development can lead to unwanted life-long bone defects, diseases, and disorders [13]. Therefore, it is necessary to understand how exposure can impact the mechanisms of bone development and result in the developmental toxicity of bone.
3. Osteogenesis and Its Hormone Regulation
Hormones are major contributors to osteogenesis and deviations in hormone expression can lead to an undesired bone formation outcome. Bone development is hormone dependent, with each hormone having its own receptor in bone tissue and controlled by several endocrine glands [14]. Therefore, these pathways are susceptible to endocrine disruption by environmental insults, including EDCs, that can cause osteogenic defects.
Thyroid hormone. Thyroid signaling plays an important role in many cells within the human body and is involved in metabolism maintenance as well as body growth and development [15][16]. Thyroid hormones (TH) aid in osteoblast formation in the early stages of skeletal development, as well as bone growth and maturation. There are three subtypes make up thyroid receptors (TRs): TRα1, TRβ1, and TRβ2, where TRα1 and TRβ1 are most expressed in bone [17]. Thyroid hormones positively regulate osteoblast differentiation via bone morphogenetic protein (BMP) and IGF1 signaling as seen in Figure 1. Positive osteoblast development is supported through the BMP/SMAD signaling pathway observed in mouse osteoblasts treated with TH (T3). Hormones triidothyronin (T3) and thyroxine (T4) are the two main forms of TH, where T4 is the primary form. Secondary, T3 is produced through the enzymatic conversion of T4 [15][16][17]. T3 led to BMP activation and SMAD1/5/8 phosphorylation that yielded enhanced osteoblast differentiation potential [18]. In differentiating MC3T3-E1 pre-osteoblast cells, T3 and T4 treatments increased Igf-1 mRNA levels supporting osteoblast differentiation [19]. TH has been shown to regulate osteoblast differentiation through WNT/β-catenin signaling pathway stimulation or inhibition. The crosstalk between THs and WNT signaling needs to be fully delineated in bone compared to more established mechanisms in other tissues [20]. When treated with T3, WNT signaling activity was decreased in mouse osteoblast cells [21]. In vivo, β-catenin levels were stabilized with a mutant thyroid hormone receptor to activate WNT signaling in the presence of TH and increase bone mass [21]. In contrast, Tsourdi et al. [22] found WNT signaling inhibitor DKK1 serum levels were increased in hypothyroid mice, which correlated with decreased bone formation [22]. In addition, BMP signaling can regulate WNT/β-catenin signaling to regulate osteoblast differentiation and bone formation [23].
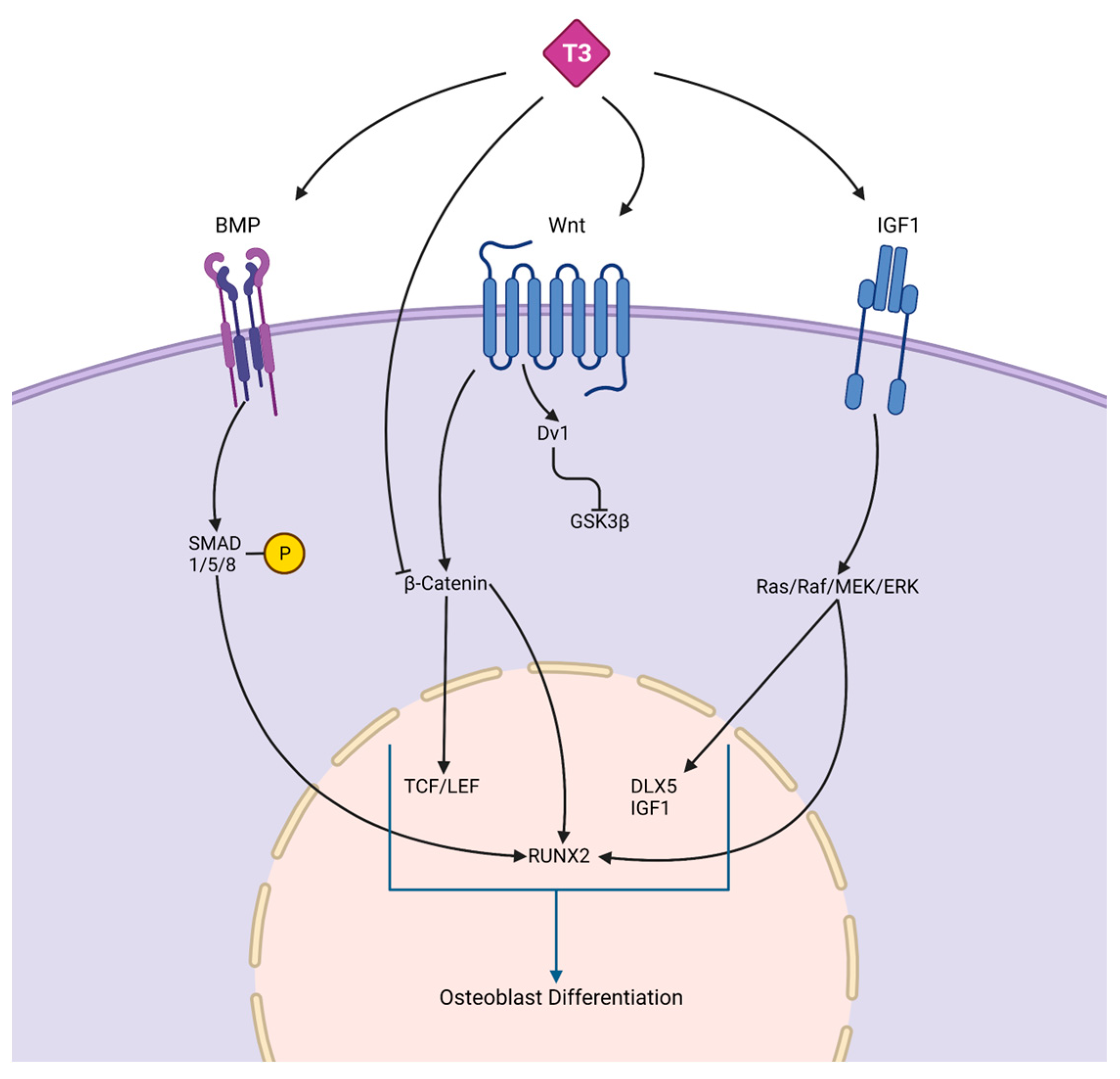
Figure 1. Crosstalk between thyroid hormones and signaling pathways. The schematic depicts the complex interaction between thyroid hormones, such as T3, and BMP, WNT, and IGF1 signaling, which are responsible for osteoblast differentiation. BMPs bind to receptors on osteoblast progenitors to activate SMADS, leading to increased RUNX2. RUNX2 is an osteogenesis specific transcription factor that promotes osteogenic related genes expression. In the WNT/β-catenin pathway, TH regulates osteoblast differentiation through either inhibiting β-catenin, which prevents osteoblast differentiation, or binding to WNT, which promotes osteoblast differentiation through accumulating β-catenin, increasing the levels of TCF/LEF and RUNX2. IGF-1 receptor-induced osteogenesis activates the Ras/Raf/MEK/ERK pathway, leading to an increase in osteogenic genes.
Parathyroid hormone. The parathyroid hormone (PTH) is an 84-amino acid peptide hormone secreted by the parathyroid glands. PTH mainly acts on the bone and kidney. It is crucial for osteoblast differentiation and post-natal bone calcium and phosphorus maintenance. PTH-related protein (PTHrP) is crucial for endochondral bone formation during pre- and post-natal bone formation [24]. PTH and PTHrP are similar peptide hormones that share interaction with a single common receptor, PTH type I receptor (PTH1R), predominantly through cyclic adenosine monophosphate/protein kinase A (cAMP) [24]. These receptors are found in progenitor and osteoblast cells. Figure 2 demonstrates PTH stimulation of osteoblast development mediated through the cyclic AMP and BMP signaling pathways downstream of the PTH1R [24][25][26][27][28]. PTH-induced BMP signaling stimulation phosphorylates SMAD1, which prevents the inhibitory effect of NOGGIN and increases the endocytosis of PTH/PTH1R/LRP6, which induces β-catenin stabilization. Increased PTH enhances MSC differentiation into osteoblasts through BMP signaling [29]. PTH and PTHrP stimulate pro-osteogenic genes, RUNX2, ALP, and OCN. Expressed at the correct timing of development, PTH increases osteoblast differentiation. PTH receptor (PTHR) deletion in bone marrow cells resulted in an increase in bone marrow adiposity and bone resorption, along with a physically visible low bone mass in mice [30].
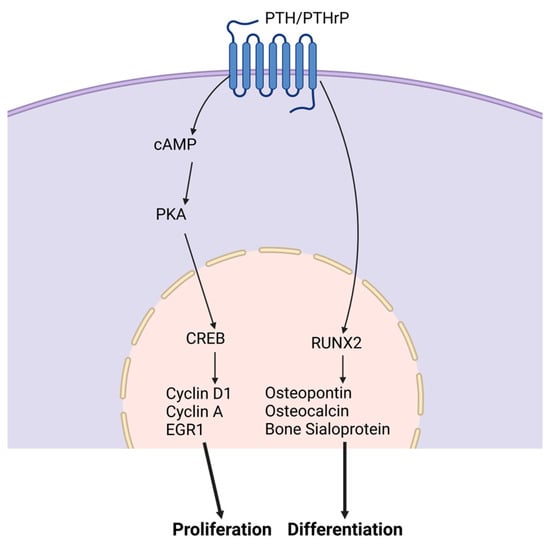
Figure 2. PTH and PTHrP signaling pathways. PTH and PTHR stimulate the proliferation and differentiation of osteoblasts. To promote proliferation, cAMP is activated followed by an increase in PKA levels. These cellular outcomes are mediated through elevation of intracellular cAMP via the PTH receptor. This increase leads to the activation of CREB in osteogenic cells.
Vitamin D. Cells of the osteoblast lineage are responsive to systemic hormones such as 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). Vitamin D is a steroid hormone with an essential role in bone metabolism. The active form of vitamin D, 1,25(OH)2D3, binds to the vitamin D receptor (VDR), which heterodimerizes with the retinoic X receptor (RXR) and activates target genes. Increased vitamin D levels enhance bone formation by promoting osteoblast differentiation and mineralization [31], provided in Figure 3. Mouse overexpression of the human VDR gene increased cortical and trabecular bone supporting 1,25(OH)2D3 impact on bone. Similarly, in antigen-induced arthritis (AIA) rats that have significant bone loss, 1,25(OH)2D3 treatment increased trabecular bone volume compared to untreated AIA rats and healthy control rats [32]. However, the 1,25(OH)2D3 administration did not have any anti-inflammatory effect. MSCs treated with exogenous 1,25(OH)2D3 differentiate into osteoblasts that produce a mineralized extracellular matrix that enhanced differentiation. Cell culture medium supplementation with 1,25(OH)2D3 triggers human embryonic and induces pluripotent stem cell osteoblast differentiation [33][34]. Human MSC and mouse embryonic stem cell studies resemble human pluripotent stem cell studies showing increased osteoblast differentiation with 1,25(OH)2D3 treatment [35][36].
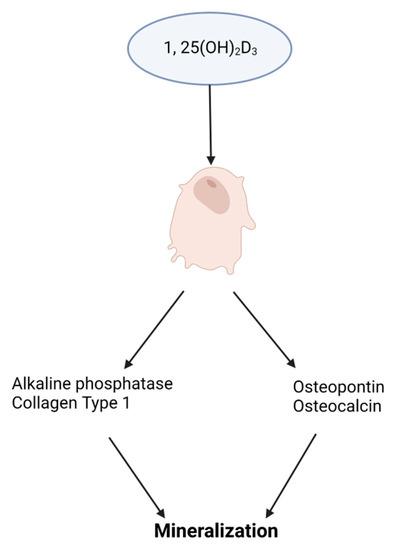
Figure 3. Impact of vitamin D3, 1,25(OH)2D3, on osteoblast differentiation. A schematic illustrating 1,25(OH)2D3 stimulating the expression of osteoblast promoting genes for extracellular matrix mineralization.
Estrogen. Estrogen is a key hormone involved in the development and homeostasis of bone tissue in both males and females. Estradiol is the most potent estrogenic hormone in the human body. Estrogen action is controlled by two main estrogen receptors (ER), alpha and beta (ERα and ERβ), encoded by ESR1 and ESR2, respectively. It regulates gene expression, metabolism, cell growth, and proliferation by acting through cytoplasmic signaling pathways or activating transcription in the nucleus, seen in Figure 4A. Estrogens bind to their receptors in the nucleus, acting as transcription factors regulating the expression of target genes. Estrogens can also bind to their receptors outside of the nucleus activating signaling pathways in the cytoplasm. The cytoplasmic signaling pathway is activated by estrogen and growth factors and acts though the kinase signaling cascade which phosphorylates substrate proteins and transcription factors [37][38]. Estrogen treatment has been found to induce osteoblast differentiation and activate ERK/JNK signaling, cell cycle regulation, cell growth, and the survival pathway in rat bone marrow-MSCs. In the WNT pathway, activation of ER signaling induces osteogenic differentiation and matrix mineralization [39][40][41]. A deficiency in estrogen is associated with reduced bone formation. Estrogen prevents bone loss by inhibiting osteoclast—the bone-resorbing cell—activity. Esr1 deletion in female mice osteoclasts resulted in increased osteoclast numbers and reduced trabecular bone mass [42]. Nakamura et al. [42] concluded that estrogen’s osteoprotective effect was through the expression of Fas ligand (FasL) in osteoblasts that induced osteoclast apoptosis, as depicted in Figure 4B [42]. Another mechanism of estrogen-mediated osteoclast inhibition involves the receptor activator of nuclear factor κB ligand (RANKL) regulation [42]. RANKL is essential for osteoclast differentiation and can be suppressed by osteoprotegerin (OPG). In estrogen deficient C57BL/6 mice, increased bone resorption activity was found due to the lack of ERα-mediated suppression of Rankl expression in bone lining cells, which RANKL binds to RANK on the surface of osteoclast progenitors to initiate the bone breakdown [43]. In addition, estrogen deficiency has been linked to oxidative stress and inflammation, which can increase bone resorption [44].
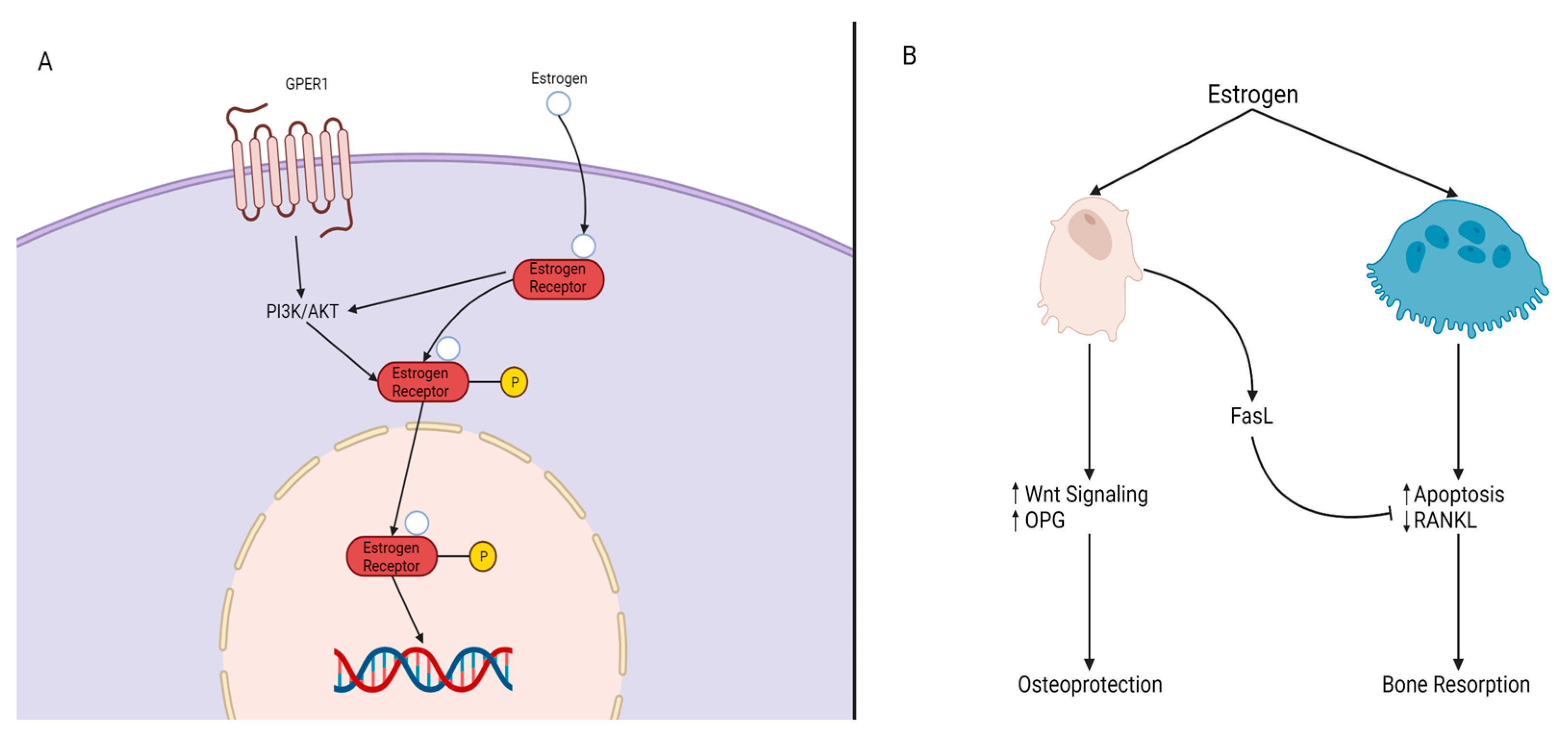
Figure 4. Mechanisms of Estrogen Signaling. (A) P13K/AKT is impacted by either G protein-coupled estrogen receptor, GPER, or by the estrogen/estrogen receptor (ER) complex, resulting in the phosphorylation of the estrogen/ER complex. The complex then crosses the nucleus and elicits its response on target genes. (B) Estrogen impacts both osteoblasts and osteoclasts. In the presence of estrogen, osteoblasts experience an increase of WNT signaling and OPG levels and produce FasL. FasL inhibits osteoclast activity through reduced RANKL expression and osteoclast apoptosis, resulting in osteoblast protection and maintenance (osteoprotection). The absence of estrogen leads to bone resorption.
References
- World Health Organization. Congenital Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/birth-defects (accessed on 26 April 2023).
- World Health Organization. Birth Defects Surveillance: A Manual for Programme Managers, 2nd ed.; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/publications-detail-redirect/9789240015395 (accessed on 31 March 2023).
- Källén, B. Genetic and Non-genetic Factors in the Origin of Congenital Malformations. In Epidemiology of Human Congenital Malformations; Springer: Cham, Switzerland, 2014; pp. 5–8.
- Breeland, G.; Sinkler, M.A.; Menezes, R.G. Embryology, Bone Ossification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539718/ (accessed on 31 March 2023).
- Sera, S.R.; Zur Nieden, N.I. microRNA Regulation of Skeletal Development. Curr. Osteoporos. Rep. 2018, 4, 353–366.
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and Transcriptional Control of Bone Formation. Oral Maxillofac. Surg. Clin. N. Am. 2011, 3, 283–293.
- Jin, S.W.; Sim, K.B.; Kim, S.D. Development and Growth of the Normal Cranial Vault: An Embryologic Review. J. Korean Neurosurg. Soc. 2016, 59, 192–196.
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In Vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus. Med. Hemotherapy 2008, 35, 228–238.
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754.
- Zur Nieden, N.I.; Kempka, G.; Rancourt, D.E.; Ahr, H.J. Induction of Chondro-, Osteo- and Adipogenesis in Embryonic Stem Cells by Bone Morphogenetic Protein-2: Effect of Cofactors on Differentiating Lineages. BMC Dev. Biol. 2005, 5, 21.
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092.
- Zur Nieden, N.I.; Price, F.D.; Davis, L.A.; Everitt, R.E.; Rancourt, D.E. Gene Profiling on Mixed Embryonic Stem Cell Populations Reveals a Biphasic Role for β-Catenin in Osteogenic Differentiation. Mol. Endocrinol. 2007, 3, 674–685.
- Darbre, P.D. Overview of Air Pollution and Endocrine Disorders. Int. J. Gen. Med. 2018, 11, 191–207.
- Yaglova, N.V.; Yaglov, V.V. Endocrine Disruptors as a New Etiologic Factor of Bone Tissue Diseases (Review). Sovrem. Tekhnologii Med. 2021, 2, 84–94.
- Sinha, R.; Yen, P.M. Cellular Action of Thyroid Hormone. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.E., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK285568/ (accessed on 22 March 2023).
- Goel, R.; Raju, R.; Maharudraiah, J.; Kumar, G.S.S.; Ghosh, K.; Kumar, A.; Lakshmi, T.P.; Sharma, J.; Sharma, R.; Balakrishnan, L.; et al. A Signaling Network of Thyroid-Stimulating Hormone. J. Proteom. Bioinform. 2011, 4, 10.
- Bassett, J.H.D.; Williams, G.R. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev. 2016, 37, 135–187.
- Lademann, F.; Weidner, H.; Tsourdi, E.; Kumar, R.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Rauner, M. Disruption of BMP Signaling Prevents Hyperthyroidism-Induced Bone Loss in Male Mice. J. Bone Miner. Res. 2020, 35, 2058–2069.
- Cheng, S.; Xing, W.; Pourteymoor, S.; Mohan, S. Effects of Thyroxine (T4), 3,5,3’-Triiodo-L-Thyronine (T3) and Their Metabolites on Osteoblast Differentiation. Calcif. Tissue Int. 2016, 99, 435–442.
- Lademann, F.; Tsourdi, E.; Hofbauer, L.C.; Rauner, M. Thyroid Hormone Actions and Bone Remodeling—The Role of the Wnt Signaling Pathway. Exp. Clin. Endocrinol. Diabetes 2020, 128, 450–454.
- O’Shea, P.J.; Kim, D.W.; Logan, J.G.; Davis, S.; Walker, R.L.; Meltzer, P.S.; Cheng, S.Y.; Williams, G.R. Advanced Bone Formation in Mice with a Dominant-Negative Mutation in the Thyroid Hormone Receptor β Gene Due to Activation of Wnt/β-Catenin Protein Signaling. J. Biol. Chem. 2012, 287, 17812–17822.
- Tsourdi, E.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Rauner, M. Hyperthyroidism and Hypothyroidism in Male Mice and Their Effects on Bone Mass, Bone Turnover, and the Wnt Inhibitors Sclerostin and Dickkopf-1. Endocrinology 2015, 156, 3517–3527.
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Chen, D.; Tsao, C.; Deng, H.-W.; Zhao, M. Wnt/β-Catenin Signaling Activates Bone Morphogenetic Protein 2 Expression in Osteoblasts. Bone 2013, 52, 145–156.
- Martin, T.J.; Sims, N.A.; Seeman, E. Physiological and Pharmacological Roles of PTH and PTHrP in Bone Using Their Shared Receptor, PTH1R. Endocr. Rev. 2021, 42, a011148.
- Pacifici, R. Role of Gut Microbiota in the Skeletal Response to PTH. J. Clin. Endocrinol. Metab. 2021, 106, 636–645.
- Silva, B.C.; Bilezikian, J.P. Parathyroid Hormone: Anabolic and Catabolic Actions on the Skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50.
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851.
- Cheloha, R.W.; Gellman, S.H.; Vilardaga, J.P.; Gardella, T.J. PTH Receptor-1 Signalling—Mechanistic Insights and Therapeutic Prospects. Nat. Rev. Endocrinol. 2015, 12, 712–724.
- Yu, B.; Zhao, X.; Yang, C.; Crane, J.; Xian, L.; Lu, W.; Wan, M.; Cao, X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J. Bone Miner. Res. 2012, 9, 2001–2014.
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672.
- Haussler, M.R.; Livingston, S.; Sabir, Z.L.; Haussler, C.A.; Jurutka, P.W. Vitamin D Receptor Mediates a Myriad of Biological Actions Dependent on Its 1,25-Dihydroxyvitamin D Ligand: Distinct Regulatory Themes Revealed by Induction of Klotho and Fibroblast Growth Factor-23. JBMR Plus 2020, 5, e10432.
- Oelzner, P.; Petrow, P.K.; Wolf, G.; Bräuer, R. 1,25-Dihydroxyvitamin D3 Prevents Bone Loss of the Secondary Spongiosa in Arthritic Rats by an Increase of Bone Formation and Mineralization and Inhibition of Bone Resorption. BMC Musculoskelet. Disord. 2014, 15, 345.
- Sparks, N.R.L.; Martinez, I.K.C.; Soto, C.H.; zur Nieden, N.I. Low Osteogenic Yield in Human Pluripotent Stem Cells Associates with Differential Neural Crest Promoter Methylation. Stem Cells 2018, 36, 349–362.
- Sparks, N.R.L.; Walker, L.M.; Sera, S.R.; Madrid, J.V.; Hanna, M.; Dominguez, E.C.; zur Nieden, N.I. Sidestream Smoke Extracts from Harm-Reduction and Conventional Camel Cigarettes Inhibit Osteogenic Differentiation via Oxidative Stress and Differential Activation of Intrinsic Apoptotic Pathways. Antioxidants 2022, 11, 2474.
- zur Nieden, N.I.; Kempka, G.; Ahr, H.J. In Vitro Differentiation of Embryonic Stem Cells into Mineralized Osteoblasts. Differentiation 2003, 71, 18–27.
- Lou, Y.R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3 Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 42816.
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The Role of Estrogen and Androgen Receptors in Bone Health and Disease. Nat. Rev. 2013, 9, 699–712.
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030.
- Thomas, S.; Jaganathan, B.G. Signaling Network Regulating Osteogenesis in Mesenchymal Stem Cells. Cell Commun. Signal. 2022, 16, 47–61.
- Wan, M.; Yang, C.; Li, J.; Wu, X.; Yuan, H.; Ma, H.; He, X.; Nie, S.; Chang, C.; Cao, X. Parathyroid Hormone Signaling through Low-Density Lipoprotein-Related Protein 6. Genes Dev. 2008, 22, 2968–2979.
- Yang, A.; Suh, W.I.; Kang, N.K.; Lee, B.; Chang, Y.K. MAPK/ERK and JNK Pathways Regulate Lipid Synthesis and Cell Growth of Chlamydomonas Reinhardtii under Osmotic Stress, Respectively. Sci. Rep. 2018, 8, 13857.
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823.
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460.
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
18 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




