Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vlatka Sotošek | -- | 1890 | 2023-05-17 09:11:28 | | | |
| 2 | Peter Tang | Meta information modification | 1890 | 2023-05-17 11:07:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Knežević, D.; Ćurko-Cofek, B.; Batinac, T.; Laškarin, G.; Rakić, M.; Šoštarič, M.; Zdravković, M.; Šustić, A.; Sotošek, V.; Batičić, L. Changes of Endothelial Glycocalyx in Cardiac Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/44415 (accessed on 02 March 2026).
Knežević D, Ćurko-Cofek B, Batinac T, Laškarin G, Rakić M, Šoštarič M, et al. Changes of Endothelial Glycocalyx in Cardiac Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/44415. Accessed March 02, 2026.
Knežević, Danijel, Božena Ćurko-Cofek, Tanja Batinac, Gordana Laškarin, Marijana Rakić, Maja Šoštarič, Marko Zdravković, Alan Šustić, Vlatka Sotošek, Lara Batičić. "Changes of Endothelial Glycocalyx in Cardiac Surgery" Encyclopedia, https://encyclopedia.pub/entry/44415 (accessed March 02, 2026).
Knežević, D., Ćurko-Cofek, B., Batinac, T., Laškarin, G., Rakić, M., Šoštarič, M., Zdravković, M., Šustić, A., Sotošek, V., & Batičić, L. (2023, May 17). Changes of Endothelial Glycocalyx in Cardiac Surgery. In Encyclopedia. https://encyclopedia.pub/entry/44415
Knežević, Danijel, et al. "Changes of Endothelial Glycocalyx in Cardiac Surgery." Encyclopedia. Web. 17 May, 2023.
Copy Citation
Cardiac surgery is one of the highest-risk procedures, usually involving cardiopulmonary bypass and commonly inducing endothelial injury that contributes to the development of perioperative and postoperative organ dysfunction. Substantial scientific efforts are being made to unravel the complex interaction of biomolecules involved in endothelial dysfunction to find new therapeutic targets and biomarkers and to develop therapeutic strategies to protect and restore the endothelium.
endothelium
endothelial dysfunction
endothelial glycocalyx
1. Introduction
Cardiac surgery involves procedures on the heart and thoracic aorta. It plays an important role in the treatment of heart diseases whose prevalence is continuously increasing [1]. Currently, more than a million cardiac surgeries are performed annually worldwide [2]. The indications for cardiac surgery are described in detail in the 2019 guidelines jointly produced by three associations: the European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Cardiothoracic Anesthesiology and Intensive Care (EACTAIC), and the Quality and Outcomes Committee of the European Board of Cardiovascular Perfusion (EBCP) [3]. Overall, the most common heart pathologies that need surgical treatment are severe valvular stenosis or regurgitation and an advanced form of ischemic heart disease.
In patients with valvular heart disease, depending on the valve affected, surgical treatment includes valve reconstructions or replacement during open heart surgery [4]. In patients with an advanced form of ischemic heart disease, when medical and/or invasive cardiological therapy is insufficient, cardiac surgery should be performed [5][6]. Possible treatment modalities include minimally invasive or open cardiac surgery [7][8].
Most cardiac surgeries are performed with cardiopulmonary bypass (CPB), which temporarily replaces the heart and lung functions with an artificial circuit consisting of a pump and an oxygenation membrane [1]. CPB allows a bloodless surgical field and quiescent heart while maintaining systemic perfusion and adequate oxygenation. Roller and centrifugal pumps on the CPB machine produce non-pulsatile flow, which is still the most frequent type of CPB [9]. Recently, the pulsatile flow has been introduced; it is thought to be more physiological because it mimics arterial pulsations. Nowadays, considerable efforts are being made to identify underlying mechanisms involved in organ dysfunction following cardiac surgery, and the non-pulsatile flow is considered one of them. Although the 2019 EACTS/EACTAIC/EBCP guidelines recommend the use of pulsatile flow during CPB in adult open-heart surgery, there is still a lack of evidence for its beneficial effect over non-pulsatile flow [3].
There are also some other mechanisms related to perioperative organ dysfunction in cardiac surgery, including the release of numerous mediators and vascular endothelial dysfunction. Inflammatory mediators such as interleukin (IL)-1, IL-6, IL-8, IL-12, and IL-18 are released due to the chronic inflammation of the myocardium caused by stenosis of the vessels and the surgical stress itself [10][11][12]. There is also a noticeable secretion of degradation products of the endothelial glycocalyx due to the activation of the pro-inflammatory cascade and the need for abundant volume compensation with the aim of maintaining hemodynamic stability during and after the procedure, which leads to the secretion of the atrial natriuretic peptide and consequently damage to the endothelial glycocalyx [13]. The moderation of resultant endothelial dysfunction has become a focus of clinical and animal research.
The disorders of endothelial glycocalyx are also detected in non-cardiac surgery, as anesthetics, fluid overload, and ischemic-reperfusion injury can affect the degradation of endothelial glycocalyx. However, disorders of endothelial glycocalyx are more pronounced in cardiac surgery where among others, the extensive contact of blood and the artificial circuits during the CPB lead to a prominent surgical stress response. Moreover, the patients undergoing cardiac surgery have higher endothelial dysfunction before the surgical procedure due to the immanent characteristic of their basic disease.
2. Basic Structure and Function of Endothelial Glycocalyx
The blood vessel wall has three layers: tunica interna or intima, tunica media, and tunica externa or adventitia. The tunica interna is located next to the lumen and covered with one layer of endothelial cells attached to the basement membrane. These cells are in direct contact with blood components and form a barrier to the tissue. As such, endothelial cells exert numerous functions, including control of extravasation of fluids, ions, and molecules and regulation of vascular tone, blood coagulation, and leukocyte activation in the inflammatory and immune response [14]. Endothelial cells also produce components of the glycocalyx, which covers their luminal (apical) side [15]. The glycocalyx and attached plasma proteins, such as albumin, orosomucoid, antithrombin III and growth factors, form the endothelial surface layer (ESL) [16][17] (Figure 1).
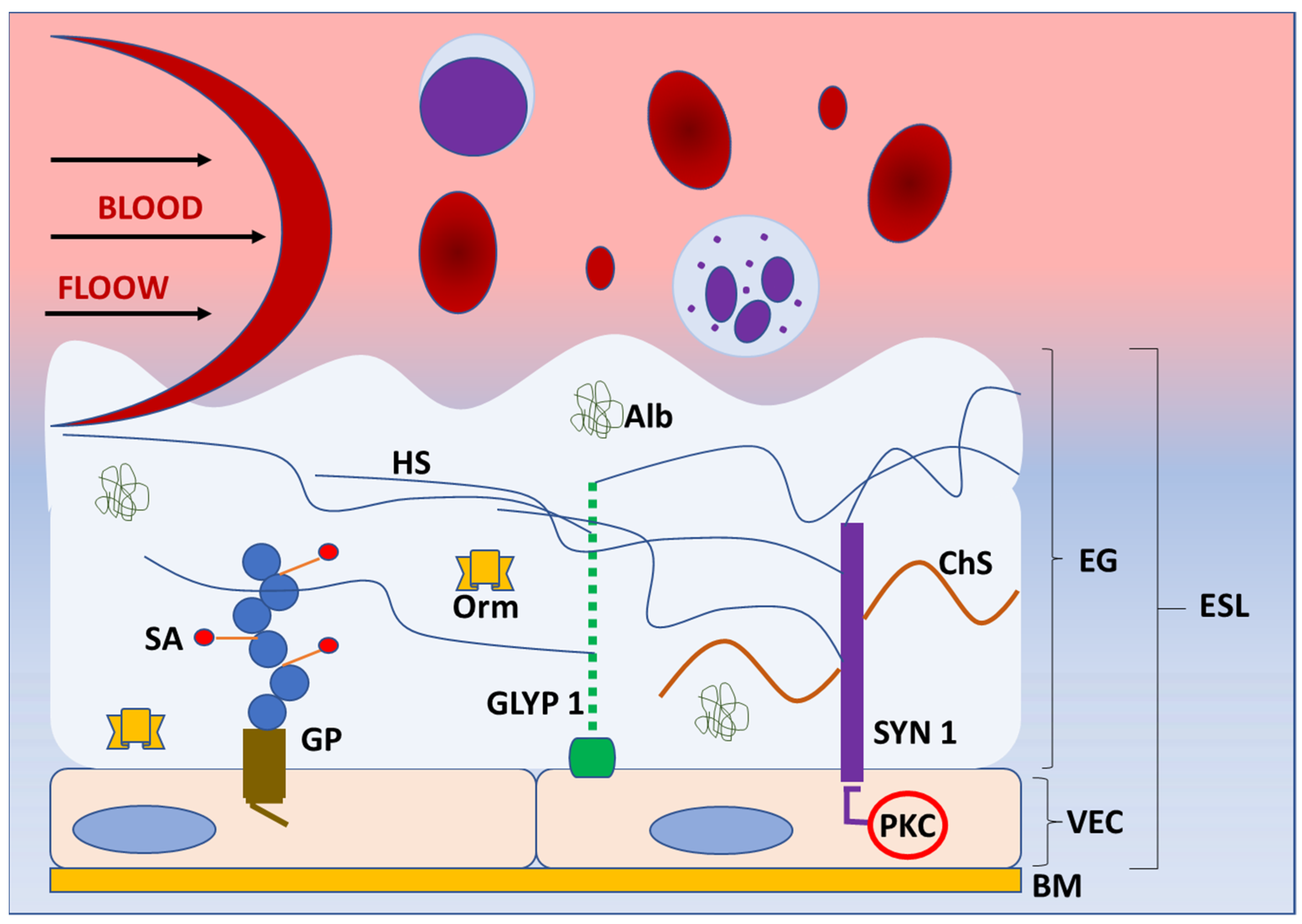
Figure 1. Structure of endothelial glycoaylyx. Schematic representation of the basic structure of endothelial glycocalyx (EG) under normal physiologycal conditions. EG forms a protective layer of glycosaminoglycans, proteoglycans (syndecans, glypicans), and incorporated proteins on the luminal side of vascular endothelial cells, preventing direct contact of blood elements with the blood vessel wall. The components of EG transmit intraluminal events to endothelial cells activating the enzymes (protein kinase C) and intracellular signaling pathways. Abbreviations: Alb—albumin, BM—basement membrane, ChS—chondroitin sulphate, EG—endothelial glycocalyx, ESL—endothelial surphace layer, GLYP 1—glypican 1, GP—glycoprotein, HS—heparan sulphate, Orm—orosomucoid, PKC—protein kinase C, SA—sialic acid, SYN 1—syndecan 1, VEC—vascular endothelial cell.
Before the use of electron and confocal microscopy, the existence of the endothelial glycocalyx was unknown. Around 70 years ago, a thin structure was discovered that is known today as the endothelial glycocalyx, which prevents the direct contact of blood elements with the blood vessel wall [16][17]. Dr Stanley Bennett was the first who proposed the term endothelial glycocalyx for this extracellular polysaccharide-rich structure [18]. Glycoproteins and proteoglycans are the main components and the basic structure of the endothelial glycocalyx. Glycoproteins are glycosylated molecular complexes containing carbohydrate groups covalently attached to the protein by covalent bonds, whereas proteoglycans are proteins attached to at least one glycosaminoglycan chain. They both anchor glycocalyx to the vascular endothelial cells, creating a matrix with incorporated soluble and insoluble components [19]. Some of these components are plasma proteins, enzymes, cofactors, superoxide dismutase, antithrombin III, thrombomodulin, and xanthine-oxidoreductase [20]. Glycoproteins and proteoglycans are mostly cell adhesion molecules that consist of variable extracellular domains, a transmembrane domain, and a cytoplasmic tail and belong to selectin, immunoglobulin, or integrin receptor families [15]. It was also noticed that the endothelial glycocalyx acts as a filter for plasma proteins depending on their size and charge [18]. The glycoproteins have short carbohydrate side chains, which are capped with sialic acid [21]. The studies showed that sialic acid in the endothelial glycocalyx significantly contributes to its negative charge and that reduction of sialic acid content results in the reduction of vascular endothelium negative surface charge [21].
Proteoglycans bind long, negatively charged, hydrophilic, unbranched glycosaminoglycan chains of disaccharide units [22][23]. Some of the glycosaminoglycans are chondroitin sulphate (associated with syndecans), heparan sulphate (associated with syndecans and glypicans), hyaluronic acid (hyaluronan; binds to surface receptors, e.g., CD44), and dermatan sulphate (covalently attached to serine residues of core proteins) [24]. Heparan sulphates are the most abundant and comprise 50–90% of all glycosaminoglycans [25]. The sulfonation of glycosaminoglycans significantly contributes to the negative charge of endothelial glycocalyx, which allows the binding of proteins from blood [26]. Syndecans and glypicans are the most significant proteoglycans, along with biglycans, decorins, mimecans, and perlecans, which are all present in the endothelial glycocalyx. So far, there are four known syndecans—syndecan-1, -2, -3, and -4 [27]. The syndecans are incorporated into the cell membrane. Their cytoplasmic tails are in contact with protein kinase C and may initiate different intracellular signaling events [27]. Through the connections with proteins of the cytoskeleton, syndecans allow for the transmission of extracellular mechanical forces to the cell [28]. Additionally, they participate in the regulation of the inflammatory response in infection and trauma. Syndecans express many glycosaminoglycan chains, which bind cytokines and initiate the inflammatory response. Animal models showed the involvement of syndecans in various aspects of inflammation, from leukocyte recruitment to the resolution of inflammation. Furthermore, the upregulation of syndecan expression during inflammation and a direct relationship between serum syndecan level and severity of inflammation were reported in humans [27]. Although the role of syndecan-1 in inflammation is the most studied, other syndecans are also involved in the inflammatory response [29].
The glypican family has six members—glypican-1 to glypican-6. Unlike syndecans, which are transmembrane structures, glypicans are connected to the cell membrane via glycosylphosphatidylinositol molecules in the areas of lipid rafts rich in signaling molecules [30]. Glypican-1 consists of the core protein and three heparan sulfate chains. It is a coreceptor in many signaling pathways, such as vascular endothelial growth factor-A, transforming growth factor-β, and bone morphogenic protein. Hence, glypican-1 modulates those pathways through the interactions with ligands and receptors on the cell surface [31]. It is also involved in signaling pathways that result in the activation of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) production [32]. It was demonstrated that glypican-1 is overexpressed in various types of cancers (breast, pancreatic, glioma) and that its high level of expression correlates with poor prognosis [30]. Additionally, the glypican-1 isoform, as the component of the glycocalyx, has a significant role in shear stress mechanosensation and mechanotransduction. [31]. The endothelial glycocalyx protrudes in the lumen of the blood vessel, and it is constantly under the shear stress generated by blood flow. However, the endothelial glycocalyx also connects to the cell membrane and cytoskeleton and includes the molecules that activate signaling pathways, such as syndecans [27]. Hence, the endothelial glycocalyx translates blood shear forces to functional and genetic changes inside the endothelial cells [24]. The results of shear force sensing and transducing are eNOS activation, NO production, and vasodilatation [33]. The cell culture and animal model study by Mahmoud et al. showed that the inhibition of glypican-1 results in endothelial cell dysfunction and inflammation through enhanced inflammatory gene expression, monocyte adhesion, and inhibited NO expression [24].
Various pathogens can be present in the cardiovascular system and blood. Therefore, the endothelial glycocalyx is also exposed to these pathogens and protects endothelial cells by providing the physical distance barrier and preventing adhesion [15]. When bacteria enter the blood, they must penetrate the endothelial cells to colonize the tissue. Since most gram-negative and gram-positive bacteria have negatively charged surfaces, and the endothelial glycocalyx is also negatively charged, it repels pathogens and prevents their access to endothelial cells [34]. Regarding the viruses, they mostly have a negative surface at pH 7.4. Accordingly, the endothelial glycocalyx represents the electrostatic charge barrier for the viruses as well [35].
Since the endothelial glycocalyx covers the luminal side of the blood vessels [36], it participates in the regulation of endothelial permeability and leukocyte and platelet adhesion [37][38][39]. Thus, it contributes to the physical and biochemical health of the endothelium and the vasculature [40][41][42][43]. Additionally, the endothelial glycocalyx is constantly exposed to the circulating enzymes, which cause the mechanical and biochemical degradation of the endothelial glycocalyx followed by the renovation process [44], making it a very dynamic structure (Figure 2).
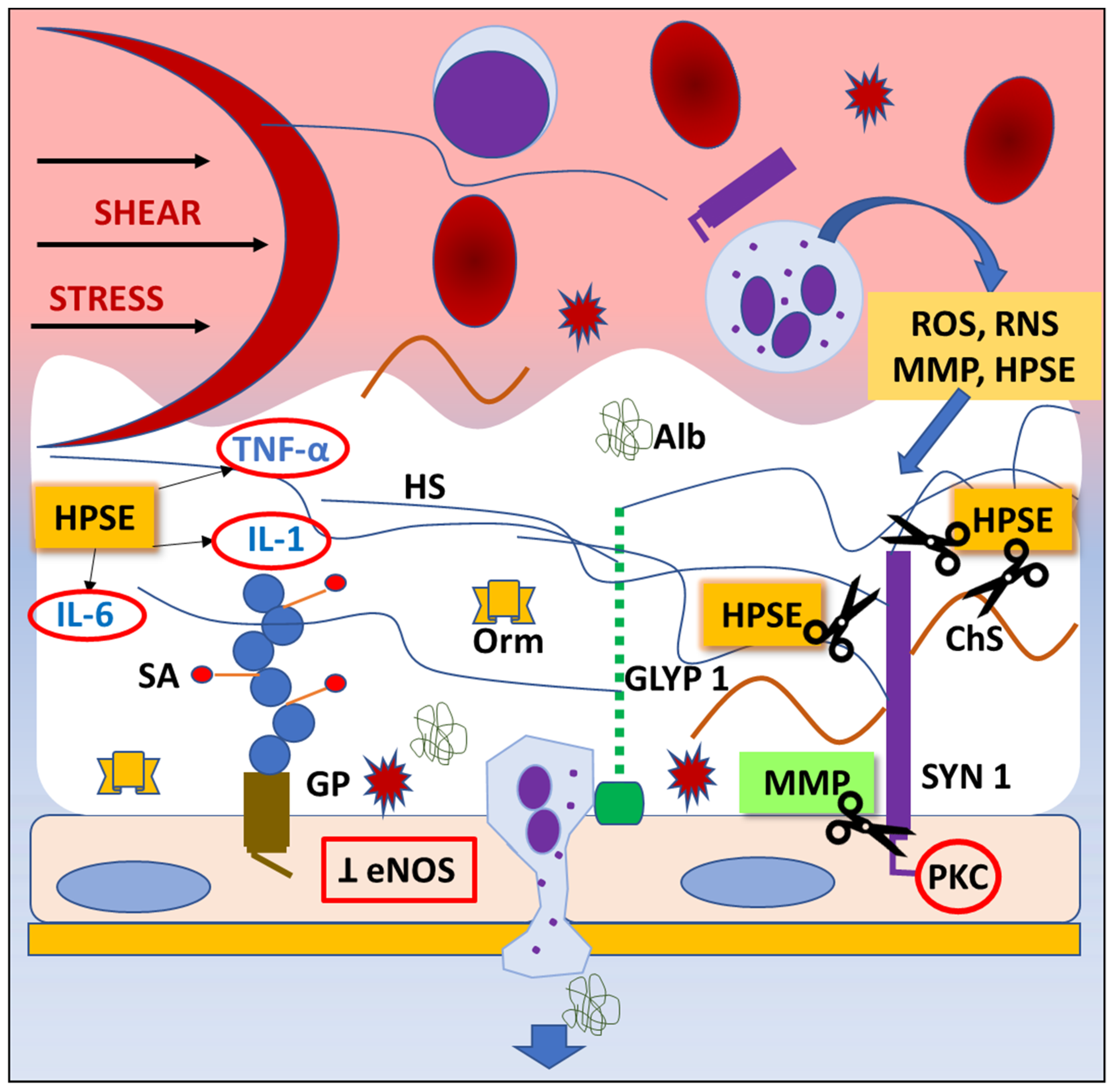
Figure 2. Mechanisms of endothelial glycocalyx degradation (“shedding”). Any pathological situation, like trauma or ischemia/reperfusion injury, can lead to degradation of endothelial glycocalyx (EG). Shear stress activates inflammatory cells, which release highly reactive chemicals (ROS, RNS), cytokines, and proteaze enzymes. Consequently, the inhibition of endothelial nitric oxide synthase (eNOS) synthesis and protein kinase C (PKC) activity result in impared ability of vasodilatation and inhibition of intracellular signaling pathways, thus leading to EG degradation, dysfunction of endothelial cells’ regulatory functions, and leukocyte and platelet adhesion. Abbreviations: Alb—albumin, ChS—chondroitin sulphate, eNOS—endothelial nitric oxyde synthase, GLYP 1—glypican 1, GP—glycoprotein, HPSE—heparanase, HS—heparan sulphate, IL-1—interleukin-1, IL-6—interleukin-6, MMP—matrix metaloproteinase, Orm—orosomucoid, PKC—protein kinase C, RNS—reactive nitrogen species, ROS—reactive oxygen species, SA—sialic acid, SYN 1—syndecan 1, TNF- α—tumor necrosis factor alpha.
References
- Verrier, E.D. Cardiac surgery. J. Am. Coll. Surg. 1999, 188, 104–110.
- Vervoort, D.; Meuris, B.; Meyns, B.; Verbrugghe, P. Global cardiac surgery: Access to cardiac surgical care around the world. J. Thorac. Cardiovasc. Surg. 2020, 159, 987.e6–996.e6.
- Wahba, A.; Milojevic, M.; Boer, C.; De Somer, F.M.J.J.; Gudbjartsson, T.; van den Goor, J.; Jones, T.J.; Lomivorotov, V.; Merkle, F.; Ranucci, M.; et al. EACTS/EACTA/EBCP Committee Reviewers. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 210–251.
- Siregar, S.; Groenwold, R.H.; de Heer, F.; Bots, M.L.; van der Graaf, Y.; van Herwerden, L.A. Performance of the original EuroSCORE. Eur. J. Cardiothorac. Surg. 2012, 41, 746–754.
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488.
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl. J. Med. 2005, 352, 1685–1695.
- Wenger, N.K.; Boden, W.E. Ischemic Heart Disease. In Institute of Medicine (US) Committee on Social Security Cardiovascular Disability Criteria Cardiovascular Disability: Updating the Social Security Listings; National Academies Press: Washington, DC, USA, 2010.
- Stevens, J.R.; Zamani, A.; Osborne, J.I.A.; Zamani, R.; Akrami, M. Critical evaluation of stents in coronary angioplasty: A systematic review. Biomed. Eng. Online 2021, 20, 46.
- Khan, M.S.; Islam, M.Y.; Ahmed, M.U.; Bawany, F.I.; Khan, A.; Arshad, M.H. On pump coronary artery bypass graft surgery versus off pump coronary artery bypass graft surgery: A review. Glob. J. Health Sci. 2014, 6, 186–193.
- Tan, A.; Newey, C.; Falter, F. Pulsatile Perfusion during Cardiopulmonary Bypass: A Literature Review. J. Extra. Corpor. Technol. 2022, 54, 50–60.
- Hadi, A.R.H.; Cornelia, S.C.; Suwaidi, J.A. Endothelial dysfunction: Cardiovascular risc factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198.
- Pesonen, E.; Passov, A.; Andersson, S.; Suojaranta, R.; Niemi, T.; Raivio, P.; Salmenperä, M.; Schramko, A. Glycocalyx Degradation and Inflammation in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 341–345.
- Song, J.W.; Goligorsky, M.S. Perioperative implication of the endothelial glycocalyx. Korean J. Anesthesiol. 2018, 71, 92–102.
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411.
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811.
- Jedlicka, J.; Becker, B.F.; Chappell, D. Endothelial Glycocalyx. Crit. Care Clin. 2020, 36, 217–232.
- Wang, G.; Tiemeier, G.L.; van der Berg, B.M.; Rabelink, T.J. Endothelial Glycocalyx Hyaluronan: Regulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790.
- Bennett, H.S. Morphological aspects of extracellular polysaccharides. J. Histochem. Cytochem. 1963, 11, 14–23.
- Pillinger, N.L.; Kam, P. Endothelial glycocalyx: Basic science and clinical implications. Anesth. Intensive Care 2017, 45, 295–307.
- Brouns, S.L.N.; Provenzale, I.; van Geffen, J.P.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Localized endothelial-based control of platelet aggregation and coagulation under flow: A proof-of-principle vessel-on-a-chip study. J. Thromb. Haemost. 2020, 18, 931–941.
- Kincses, A.; Santa-Maria, A.R.; Walter, F.R.; Dér, L.; Horányi, N.; Lipka, D.V.; Valkai, S.; Deli, M.A.; Dér, A. A chip device to determine surface charge properties of confluent cell monolayers by measuring streaming potential. Lab. Chip. 2020, 20, 3792–3805.
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Int. Med. 2016, 280, 97–113.
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx: From structure to function. Am. J. Pathol. 2020, 190, 732–741.
- Mahmoud, M.; Mayer, M.; Cancel, L.M.; Bartosch, A.M.; Mathews, R.; Tarbell, J.M. The glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2021, 117, 1592–1605.
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836.
- Esko, J.D.; Linhardt, R.J. Proteins that bind sulfated glycosaminoglycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009.
- Gopal, S. Syndecans in Inflammation at a Glance. Front. Immunol. 2020, 11, 227.
- Zeng, Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J. Cell Mol. Med. 2017, 21, 1457–1462.
- Agere, S.A.; Kim, E.Y.; Akhtar, N.; Ahmed, S. Syndecans in chronic inflammatory and autoimmune diseases: Pathological insights and therapeutic opportunities. J. Cell Physiol. 2018, 233, 6346–6358.
- Pan, J.; Ho, M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am. J. Physiol. Cell Physiol. 2021, 321, C846–C858.
- Xie, M.; Li, J.-P. Heparan sulfate proteoglycan –A common receptor for diverse cytokines. Cell Signal. 2019, 54, 115–121.
- Ebong, E.E.; Lopez-Quintero, S.V.; Rizzo, V.; Spray, D.C.; Tarbell, J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. 2014, 6, 338–347.
- Dragovich, M.A.; Chester, D.; Fu, B.M.; Wu, C.; Xu, Y.; Goligorsky, M.S.; Zhang, X.F. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am. J. Physiol. Cell Physiol. 2016, 311, C846–C853.
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta. 2016, 1858, 936–946.
- Michen, B.; Graule, T. Isoelectric points of viruses. J. Appl. Microbiol. 2010, 109, 388–397.
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.L.; Kastelein, J.J.P.; Stroes, E.S.G. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005, 16, 507–511.
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167.
- Mulivor, A.W.; Lipowsky, H.H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1282–H1291.
- Chappell, D.; Heindl, B.; Jacob, M.; Annecke, T.; Chen, C.; Rehm, M.; Conzen, P.; Becker, B.F. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011, 115, 483–491.
- Mitra, R.; O’Neil, G.L.; Harding, I.C.; Cheng, M.J.; Mensah, S.A.; Ebong, E.E. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr. Atheroscler. Rep. 2017, 19, 63.
- Davies, P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995, 75, 519–560.
- Dewey, C.F., Jr.; Bussolari, S.R.; Gimbrone, M.A., Jr.; Davies, P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981, 103, 177–185.
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers. Arch. 2007, 454, 345–359.
- Ali, M.M.; Mahmoud, A.M.; Le Master, E.; Levitan, I.; Phillips, S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H647–H663.
More
Information
Subjects:
Critical Care Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
17 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





