Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Satoru Matsuda | -- | 2497 | 2023-05-17 10:51:42 | | | |
| 2 | Dean Liu | -1 word(s) | 2496 | 2023-05-18 02:38:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Murai, T.; Matsuda, S. Pleiotropic Signaling by Reactive Oxygen Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/44423 (accessed on 13 January 2026).
Murai T, Matsuda S. Pleiotropic Signaling by Reactive Oxygen Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/44423. Accessed January 13, 2026.
Murai, Toshiyuki, Satoru Matsuda. "Pleiotropic Signaling by Reactive Oxygen Species" Encyclopedia, https://encyclopedia.pub/entry/44423 (accessed January 13, 2026).
Murai, T., & Matsuda, S. (2023, May 17). Pleiotropic Signaling by Reactive Oxygen Species. In Encyclopedia. https://encyclopedia.pub/entry/44423
Murai, Toshiyuki and Satoru Matsuda. "Pleiotropic Signaling by Reactive Oxygen Species." Encyclopedia. Web. 17 May, 2023.
Copy Citation
The excessive generation of reactive oxygen species (ROS) plays a pivotal role in the pathogenesis of diseases. ROS are central to cellular redox regulation and act as second messengers to activate redox-sensitive signals. Studies have revealed that certain sources of ROS can be beneficial or harmful to human health. Considering the essential and pleiotropic roles of ROS in basic physiological functions, future therapeutics should be designed to modulate the redox state. Dietary phytochemicals, microbiota, and metabolites derived from them can be expected to be developed as drugs to prevent or treat disorders in the tumor microenvironment.
redox signaling
tumor microenvironment
cancer stem cells
1. Introduction
Reactive oxygen species (ROS) is a term used for a family of reactive species derived from molecular oxygen. ROS are continuously generated and scavenged in the cells of all aerobic organisms. The term ROS is useful for a global description, but the name of the specific chemical species referred to must be used as and when required [1]. Superoxide anion (O2−•), hydroxyl (OH•), alkoxyl (RO•), and peroxyl (ROO•) are the major free-radical ROS, while hydrogen peroxide (H2O2), ozone (O3), singlet molecular oxygen (O21Δg), electronically excited carbonyls (RCO), and organic hydroperoxide (ROOH) are the major nonfree-radical forms of ROS.
2. Dietary Phytochemicals Regulate ROS Signaling
Plants synthesize a wide variety of chemical compounds known as secondary metabolites, such as polyphenols and flavonoids, which are essential to their physiology and growth. There is a substantial body of research that has explored the health benefits of these phytochemicals when plants are included in the diet as many of them are likely to have pleiotropic effects including those through ROS regulation, while some effects may be unknown or require further investigation [2][3][4][5] (Figure 1). Dietary polyphenols include the families of flavonoids, stilbenes, and chalcones in addition to nonflavonoid polyphenol compounds [6]. Flavonoids contain a common carbon skeleton structure of diphenyl propane in which two benzene rings connect by a linear three-carbon chain (Figure 1). The main flavonoid sources are fruits, vegetables, and tea. Among the fruits, berries, cherries, plums, and apples are the richest in flavonoids; in contrast, flavonoids are less abundant in tropical fruits [7][8]. Flavonoids are potent antioxidants that protect plants from unfavorable environmental conditions [8]. The hydroxyl groups in flavonols are responsible for their biological activities. These hydroxyl groups are capable of readily donating hydrogen electrons to stabilize a radical species [9].
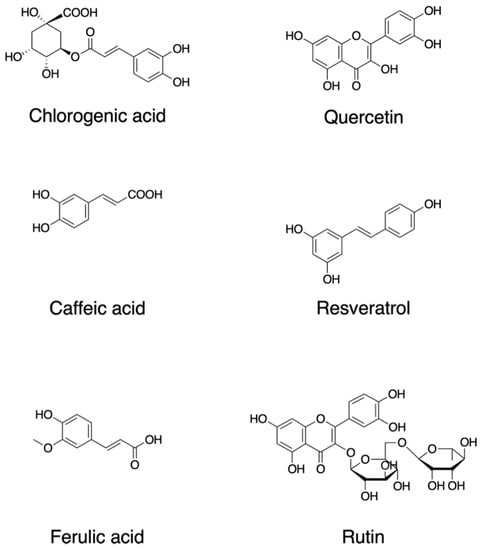
Figure 1. Chemical structures of dietary phytochemicals that act as antioxidants.
Quercetin, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one; resveratrol, 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol; rutin, quercetin-3-O-rutinoside (3′,4′,5,7-tetrahydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavone; chlorogenic acid ((1S,3R,4R,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxycyclohexane-1-carboxylic acid; caffeic acid, (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid; ferulic acid, (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid.
Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) (Figure 1) is a major plant flavonol from the flavonoid group of polyphenols with a bitter flavor and is found in many fruits, vegetables, leaves, and seeds such as capers and red onions. Quercetin has scavenging activity against hydrogen peroxide (H2O2), hydroxyl radical (OH•), and superoxide anion (O2−•) and exhibits neuroprotective and anti-inflammatory activities. The neuroprotective effects of quercetin are mainly attributed to signaling activities via the nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), Jun N-terminal kinase (JNK), protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathways [10]. Quercetin attenuates inflammation through the NF-κB and Nrf2 pathways [11].
Resveratrol, trans-3,4′,-5-trihydroxystilebene, (5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol) (Figure 1) is an efficient ROS scavenger and exhibits a protective effect against lipid peroxidation in plasma membranes and DNA damage caused by ROS [12]. Resveratrol is rich in the skin of fruits such as grapes, blueberries, and raspberries, and the uptake of resveratrol by red wine consumption could be behind the so-called French paradox [13]. Resveratrol elicits a wide variety of pharmacological effects including cellular defense, which is attributed in part to resveratrol’s activity as a direct antioxidant [14]. Resveratrol activates sirtuin 1 via the AMP-activated protein kinase pathway and prevents inflammation via the inhibition of the NF-κB pathway [14]. Resveratrol attenuates ROS production and the MAPK and NF-κB signaling pathways, exhibiting an anti-inflammatory function [15].
Rutin, quercetin-3-O-rutinoside (3′,4′,5,7-tetrahydroxy-3-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyloxy]flavone) is a common dietary flavonoid glycoside found in many plants, including buckwheat and asparagus, exhibiting pharmacological activities including anti-oxidation [16]. Rutin provides a protection effect against neurotoxicity via the activation of the PI3K/AKT/glycogen synthase kinase 3β (GSK-3β) pathway by regulating phosphorylation by scavenging free-radical generation [17].
While many reports focused on the antioxidant properties of flavonoids, recent research suggests an emerging view that flavonoids and their metabolites do not solely act as conventional hydrogen-donating antioxidants but rather may exert modulatory actions via intracellular signaling pathways [18]. In particular, flavonoids and their metabolites have been reported to act as modulators for the PKC pathway in addition to the PI3K/AKT and MAPK signaling cascades, which are likely to affect profound cellular function including gene expression.
One of the major types of phytochemicals are polyphenols, which have attracted the attention of nutritionists and biochemists due to epidemiological studies supporting their beneficial effects on human health [19][20][21][22][23]. Foods such as coffee, tea, cocoa, fruits, seeds, and their oils are rich in nonflavonoid polyphenols. As an example, coffee is a rich source of various secondary metabolites and is abundant with several phenolic compounds such as chlorogenic acid, caffeic acid, lactones, and diterpenes [24]. Chlorogenic acid (3-O-caffeoylquinic acid; 3-(3,4-dihydroxycinnamoyl)quinic acid (1S,3R,4R,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxycyclohexane-1-carboxylic acid) is a polyphenol and the cinnamate ester of quinic acid and caffeic acid (Figure 1). The beneficial effects of these compounds are attributed in part to their antioxidant activities. Chlorogenic acid exerts its antioxidant effects due to its polyhydroxyl-based structure which consists of several hydroxyl groups that readily react with free radicals to form hydrogen free radicals, which eliminate hydroxyl radicals (OH•) and superoxide anions (O2−•) to exhibit a significant antioxidant activity; therefore, they regulate the activities of the endogenous oxidase system and its associated proteins which prevent oxidative damage to cell organelles, proteins, nucleic acids, and lipids, thereby effecting the pathogenesis of cancer, inflammation, and neurological diseases [25][26].
Dietary polyphenols including the chemical compounds described above are found in various fruits, vegetables, and seeds and have been studied for their potential health benefits [27]. Currently, available evidence supports this association, and recent clinical studies regarding the use of polyphenol supplementation, which include the doses, duration of the studies, and the specific health outcomes that were measured, have also been summarized [28]. However, a safety assessment of the dose of polyphenols should be performed to prevent adverse effects [29]. Therefore, it would be helpful to refer to the possible side effects of polyphenols which have been comprehensively summarized in [30].
3. ROS as Developmental Signals in Oocytes
ROS signaling may play important roles in embryo development. Ovarian tissue is composed of two components, i.e., the ovarian cortex and the ovarian medulla. The ovarian medulla consists of connective tissues and blood vessels in addition to the fibrous tissues [31]. The interaction between oocytes and their surrounding cells is supported by direct association between them through ECM molecules, signaling proteins such as growth factors, and metabolite molecules [31]. The ovarian micro-environment may regulate the status of oocytes and enhance the aging of oocytes which lead to disorders including infertility [31]. High ROS concentration over physiological concentration might destabilize mitosis-promoting factor (MPF) and reduce certain survival factors which lead to the programmed cell death of oocytes mediated by the mitochondrion [32]. Oocytes are active in metabolism in dormancy and therefore have to keep mitochondrial activity for the generation of essential factors, while the mitochondrion is the main generator of ROS, producing ROS just as side products of oxidative respiratory reactions [33]. While ROS are able to act as signaling molecules, highly concentrated ROS promote DNA mutagenesis and thus are harmful to the cell [33]. Mitochondria of early oocytes in culture lack ROS and have low membrane potential, basal respiration rates, and resistance to rotenone indicating that they have evolved to balance their metabolism to enhance longevity, and hence primordial oocytes in humans and Xenopus laevis (frogs) lack any detectable ROS signals [33]. While it is true that primordial oocytes in humans and Xenopus laevis lack detectable ROS signals, it is important to note that ROS levels increase as oocytes mature. This can have implications for the quality of the resulting embryos. Early oocytes exhibit greatly reduced levels of mitochondrial complex I [33]. Complex I (respiratory complex I) is the first enzyme of the mitochondrial electron transport chain, a proton-pumping oxidoreductase key to bioenergetic metabolism. Primary sources of ROS occur from the transfer of electrons to molecular oxygen at Complex I. Furthermore, artificial induction of ROS synthesis in eggs caused them to degenerate rapidly, suggesting that they may have poorly developed protective mechanisms against ROS-mediated damage and that they use ROS as signals in a self-defense strategy for ensuring a longer life [34].
4. ROS Signaling in the Tumor Microenvironment
A tumor is not merely a mass of cancer cells, but rather a heterogeneous assembly of infiltrating and resident host cells, various secreted factors, and the extracellular matrix, forming a tumor microenvironment. The tumor microenvironment is a highly complex and dynamic ensemble of cells of which a variety of immune cells and cancer-associated fibroblasts (CAF) are a major component. Currently, increasing evidence indicates that various kinds of immune cells reside within the tumor microenvironment: innate immune cells including neutrophils, macrophages/dendritic cells, and innate lymphoid cells (ILCs) and acquired immune cells including T- and B-lymphocytes. Neutrophils reconstitute the ECM in the tumor microenvironment by producing matrix metalloproteinases and ROS, and macrophages/dendritic cells modulate the migration of cancer cells within the tumor microenvironment by interacting with them. The details of the subtypes of these tumor-microenvironment-related immune cells have been summarized in [35]. These cells secrete vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and hepatocyte growth factor (HGF) and the subsequent activation of their receptor tyrosine kinase (RTK) signaling pathways in enhancing ECM degradation, angiogenesis, and metastasis. At the early stage of tumor growth, dynamic interactions occur within the tumor microenvironment, where cancer cells are supported by the other components to survive, locally invade, and form metastatic dissemination. The development of secondary tumors in bodily sites distant from the primary tumor is termed metastasis. Though metastasis accounts for the greatest proportion of cancer-associated deaths, it remains the most complex and least understood aspect of cancer biology. Cancer stem cells were defined as a small subpopulation of cells within a tumor that possess the capacity to self-renew and to initiate the heterogeneous lineages of cancer cells that constitute a tumor.
Cancer stem cells are identified and can be isolated based on the expression of specific cell-surface proteins that act as molecular biomarkers. The markers most frequently used to identify cancer stem cells in solid tumors are CD44, CD133, CD24, epithelial cell adhesion molecule (EpCAM), interleukin 6 (IL-6) receptor, and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5). The expression profiles of those markers are typically conserved across the cancer cell types of hematogenous and solid tumors. However, the clinical use of cancer stem-cell-specific biomarkers in solid tumors is relatively limited because most markers expressed in cancer stem cells are also expressed in stem cell populations within normal adult tissues. Moreover, there is accumulating evidence that does not support the conventional cancer stem cell hypothesis [36][37].
Although cancer stem cells have been considered as a relatively very small subpopulation of tumor cells in certain malignancies, relative rarity is not a defining criterion within the consensual cancer stem cell definition: relative rarity is not necessarily a common feature that defines all cancer stem cells that have been identified. A characteristic of cancerous stem cells is their adaptability to the heterogeneous tumor microenvironment which includes low pH, hypoxia, nutritional state [38][39], and acidosis which is a consequence of exacerbated glycolysis and the impaired extrusion of acidic waste products such as lactate [40]. Aberrant metabolic changes in cancer cells such as enhanced glucose metabolism induce the generation of excess protons; hence, cancer cells rely on proton exchangers and transporters to export the protons into the microenvironment, allowing them to survive the hostile environment that they create [41]. Therefore, pH regulators expressed in cancer cells such as carbonic anhydrase are possible therapeutic targets, and inhibitors of carbonic anhydrase such as sulfonamides and coumarins displayed inhibitory effects on breast cancer stem cells [41].
However, the adoption of glycolysis metabolism may be promoted in an unforced manner that can occur even in a microenvironment where oxygen is available and cells have proper mitochondrial function [42]. This metabolic reprogramming forces cancer cells to maintain anabolism while decreasing ROS overproduction, which is highly toxic for them as they have low levels of ROS detoxification enzymes [42]. In addition, glucose, fatty acids, and extracellular catabolites can support metabolism in cancer stem cells by serving as alternative fuel. Since cancer cells utilize fatty acid synthesis for obtaining energy from fatty acid metabolism to support cell growth and proliferation and fatty acid oxidation to produce nicotinamide adenine dinucleotide (NADH) and ATP, various candidate drugs targeting lipid metabolism in cancer stem cells, such as fatty acid synthase inhibitors, have entered clinical investigations in recent years [43].
Although metastasis, which is the development of secondary tumors at sites distant from the primary tumor, accounts for the highest proportion of cancer-associated deaths, it remains the most complex and least understood aspect of cancer biology [44]. According to the cancer stem cell model, to establish a distant metastasis, cancer stem cells must intravasate into the bloodstream or lymphatic system for which they must first lose their epithelial characteristics such as cell–cell interactions by tight junctions and adherens junctions, to exhibit mesenchymal characteristics such as a typical fibroblastic morphology, enhanced migration, and augmented invasion capabilities; this epithelial-to-mesenchymal transition (EMT) is triggered by signals that the cells receive from their microenvironment [45][46][47][48]. The EMT International Association (TEMTIA) has noted that cancer cells may be able to migrate without promoting the EMT, possibly by collective cell migration in a manner that frequently occurs in organismal development. However, it is still unknown whether cancer cells in the primary site are able to conduct all the steps of metastatic dissemination cascades without the transient activation of the EMT to some extent [18]. Despite a continued scientific debate on the involvement of the EMT in cancer progression, it appears to be a major strategy utilized by cancer cells to acquire cancer stem-cell-specific phenotype, making this process an attractive target for developing novel cancer therapies [49]. Cancer cells are thought to accomplish metastasis not merely by the contribution of cancer stem cells or tumor-initiating cells but also by the ability of cells to exert capacity in severely adverse conditions [50]. The small population of disseminated tumor cells that are capable of the initiation of distant tumor growth are called metastasis-initiating cells, or metastatic stem cells [51]. The challenge associated with the development of therapies for the prevention or treatment of metastasis is the plasticity and heterogeneity of cancer stem cell populations.
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Sapian, S.; Budin, S.B.; Taib, I.S.; Mariappan, V.; Zainalabidin, S.; Chin, K.Y. Role of Polyphenol in Regulating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis in Diabetic Nephropathy. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 453–470.
- De Nisco, M.; Manfra, M.; Bolognese, A.; Russo, M.T. Nutraceutical properties polyphenolic profile of berry skin wine of Vitis vinifera L. Food Chem. 2013, 140, 623–629.
- Pawlowska, E.; Szczepanska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary polyphenols in age-related macular degeneration: Protection against oxidative stress, beyond. Oxidative Med. Cell. Longev. 2019, 2019, 9682318.
- Lamy, E.; Rawel, H.; Schweigert, F.J.; Capela e Silva, F.; Ferreira, A.; Costa, A.R. The effect of tannins on Mediterranean ruminant ingestive behavior: The role of the oral cavity. Molecules 2011, 16, 2766–2784.
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457.
- Trumbeckaite, S.; Bernatoniene, J.; Majiene, D.; Jakstas, V.; Savickas, A.; Toleikis, A. The effect of flavonoids on rat heart mitochondrial function. Biomed. Pharmacother. 2006, 60, 245–248.
- Bors, W.; Michel, C.; Stettmaier, K. Structure–activity relation- ships governing antioxidant capacities of plant polyphenols. Methods Enzymol. 2001, 335, 166.
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119.
- Bahar, E.; Kim, J.-Y.; Yoon, H. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNoS/NF-κB and HO-1/Nrf2 pathways. Int. J. Mol. Sci. 2017, 18, 1989.
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026.
- Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur. J. Endocrinol. 1998, 13, 619–620.
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49.
- Sadeghi, A.; Seyyed Ebrahimi, S.S.; Golestani, A.; Meshkani, R. Resveratrol Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Attenuating Oxidative Stress and JNK/NF-κB Pathway in a SIRT1-Independent Mechanism. J. Cell. Biochem. 2017, 118, 2654–2663.
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817.
- Thabet, N.M.; Moustafa, E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: Role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018, 124, 185–193.
- Singh, S.; Nagalakshmi, D.; Sharma, K.K.; Ravichandiran, V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: A review. Heliyon 2021, 7, e06216.
- Jones, Q.R.D.; Warford, J.; Rupasinghe, H.P.V.; Robertson, G.S. Target-based selection of flavonoids for neurodegenerative, disorders. Trends Pharmacol. Sci. 2012, 33, 602–610.
- Sreevalsan, S.; Safe, S. Reactive oxygen species and colorectal cancer. Curr. Color. Cancer Rep. 2013, 9, 350–357.
- Ghiringhelli, F.; Rébé, C.; Hichami, A.; Delmas, D. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anti-Cancer Agents Med. Chem. 2012, 12, 852–873.
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress plant natural, antioxidants obesity. Int. J. Mol. Sci. 2021, 22, 1786.
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic Biol. Med. 2004, 36, 838.
- Murai, T.; Matsuda, S. The chemopreventive effects of chlorogenic acids, phenolic compounds in coffee, against inflammation, cancer, and neurological diseases. Molecules 2023, 28, 2381.
- Weidinger, A.; Milivojev, N.; Hosmann, A.; Duvigneau, J.C.; Szabo, C.; Törö, G.; Rauter, L.; Vaglio-Garro, A.; Mkrtchyan, G.V.; Trofimova, L.; et al. Oxoglutarate dehydrogenase complex controls glutamate-mediated neuronal death. Redox Biol. 2023, 62, 102669.
- Weihs, W.; Kozlov, A.V.; Saito, H.; Duvigneau, J.C. Editorial: Impaired oxygen delivery in experimental disease models: Pathogenesis, diagnostics and treatment strategies. Front. Med. 2022, 9, 995958.
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216.
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829.
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S.
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536.
- Zhu, Z.; Xu, W.; Liu, L. Ovarian aging: Mechanisms and intervention strategies. Med. Rev. 2022, 2, 590–610.
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36.
- Rodríguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martínez-Zamora, M.A.; Böke, E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 2022, 607, 756–761.
- Adhikari, D.; Carroll, J. Eggs remodel energy production to protect themselves from harm. Nature 2022, 607, 664–665.
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566.
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nature 2008, 456, 593–598.
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat Med. 2017, 23, 1124–1134.
- Nallasamy, P.; Nimmakayala, R.K.; Parte, S.; Are, A.C.; Batra, S.K.; Ponnusamy, M.P. Tumor microenvironment enriches the stemness features: The architectural event of therapy resistance and metastasis. Mol. Cancer 2022, 21, 225.
- Ganguly, K.; Shah, A.; Atri, P.; Rauth, S.; Ponnusamy, M.P.; Kumar, S.; Batra, S.K. Chemokine-mucinome interplay in shaping the heterogeneous tumor microenvironment of pancreatic cancer. Semin. Cancer Biol. 2022, 86, 511–520.
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222.
- Granja, S.; Tavares-Valente, D.; Queirós, O.; Baltazar, F. Value of pH regulators in the diagnosis, prognosis and treatment of cancer. Semin. Cancer Biol. 2017, 43, 17–34.
- Daniel, Y.; Lelou, E.; Aninat, C.; Corlu, A.; Cabillic, F. Interplay between metabolism reprogramming and epithelial-to-mesenchymal transition in cancer stem cells. Cancers 2021, 13, 1973.
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232.
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691.
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. EMT International Association (TEMTIA) Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2020, 21, 341–352.
- Tian, B.; Du, X.; Zheng, S.; Zhang, Y. The role of tumor microenvironment in regulating the plasticity of osteosarcoma cells. Int. J. Mol. Sci. 2022, 23, 16155.
- Mortezaee, K.; Majidpoor, J.; Kharazinejad, E. Epithelial-mesenchymal transition in cancer stemness and heterogeneity: Updated. Med. Oncol. 2022, 39, 193.
- Garg, M. Emerging roles of epithelial-mesenchymal plasticity in invasion-metastasis cascade and therapy resistance. Cancer Metastasis Rev. 2022, 41, 131–145.
- Shibue, T.; Weinberg, R.A. EMT, CSCS, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629.
- Oskarsson, T.; Batlle, E.; Massagué, J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell 2014, 14, 306–321.
- Celià-Terrassa, T.; Liu, D.D.; Choudhury, A.; Hang, X.; Wei, Y.; Zamalloa, J.; Alfaro-Aco, R.; Chakrabarti, R.; Jiang, Y.Z.; Koh, B.I.; et al. Normal and cancerous mammary stem cells evade interferon-induced constraint through the miR-199a-LCOR axis. Nat. Cell Biol. 2017, 19, 711–723.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
705
Revisions:
2 times
(View History)
Update Date:
18 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




