
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jessica Kearney | -- | 2930 | 2023-05-17 15:43:26 | | | |
| 2 | Beatrix Zheng | + 10 word(s) | 2940 | 2023-05-18 03:25:50 | | |
Video Upload Options
The key approach to reduce diabetic kidney disease (DKD)-mediated end-stage renal disease (ESRD) is to prevent and delay the renal function decline, as once a fall in renal function occurs, it is difficult to regain, apart from when normoglycemic conditions are implemented for a long time, such as following pancreas transplantation. In parallel to lifestyle, glycemic and blood pressure control, all cornerstones for the prevention of DKD, the researchers outline five major treatment “pillars” that possess major renal protective properties
1. Overview
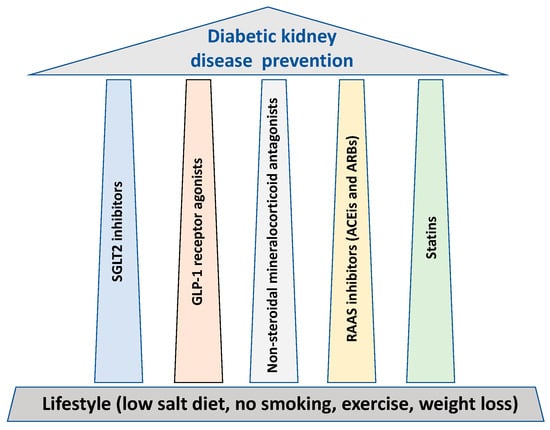
2. SGLT2 Inhibitors
Side Effects
3. GLP1 Receptor Agonists
Side Effects
4. Non-Steroidal Mineralocorticoid Receptor Antagonists
Side Effects
5. RAAS Inhibitors
Side Effects
6. Statins
An inverse relationship exists between GFR and cardiovascular disease, with cardiovascular disease being the predominant cause of increased mortality in patients with CKD [61]. Renal dysfunction alters the composition of lipids to a more atherogenic profile; hypertriglyceridemia, reduced HDL cholesterol, and variable LDL and total cholesterol are seen [62]. Dyslipidemia per se is also a recognized risk for CKD and its progression [63]. Association of British Clinical Diabetologist and UK Renal Association guidelines recommend annual lipid profiles for patients with DKD [64].
Statins are 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors that act to disrupt the liver’s production of cholesterol. NICE guidelines state that adults with CKD should be offered a statin to reduce their risk of cardiovascular events [8]. A renoprotective effect of statins, independent from cholesterol lowering, has also been postulated and a reduction inmicroalbuminuria has been observed [101–105]. Atorvastatin has been seen to reduce cardiovascular disease by 42% in those with GFR 30–60 mL/min/1.73 m2 [106]. The Association of British Clinical Diabetologist and UK Renal Association suggest the use of a statin in patients with DKD and that treatment targets should be a total cholesterol of 4 mmol/L, non-HDL cholesterol 2.5 mmol/L, and LDL cholesterol to 2 mmol/L [100].
Side-Effects
Listed side-effects of statins include myalgia, gastrointestinal symptoms, hyperglycemia, nasopharyngitis, headache, and hepatotoxicity [65]. NICE guidelines advise measuring liver function tests at 3 and 12 months after initiation of treatment. Many statins are metabolized renally and so their doses should be reduced at lower creatinine clearance levels. Statin use can cause myopathy and rhabdomyolysis that can lead to their discontinuation; however, the rates of these adverse effects are low (1.6 cases per 100,000 person years and 5 cases per 100,000 person years, respectively) [66]. If symptoms of myalgia or cramps occur then creatinine kinase levels should be measured, and if they are >5 times the upper limit of normal, then a statin should be held [65].
Conclusion
The fight against diabetic kidney disease is centred on the prevention of its development and progression. In recent years, significant new tools have become available to prevent and treat DKD. Prevention requires aggressive treatment and close follow up of patients with diabetes. Early intervention will improve patients' health outcomes, quality of life, and health- and society-related costs. Studies aimed at better understanding the mechanisms of these new molecules may open new opportunities for patient's personalized treatments and for the development of new therapeutic targets.
References
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107.
- Crapo, P.A. Simple versus complex carbohydrate use in the diabetic diet. Annu. Rev. Nutr. 1985, 5, 95–114.
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551.
- Zainordin, N.A.; Eddy Warman, N.A.; Mohamad, A.F.; Abu Yazid, F.A.; Ismail, N.H.; Chen, X.W.; Koshy, M.; Abdul Rahman, T.H.; Mohd Ismail, N.; Abdul Ghani, R. Safety and efficacy of very low carbohydrate diet in patients with diabetic kidney disease-A randomized controlled trial. PLoS ONE 2021, 16, e0258507.
- Onyenwenyi, C.; Ricardo, A.C. Impact of Lifestyle Modification on Diabetic Kidney Disease. Curr. Diabetes Rep. 2015, 15, 60.
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794.
- Solini, A. Role of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Acta Diabetol. 2016, 53, 863–870.
- Ferrannini, E.; Solini, A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat. Rev. Endocrinol. 2012, 8, 495–502.
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005, 54, 3427–3434.
- Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front. Med. 2021, 8, 777861.
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128.
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657.
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334.
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704.
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306.
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446.
- National Institute for Health and Care Excellence. Diabetes-Type 2. 2023. Available online: https://cks.nice.org.uk/topics/diabetes-type-2/management/management-adults (accessed on 20 April 2023).
- Georgianos, P.I.; Agarwal, R. Ambulatory Blood Pressure Reduction With SGLT-2 Inhibitors: Dose-Response Meta-analysis and Comparative Evaluation with Low-Dose Hydrochlorothiazide. Diabetes Care 2019, 42, 693–700.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461.
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098.
- Lo, K.B.; Gul, F.; Ram, P.; Kluger, A.Y.; Tecson, K.M.; McCullough, P.A.; Rangaswami, J. The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2020, 10, 1–10.
- Rossing, P.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: An update based on rapidly emerging new evidence. Kidney Int. 2022, 102, 990–999.
- Cherney, D.Z.I.; Cooper, M.E.; Tikkanen, I.; Pfarr, E.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Lund, S.S. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018, 93, 231–244.
- Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 2015, 66, 255–270.
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diabetes Rep. 2022, 22, 39–52.
- Garofalo, C.; Borrelli, S.; Liberti, M.E.; Andreucci, M.; Conte, G.; Minutolo, R.; Provenzano, M.; De Nicola, L. SGLT2 Inhibitors: Nephroprotective Efficacy and Side Effects. Medicina 2019, 55, 268.
- Musso, G.; Saba, F.; Cassader, M.; Gambino, R. Diabetic ketoacidosis with SGLT2 inhibitors. BMJ 2020, 371, m4147.
- Medicines and Healthcare Products Regulatory Agency. Drug Safety Update. 2020. Available online: https://www.gov.uk/drug-safety-update/sglt2-inhibitors-monitor-ketones-in-blood-during-treatment-interruption-for-surgical-procedures-or-acute-serious-medical-illness (accessed on 20 April 2023).
- Dashora, U.; Gregory, R.; Winocour, P.; Dhatariya, K.; Rowles, S.; Macklin, A.; Rayman, G.; Nagi, D.; Whitehead, K.; Beba, H.; et al. Association of British Clinical Diabetologists (ABCD) and Diabetes UK joInt. position statement and recommendations for non-diabetes specialists on the use of sodium glucose co-transporter 2 inhibitors in people with type 2 diabetes (January 2021). Clin. Med. 2021, 21, 204–210.
- Yu, J.H.; Park, S.Y.; Lee, D.Y.; Kim, N.H.; Seo, J.A. GLP-1 receptor agonists in diabetic kidney disease: Current evidence and future directions. Kidney Res. Clin. Pract. 2022, 41, 136–149.
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742.
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135.
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102.
- Kristensen, S.L.; Rorth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Kober, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785.
- Granata, A.; Maccarrone, R.; Anzaldi, M.; Leonardi, G.; Pesce, F.; Amico, F.; Gesualdo, L.; Corrao, S. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: State of the art. Clin. Kidney J. 2022, 15, 1657–1665.
- Buse, J.B.; Nauck, M.; Forst, T.; Sheu, W.H.; Shenouda, S.K.; Heilmann, C.R.; Hoogwerf, B.J.; Gao, A.; Boardman, M.K.; Fineman, M.; et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): A randomised, open-label study. Lancet 2013, 381, 117–124.
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662.
- Patoulias, D.I.; Boulmpou, A.; Teperikidis, E.; Katsimardou, A.; Siskos, F.; Doumas, M.; Papadopoulos, C.E.; Vassilikos, V. Cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors: A meta-analysis of cardiovascular outcome trials. World J. Cardiol. 2021, 13, 585–592.
- Brunton, S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: Is one approach more successful or preferable than the other? Int. J. Clin. Pract. 2014, 68, 557–567.
- Kawanami, D.; Takashi, Y. GLP-1 Receptor Agonists in Diabetic Kidney Disease: From Clinical Outcomes to Mechanisms. Front. Pharmacol. 2020, 11, 967.
- Wharton, S.; Davies, M.; Dicker, D.; Lingvay, I.; Mosenzon, O.; Rubino, D.M.; Pedersen, S.D. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: Recommendations for clinical practice. Postgrad. Med. 2022, 134, 14–19.
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230.
- Zhang, M.Z.; Bao, W.; Zheng, Q.Y.; Wang, Y.H.; Sun, L.Y. Efficacy and Safety of Finerenone in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2022, 13, 819327.
- Bakris, G.; Filippatos, G.S.; Farmakis, D.; Epstein, M.; Pitt, B. Aldosterone Antagonists and CVD. 2021. Available online: https://www.acc.org/latest-in-cardiology/articles/2021/07/19/13/42/aldosterone-antagonists-and-cvd (accessed on 20 April 2023).
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161.
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229.
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263.
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484.
- National Institute for Health and Care Excellence. British National Formulary. Finerenone. 2022. Available online: https://bnf.nice.org.uk/drugs/finerenone (accessed on 20 April 2023).
- Gnudi, L.; Gentile, G.; Ruggenenti, P. The patient with diabetes mellitus. In Oxford Textbook of Clinical Nephrology; Turner, N., Lamiere, N., Goldsmith, D.J., Wineearls, C.G., Himmelfarb, J., Remuzzi, G., Eds.; Oxford University Press: Oxford, UK, 2016; Volume 2, pp. 1199–1247.
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462.
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903.
- Navaneethan, S.D.; Nigwekar, S.U.; Sehgal, A.R.; Strippoli, G.F. Aldosterone antagonists for preventing the progression of chronic kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 542–551.
- Bianchi, S.; Bigazzi, R.; Campese, V.M. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006, 70, 2116–2123.
- Van den Meiracker, A.H.; Baggen, R.G.; Pauli, S.; Lindemans, A.; Vulto, A.G.; Poldermans, D.; Boomsma, F. Spironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal function. J. Hypertens. 2006, 24, 2285–2292.
- National Institute for Health and Care Excellence. Angiotensin-Convertine Enzyme Inhibitors. 2022. Available online: https://cks.nice.org.uk/topics/hypertension/prescribing-information/angiotensin-converting-enzyme-inhibitors (accessed on 20 April 2023).
- Khosla, N.; Kalaitzidis, R.; Bakris, G.L. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am. J. Nephrol. 2009, 30, 418–424.
- Weir, M.R.; Rolfe, M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin. J. Am. Soc. Nephrol. 2010, 5, 531–548.
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305.
- Visconti, L.; Benvenga, S.; Lacquaniti, A.; Cernaro, V.; Bruzzese, A.; Conti, G.; Buemi, M.; Santoro, D. Lipid disorders in patients with renal failure: Role in cardiovascular events and progression of chronic kidney disease. J. Clin. Transl. Endocrinol. 2016, 6, 8–14.
- Schaeffner, E.S.; Kurth, T.; Curhan, G.C.; Glynn, R.J.; Rexrode, K.M.; Baigent, C.; Buring, J.E.; Gaziano, J.M. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 2003, 14, 2084–2091.
- Zac-Varghese, S.; Mark, P.; Winocour, P.; Association of British Clinical Diabetologist and UK Renal Association. Clinical Practice Guidelines for Management of Lipids in Adults with Diabetic Kidney Disease. 2021. Available online: https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/Management-of-lipids-in%20adults-with-DKD.pdf (accessed on 20 April 2023).
- National Institute for Health and Care Excellence. Lipid Modification and Cardiovascular Disease Prevention. 2023. Available online: https://cks.nice.org.uk/topics/lipid-modification-cvd-prevention (accessed on 20 April 2023).
- Ezad, S.; Cheema, H.; Collins, N. Statin-induced rhabdomyolysis: A complication of a commonly overlooked drug interaction. Oxf. Med. Case Rep. 2018, 2018, omx104.




