Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Pérez de la Lastra | -- | 2873 | 2023-05-17 13:35:43 | | | |
| 2 | Rita Xu | Meta information modification | 2873 | 2023-05-18 05:40:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pérez De La Lastra, J.M.; Curieses Andrés, C.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Hydroxytyrosol and Arginine. Encyclopedia. Available online: https://encyclopedia.pub/entry/44438 (accessed on 06 March 2026).
Pérez De La Lastra JM, Curieses Andrés CM, Andrés Juan C, Plou FJ, Pérez-Lebeña E. Hydroxytyrosol and Arginine. Encyclopedia. Available at: https://encyclopedia.pub/entry/44438. Accessed March 06, 2026.
Pérez De La Lastra, José Manuel, Celia María Curieses Andrés, Celia Andrés Juan, Francisco J. Plou, Eduardo Pérez-Lebeña. "Hydroxytyrosol and Arginine" Encyclopedia, https://encyclopedia.pub/entry/44438 (accessed March 06, 2026).
Pérez De La Lastra, J.M., Curieses Andrés, C.M., Andrés Juan, C., Plou, F.J., & Pérez-Lebeña, E. (2023, May 17). Hydroxytyrosol and Arginine. In Encyclopedia. https://encyclopedia.pub/entry/44438
Pérez De La Lastra, José Manuel, et al. "Hydroxytyrosol and Arginine." Encyclopedia. Web. 17 May, 2023.
Copy Citation
Phytochemicals from plant extracts are becoming increasingly popular in the world of food science and technology because they have positive effects on human health. In particular, several bioactive foods and dietary supplements are being investigated as potential treatments for chronic COVID. Hydroxytyrosol (HXT) is a natural antioxidant, found in olive oil, with antioxidant anti-inflammatory properties that has been consumed by humans for centuries without reported adverse effects.
arginine
hydroxytyrosol
innate and adaptive immunity
1. Introduction
Humanity is always exposed to the emergence of new pathogens that can compromise its economy and survival, as was the case with the pandemic caused by the SARS-CoV-2 virus [1]. According to the WHO (https://covid19.who.int/), accessed on 1 April 2023, 762 million people worldwide have had COVID-19 and the number of deaths is 6,893,190, with the highest number of reported cases and deaths in the United States of America [2]. The current coronavirus disease (COVID-19) is classified as a primary respiratory and vascular disease that can cause endothelial dysfunction (ED), acute lung injury (ALI), and acute respiratory distress syndrome (ARDS) in extreme situations [3]. The mortality rate among COVID-19 survivors is relatively low, but some people develop persistent symptoms that doctors called “long COVID” or “post-COVID syndrome” (PCS) [4]. Long-term COVID is characterized by a constellation of respiratory, cardiovascular, gastrointestinal, and neurological signs and symptoms, including dyspnea, fatigue, cardiac arrhythmias, heartburn, and memory and concentration problems (sometimes known as “brain fog”) with a substantial impact on quality of life [5]. Several mechanisms involved in the pathogenesis of long COVID are currently being explored, including viral persistence, chronic inflammation, autoimmune disease, metabolic and redox balance disturbances, and endothelial dysfunction [6]. A study on patients with COVID-19, who were followed up to 6 months after the disease, found that approximately 30% reported persistent post-COVID symptoms [7]. Although the development of long-term symptoms following SARS-CoV-2 infection may seem new or surprising, it is in fact an expected phenomenon. A majority of the viral or bacterial pathogens already studied were associated with the development of chronic symptoms in a subset of patients who have been infected [8]. These patients suffer from prolonged COVID or post-acute sequelae (PASC) of COVID-19 [9][10].
Some PASC patients meet the diagnostic criteria for those suffering from myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a form of neuroinflammation characterized by chronic debilitating symptoms such as severe fatigue, musculoskeletal pain, and discomfort following physical exertion or exercise [10][11]. The delay in developing specific treatments for long COVID is due to heterogeneity inthe clinical symptoms, so treatment has focused primarily on symptomatic medications and healthy lifestyle suggestions [12].
2. Plant Polyphenols and Flavonoids—Importance of the Concept of Bioavailability in the Function of Polyphenols
There are three types of phenols, which can be distinguished by their chemical composition: polyphenols (which include tannins and flavonoids), simple phenols (which include phenolic acids), and a third class that includes everything else [13]. Polyphenols are a category of plant-based chemical compounds characterized by the presence of multiple phenolic units or building blocks per molecule [14]. Antioxidants such as polyphenols can help prevent or neutralize free radical damage when it occurs [15][16]. They also impart colour to edible plants such as flowers, fruits, and vegetables. Polyphenols are capable of modulating biological events in different diseases, based on their antioxidant and anti-inflammatory capacity. Electrostatic interactions, hydrogen bonds, and ionic bonds between phenolic hydroxyl groups and the positively charged amino groups in proteins are possible. Targeting enzymatic sources of ROS without affecting the physiological redox state is an important purpose [17][18].
In general, any phytochemical with a 15-carbon (C6-C3-C6) backbone is called a flavonoid, and more than 4500 different flavonoids have been isolated from plants. They can be classified into several subgroups, such as flavones, flavanones, flavonols, dihydroflavonols, isoflavonoids aurones, chalcones, biflavonoids, etc. [19]. Olive plants contain three primary phenolic chemicals: oleuropein (OLE), hydroxytyrosol (HXT), and tyrosol [20][21]. Oleuropein, a heterosidic ester of β-glucosylatedelenolic acid and 3,4-dihydroxy-phenylethanol, is derived from the secondary metabolism of terpenes and belongs to the secoiridoids, and it is found in concentrations of up to 140 mg/g in young olives and 60 mg/g in their leaves (in dry weight basis, in both cases) [22]. In both olive fruit and the human body, HXT and tyrosol are formed as byproductsfrom the breakdown of oleuropein. After consumption, the oleic acid in the fruit is hydrolyzed during maturation and processing and then further processed to hydroxytyrosol by lipase activity. The HXT content in extra virgin olive oil (EVOO) and table olives can be influenced by a number of variables, including the region where the olives were grown, the plant species used, the harvest date, and the processing methods used [22]. The HXT concentration in EVOO is 14.32 ± 3.01 mg/kg, while in refined virgin olive oil, it is only 1.74 ± 0.84 mg/kg. Spanish green olives have an HXT content of 170–510 mg/kg, Greek black olives of 100–340 mg/kg, and Greek Kalamata olives of 250–760 mg/kg [23]. HXT found in olive oil is an extremely potent natural antioxidant, twice as effective as coenzyme Q10. Some classes of chemicals containing amino groups are structurally attractive to HXT. The basic structure of HXT ensures that it is readily absorbed by the human body. Within 15 to 20 min of absorption, it reaches the plasma without causing toxicity problems [24][25]. Since it can easily cross cell membranes, this carrier is very effective for transporting substances through the body. The advantageous structural and transport properties of HXT at the molecular level contribute to a wide range of beneficial effects on the organism [24]. In 2012, HXT was approved by the European Food Safety Authority as a protective natural agent for the cardiovascular system [26].
Polyphenols are gaining increasing interest, particularly because of the link between their consumption and the prevention of various diseases including cardiovascular disorders, cancer, neurodegenerative dysfunctions, diabetes, and others [27]. Although there is a link between polyphenol consumption and reduced risk of chronic diseases, it should be appreciated that the discrepancies and contradictions obtained in different studies in explaining this possible beneficial effect are based on their bioavailability [28].
Several clinical analyses on the use of polyphenols have limitations, such as small numbers of participants, lack of controls, use of different methods, and heterogeneity in the types of correlations in the databases [29]. Other studies have been conducted on a broader basis and were well-designed (such as PREDIMED). This analysis showed that the Mediterranean diet has a lower cardiovascular risk and improves cognitive function in the elderly. The beneficial effects of this study may be due to the water solubility of hydroxytyrosol and oleuropein [30].
In addition to the importance of bioavailability on health effects, the average daily intake should also be taken into account. A recent systematic review of the literature, which included more than 90 human studies, estimated the intake of polyphenols based on dietary habits and identified an average daily intake of polyphenols in the general population (including adolescents, adults, and the elderly) of 0.9 g/day, the main sources being coffee, tea, red wine, fruits, and vegetables. This intake was associated with a reduction in cardiovascular disease (CVD) and type 2 diabetes mellitus [31].
Polyphenols are more soluble in organic solvents (less polar) than in water, and the solubility is dependent on the polar properties of the polyphenols. HXT has a water solubility of 50 g/L. In addition, HXT has a bioavailability of 99%, so it is easily integrated by the human body [32] and is resistant to gastric juices as it passes through the digestive system and is recovered in the urine mainly in the form of 3’-sulphate [33].
3. Antiviral Properties of Hydroxytyrosol
The antiviral properties of secondary metabolites found in plants are well documented [34]. These compounds are known to affect all stages of the virus life cycle (host contact, entry, replication, assembly, and release). Sometimes, polyphenols interfere with the attachment of a virus to host cells by binding to the viral capsid (envelope). Hydroxytyrosol is particularly effective against enveloped viruses such as influenza, human immunodeficiency virus, and coronavirus [35][36].
The antiviral activities of HXT were studied by Kentaro Yamada and colleagues with influenza A virus, New Castle disease virus, bovine rotavirus, and chicken adenovirus [35]. Although HXT proved successful in combating enveloped viruses, it proved ineffective with non-enveloped viruses. For example, the structure of the H9N2 virus was altered and surface spines were absent when the virus was treated with HXT, as observed using electron microscopy. These results indicate that the viral envelope is the primary target of HXT. The H9N2 virus treated with HXT showed antiviral properties, including a reduction in mRNA production and the absence of viral nucleoproteins [35].
In a study using an animal model, the HXT of olive oil showed anti-inflammatory effects [37]. HXT decreased the expressions of pro-inflammatory cytokines such as TNF and IL-1 in inflammatory disorders. HXT suppressed the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), and tumour necrosis factor (TNF-α) in LPS-challenged human monocytic THP-1 cells in vitro. HXT has been shown to minimize the oxidative damages caused by inflammation by inhibiting the AA-derived lipoxygenase (LOX) and cyclooxygenase enzymes [38]. Because of its anti-inflammatory effects, HXT has been considered a non-toxic drug for inflammation regulation.
The nuclear factor NF-κβ signalling pathway is activated by numerous discrete stimuli and is a master regulator of the inflammatory response to pathogens and cancerous cells, as well as a key regulator of autoimmune diseases.NF-κβ signalling is crucial for the development and activation of adaptive immune cells [39][40][41]. NF-κβ occupies a vital upstream position in the inflammatory cascade, where it controls the production of countless proinflammatory mediators. ROS (primarily H2O2 and •O2−) [42] can modulate intracellular signalling pathways, activating the NF-κβ factor. Enrico Sangiovanni et al. evaluated the effect of olive oil phenols on NF-κβ activity in human gastric adenocarcinoma cells [43]. Olive oil phenol extracts inhibited NF-κβ in a concentration-dependent manner: IC(50) for the Italian and the Spanish extract were 0.86 and 1.28 µg/mL, respectively. The IC(50) for individual compounds ranged from 4.5 to 13 µM. These extract compounds inhibited nuclear translocation as well. The data suggest that consumption of extra-virgin olive oil may be beneficial for preventing the onset of inflammation leading to more serious diseases [43].
Hydroxytyrosol is also a powerful anti-inflammatory compound. The secretion of cytokines (IL-1, IL-1, IL-6, IL-12, and TNF-α) and chemokines (CXCL10/IP-10 and CCL2/MPC-1) was reduced by effectively inhibiting the production of •NO and prostaglandin E2 (PGE2) in a study highlighting this property [44]. Reducing iNOS and COX-2 gene expression, as well as blocking NF-kβ, the signal transducer and activator of transcription 1 (STAT-1), and the interferon regulatory factor (IRF-1) activation are all effects of HXT therapy [45].
The hydroxyl groups on the aromatic rings in HXT have the capacity to donate an H+ to a variety of radicals, including superoxide anion, hydroxyl, peroxyl, etc., which lose reactivity as a result of stabilization, creating a r a relatively stable radical [46], as shown in Figure 1. Superoxide anion is the first by-product of O2 reduction [47]. Classically, it has a dual role, as it can induce cell death pathways, apoptosis, necrosis, ferroptosis, pyroptosis, and autophagic cell death. However, at the same time, it is involved in physiological processes and is beneficial for the oxidative death of microbial pathogens, which are subsequently engulfed by specialised immune cells, such as neutrophils or macrophages, during the activation of the innate immune system [48].
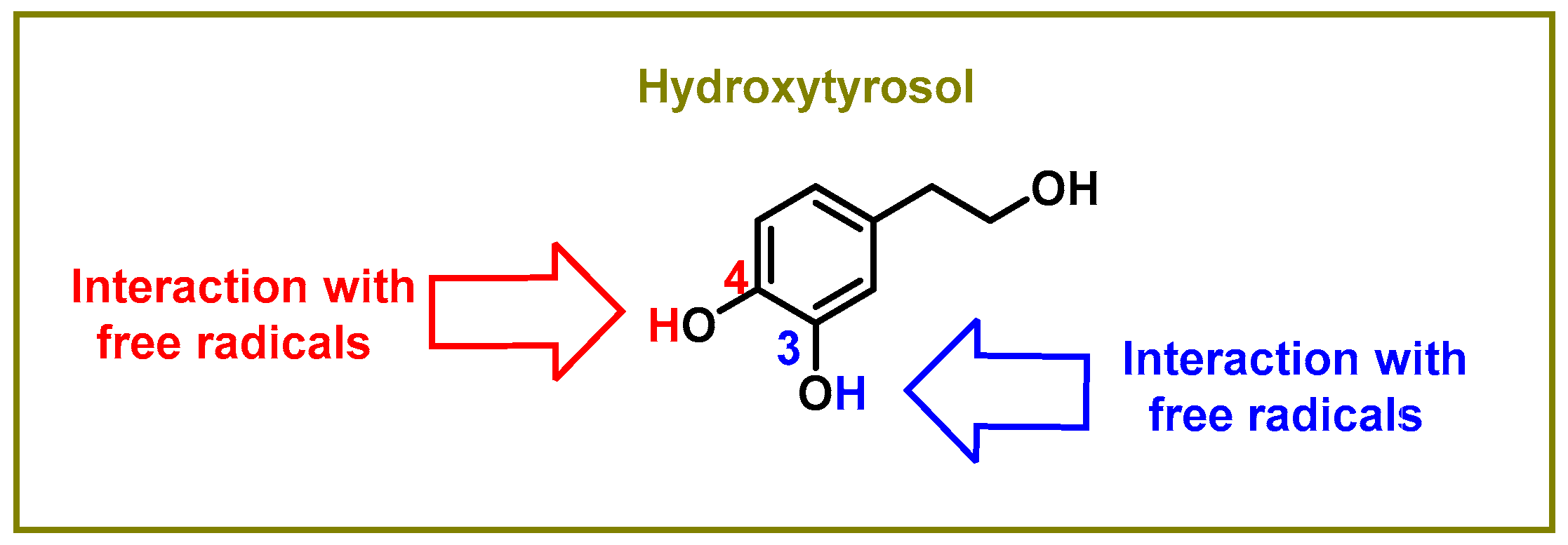
Figure 1. Sites of the interaction between the hydroxytyrosol molecule and free radicals.
The delocalisation of electrons throughout the aromatic ring is caused by an interaction with a free radical •X at the hydrogen in the OH of the C-3 and C-4 positions, as shown in Figure 2. An oxygen, nitrogen, or chlorine radical, such as hydroxyl, peroxyl, superoxide, or peroxynitrous acid, can be the •X radical [46].
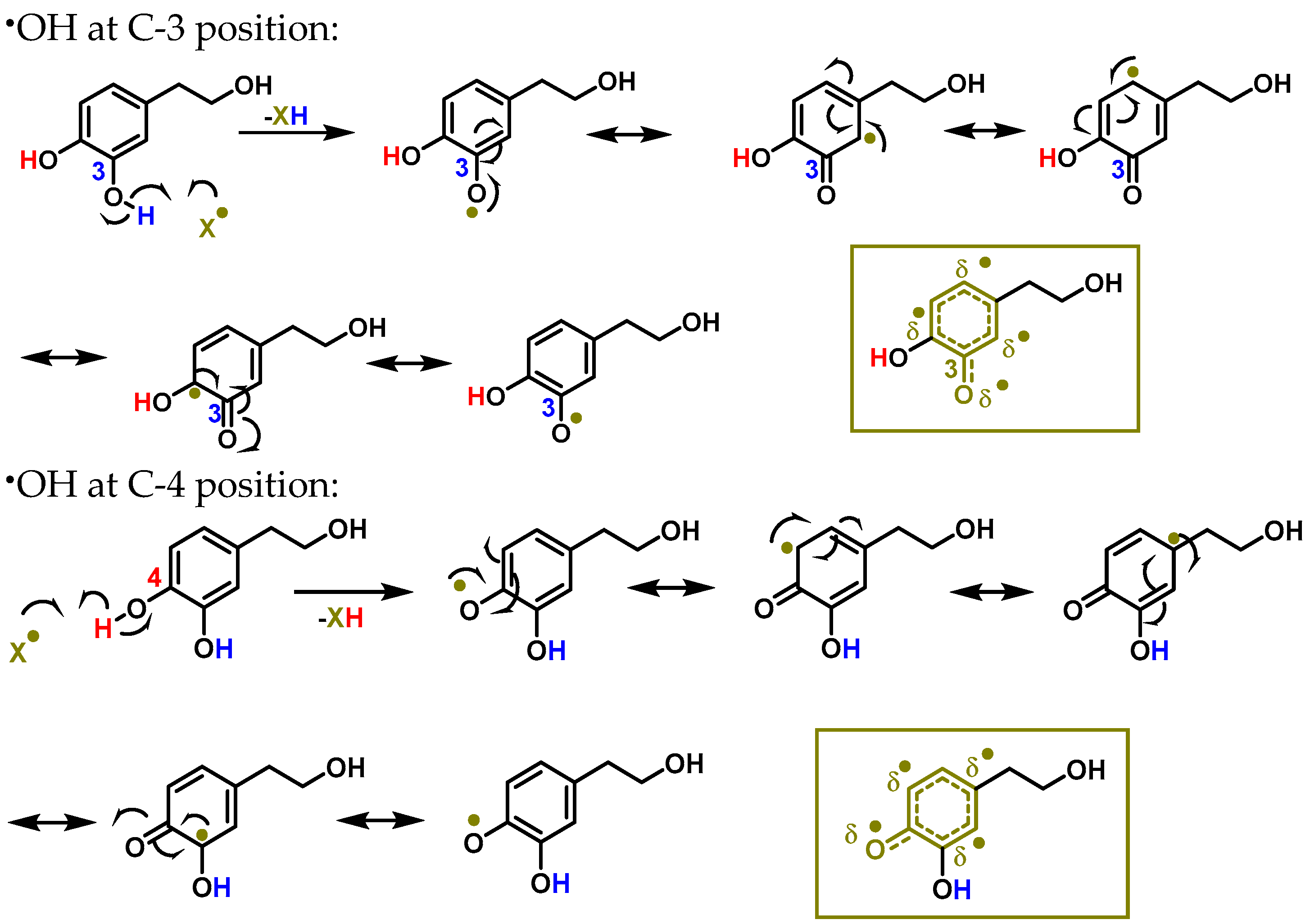
Figure 2. The interaction between hydroxytyrosol (HXT) and a free radical •X at the hydrogen in the OH of C-3 and C-4 positions results in electron delocalisation throughout the aromatic ring.
Scientists have discovered that this chemical regulates the redox state in cells and prevents oxidative stress from damaging cells. Vilaplana-Pérez et al., 2014, examined the properties of HXT and its mechanisms of action, which include potent antioxidant and anti-inflammatory effects, among others [26]. They highlighted the importance of HXT in the protection of low-density lipoproteins and, consequently, its involvement in reducing the risk of cardiovascular disease, which was also highlighted by the European Food Safety Authority. They concluded that 5 mg of HXT and its derivatives should be consumed daily to achieve this effect at the physiological level [26].
Physiological and pathological inflammatory responses are associated with the production of cytokines (TNF-α, IL -1, IL -6, and IL -17), chemokines, and adhesion molecules.Another study found that hydroxytyrosol affected the activation of the transcription factor NF-kB. SIRT1 activation increased the effect of HXT on endothelial nitric oxide synthase (eNOS) phosphorylation in glucose-stimulated human umbilical vein endothelial cells (HUVECs). However, the promoting effect of HXT on eNOS phosphorylation was abolished by the deactivation of sirtuin SIRT1. Most importantly, HXT inhibited the production of reactive oxygen species (ROS) via SIRT1 in HUVECs. The ROS scavenger potentiated the effect of HXT on eNOS phosphorylation [49].
Shan and Miao, 2022, evaluated the immunomodulatory and antioxidant effects of HXT in immunosuppressed broilers. Immunosuppressed broiler models were established with intraperitoneal injection of 80 mg/kg cyclophosphamide. The HXT groups were treated with 0.5 mL of 200 mg/L HXT solution, once daily, for 7 days. HXT was found to increase the villus height (HV)/crypt depth (CD) ratio in the duodenum and suppressed serum levels of TNF-α and interleukin-6 (IL-6). In addition, it elevated CD4+ and CD8+ T-lymphocyte expression. HXT increased the mRNA expression levels of IL-2, IL-4, and IL-10, increased the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and reduced the levels of malondialdehyde (MDA) in the cyclophosphamideCy-induced immunosuppressed broilers. They concluded that HXT alleviates immunosuppression, as well as improves immunity and antioxidant activities in the local small intestinal mucosa of broiler chickens. Therefore, it was proposed that HXT can be used as an immune stimulant [50].
These properties have earned hydroxytyrosol much attention as a potential agent to defend against COVID-19 infections [51][52].
Arginase catalyses the last step in the urea cycle, a series of biochemical reactions in which the mammalian organism eliminates harmful ammonia.However, arginase activity is increased during inflammatory processes and with reactive oxygen species associated with disease states. Although plant polyphenolic compounds belonging to the dihydroxyphenyl group show little inhibitory effect on arginase and are not specific, they still exhibit antioxidant activity and potentially cardiovascular protective effects [53].
4. The Importance of Amino Acids in the Immune Response
In their simplest form, amino acids were the building blocks for life on Earth. The combination of amino acids to form peptides and eventually proteins that mediate biological functions was a crucial evolutionary step [54]. Before cellular biosynthesis evolved, only a select group of 10 amino acids may have been used for protein synthesis [55]. In nature, there are many more amino acids that affect cellular function, but mammals use only a subset of 20 for protein synthesis [56][57]. Nine of the twenty amino acids used to make proteins are considered “essential” because the body cannot make enough of them on its own. In contrast, the body can make sufficient amounts of the non-essential amino acids. Thus, they are not needed but may become temporarily necessary if their demand exceeds their supply [58][59].
Amino acids are important not only for protein production but also for a variety of actions that promote growth and cell division. Protection against infection and tolerance to harmless environmental antigens are normal results of the immune response [59]. The vertebrate immune system is divided into innate and adaptive immune systems, which were once considered independent but are now known to be closely linked through the release of cytokines and chemokines. The establishment of an efficient immune response depends on the interaction between and regulation of innate and adaptive immunity [60].
The metabolic status of immune cells is critical because their activation status is constantly changing in response to infections and changes in their tissue environment. Immune cells rely on amino acids for adequate nutrition, and amino acid availability controls immune cell activity. Immune cells have specific amino acid requirements. During an immune response, non-essential amino acids can become conditionally essential as immune cells undergo dramatic changes in cell growth and proliferation in response to changes in their extracellular environment [59]. Amino acids stored in lysosomes can be released or restricted as needed to trigger autophagy as a protective mechanism during times of nutrient scarcity [61]. Stimulation by growth factors and activation of T cells drives their rapid proliferation, which in turn increases the need for an increased amino acid supply [62]. Abnormal proliferation and polarization of Th1, Th2, Th9, Th17, or Th22 cell populations are hallmarks of the chronic proinflammatory state that results from an active immune response in many diseases [63][64]. Withtheir involvement in central energy metabolism, redox balance, epigenetic modifications, and post-translational modifications (PTMs), amino acids can influence immunity through multiple mechanisms [59]. Different immune cells are thought to respond differently to amino acid perturbations when an immune cell expresses transporters and internal metabolic enzymes to process the amino acids [64]. Viruses have adapted to specifically target amino acid transporters because amino acid uptake is an early checkpoint for manipulating amino acid metabolism. Targeting amino acid metabolism in immune cells is a useful technique to enhance or antagonize immune responses [65][66]. Many new amino acid metabolic pathways remain to be studied for their potential effects on immune cells, and a better understanding of amino acid metabolism in immune cells should be of great therapeutic benefit [65].
References
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98.
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E. Coronavirus pandemic (COVID-19). Our World Data 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 11 April 2023).
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329.
- Raveendran, A.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875.
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648.
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID syndrome: An insight on its pathogenesis. Vaccines 2021, 9, 497.
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open 2021, 4, e210830.
- Bannister, B. Post-infectious disease syndrome. Postgrad. Med. J. 1988, 64, 559–567.
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615.
- Ramakrishnan, R.K.; Kashour, T.; Hamid, Q.; Halwani, R.; Tleyjeh, I.M. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front. Immunol. 2021, 12, 2574.
- Sanyaolu, A.; Marinkovic, A.; Prakash, S.; Zhao, A.; Balendra, V.; Haider, N.; Jain, I.; Simic, T.; Okorie, C. Post-acute Sequelae in COVID-19 Survivors: An Overview. SN Compr. Clin. Med. 2022, 4, 91.
- Frontera, J.A.; Thorpe, L.E.; Simon, N.M.; de Havenon, A.; Yaghi, S.; Sabadia, S.B.; Yang, D.; Lewis, A.; Melmed, K.; Balcer, L.J. Post-acute sequelae of COVID-19 symptom phenotypes and therapeutic strategies: A prospective, observational study. PLoS ONE 2022, 17, e0275274.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 1–25.
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecular 2009, 14, 2202–2211.
- Visioli, F.; Lastra, C.A.D.L.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546.
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879.
- Adamczyk, B.; Adamczyk, S.; Smolander, A.; Kitunen, V. Tannic acid and Norway spruce condensed tannins can precipitate various organic nitrogen compounds. Soil Biol. Biochem. 2011, 43, 628–637.
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.d.l.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions. Molecules 2017, 22, 669.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive tree derivatives and hydroxytyrosol: Their potential effects on human health and its use as functional ingredient in meat. Foods 2021, 10, 2611.
- Alarcón-de-la-Lastra, C. Olive Oil Antioxidants. Antioxidants 2022, 11, 996.
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154.
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242.
- Cvorovic, J.; Ziberna, L.; Tramer, F.; Passamonti, S.; Daglia, M.; F Nabavi, S.; Sobarzo-Sanchez, E.; M Nabavi, S. Hydroxytyrosol, a Phenyl Ethyl Alcohol with Health Effects. Curr. Org. Chem. 2017, 21, 325–332.
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417.
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18.
- Żyżelewicz, D.; Oracz, J. Bioavailability and Bioactivity of Plant Antioxidants. Antioxidants 2022, 11, 2336.
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Adv. Chronic. Dis. 2012, 3, 87–106.
- Xu, Y.; Le Sayec, M.; Roberts, C.; Hein, S.; Rodriguez-Mateos, A.; Gibson, R. Dietary Assessment Methods to Estimate (Poly)phenol Intake in Epidemiological Studies: A Systematic Review. Adv. Nutr. 2021, 12, 1781–1801.
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. 2014, 5, 330s–336s.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Schaffer, S.; Müller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharm. Res. 2010, 62, 322–327.
- Khymenets, O.; Crespo, M.C.; Dangles, O.; Rakotomanomana, N.; Andres-Lacueva, C.; Visioli, F. Human hydroxytyrosol’s absorption and excretion from a nutraceutical. J. Funct. Foods 2016, 23, 278–282.
- Pérez de la Lastra, J.M.; Andrés-Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Impact of zinc, glutathione, and polyphenols as antioxidants in the immune response against SARS-CoV-2. Processes 2021, 9, 506.
- Yamada, K.; Ogawa, H.; Hara, A.; Yoshida, Y.; Yonezawa, Y.; Karibe, K.; Nghia, V.B.; Yoshimura, H.; Yamamoto, Y.; Yamada, M. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antivir. Res. 2009, 83, 35–44.
- Bedoya, L.M.; Beltrán, M.; Obregon-Calderon, P.; García-Pérez, J.; Humberto, E.; González, N.; Pérez-Olmeda, M.; Auñón, D.; Capa, L.; Gómez-Acebo, E. Hydroxytyrosol: A new class of microbicide displaying broad anti-HIV-1 activity. AIDS 2016, 30, 2767.
- Achmon, Y.; Fishman, A. The antioxidant hydroxytyrosol: Biotechnological production challenges and opportunities. Appl. Microbiol. Biotechnol. 2015, 99, 1119–1130.
- Burayk, S.; Oh-Hashi, K.; Kandeel, M. Drug Discovery of New Anti-Inflammatory Compounds by Targeting Cyclooxygenases. Pharmaceuticals 2022, 15, 282.
- Killeen, M.J.; Linder, M.; Pontoniere, P.; Crea, R. NF-κβ signaling and chronic inflammatory diseases: Exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov. Today 2014, 19, 373–378.
- Kaileh, M.; Sen, R. NF-κB function in B lymphocytes. Immunol. Rev. 2012, 246, 254–271.
- Cildir, G.; Low, K.C.; Tergaonkar, V. Noncanonical NF-κB signaling in health and disease. Trends Mol. Med. 2016, 22, 414–429.
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Sangiovanni, E.; Colombo, E.; Fumagalli, M.; Abbiati, F.; Caruso, D.; Dell’Agli, M. Inhibition of NF-κB Activity by Minor Polar Components of Extra-Virgin Olive Oil at Gastric Level. Phytother. Res. 2012, 26, 1569–1571.
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897.
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 581–586.
- Napolitano, A.; De Lucia, M.; Panzella, L.; d’Ischia, M. The chemistry of tyrosol and hydroxytyrosol: Implications for oxidative stress. In Olives and Olive oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1225–1232.
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841.
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735.
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215.
- Shan, C.; Miao, F. Immunomodulatory and antioxidant effects of hydroxytyrosol in cyclophosphamide-induced immunosuppressed broilers. Poult. Sci. 2022, 101, 101516.
- Takeda, Y.; Jamsransuren, D.; Matsuda, S.; Crea, R.; Ogawa, H. The SARS-CoV-2-inactivating activity of hydroxytyrosol-rich aqueous olive pulp extract (HIDROX®) and its use as a virucidal cream for topical application. Viruses 2021, 13, 232.
- Ergoren, M.C.; Paolacci, S.; Manara, E.; Dautaj, A.; Dhuli, K.; Anpilogov, K.; Camilleri, G.; Suer, H.K.; Sayan, M.; Tuncel, G. A pilot study on the preventative potential of alpha-cyclodextrin and hydroxytyrosol against SARS-CoV-2 transmission. Acta Bio Med. Atenei Parm. 2020, 91, e2020022.
- Sun, Y. Antioxidant and Biological Activities of Tyrosol, Hydroxytyrosol and Their Esters. Ph.D. Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2017.
- Meierhenrich, U. Amino Acids and the Asymmetry of Life: Caught in the Act of Formation; Springer: Berlin/Heidelberg, Germany, 2008.
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153.
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227S–231S.
- Heger, J.; Frydrych, Z. Efficiency of utilization of amino acids. In Absorption and Utilization of Amino Acids; CRC Press: Boca Raton, FL, USA, 2019; pp. 31–56.
- Eagle, H. Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437.
- Kelly, B.; Pearce, E.L. Amino assets: How amino acids support immunity. Cell Metab. 2020, 32, 154–175.
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.; Schultze, J.L. Innate and adaptive immune memory: An evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 2019, 25, 13–26.
- Mizushima, N.; Klionsky, D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007, 27, 19–40.
- Dimeloe, S.; Burgener, A.V.; Grählert, J.; Hess, C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 2017, 150, 35–44.
- Zielinski, C.E.; Corti, D.; Mele, F.; Pinto, D.; Lanzavecchia, A.; Sallusto, F. Dissecting the human immunologic memory for pathogens. Immunol. Rev. 2011, 240, 40–51.
- Wang, W.; Zou, W. Amino acids and their transporters in T cell immunity and cancer therapy. Mol. Cell 2020, 80, 384–395.
- Almutairi, S.M.; Ali, A.K.; He, W.; Yang, D.-S.; Ghorbani, P.; Wang, L.; Fullerton, M.D.; Lee, S.-H. Interleukin-18 up-regulates amino acid transporters and facilitates amino acid–induced mTORC1 activation in natural killer cells. J. Biol. Chem. 2019, 294, 4644–4655.
- Camargo, S.M.; Vuille-dit-Bille, R.N.; Meier, C.F.; Verrey, F. ACE2 and gut amino acid transport. Clin. Sci. 2020, 134, 2823–2833.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
968
Revisions:
2 times
(View History)
Update Date:
18 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





