
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luís R. Silva | -- | 1652 | 2023-05-16 11:18:31 | | | |
| 2 | Peter Tang | Meta information modification | 1652 | 2023-05-16 12:58:07 | | |
Video Upload Options
Diabetes mellitus (DM) is a metabolic disease characterized by abnormal blood glucose levels-hyperglycemia, caused by a lack of insulin secretion, impaired insulin action, or a combination of both. The incidence of DM is increasing, resulting in billions of dollars in annual healthcare costs worldwide. Therapeutics aim to control hyperglycemia and reduce blood glucose levels to normal. However, most modern drugs have numerous side effects, some of which cause severe kidney and liver problems. On the other hand, natural compounds rich in anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin) have also been used for the prevention and treatment of DM.
1. Introduction
2. Structure and Function
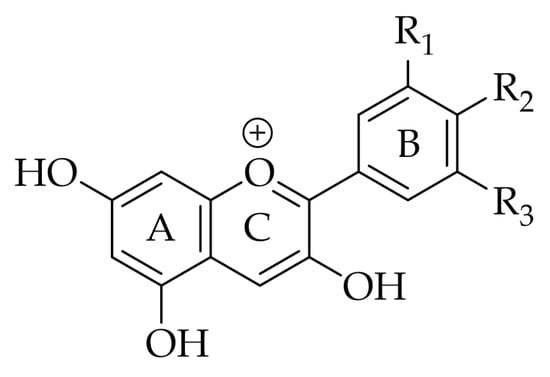
|
Anthocyanidin |
R1 |
R2 |
R3 |
Natural Sources |
|---|---|---|---|---|
|
Cyanidin |
-OH |
-OH |
-H |
Apple, blackberry, elderberry, plum, peach, nectarine |
|
Delphinidin |
-OH |
-OH |
-OH |
Oranges, grapes, beans |
|
Pelargonidin |
-H |
-OH |
-H |
Strawberries, red radishes |
|
Malvidin |
-OCH3 |
-OH |
-OCH3 |
Grapes |
|
Peonidin |
-OCH3 |
-OH |
-H |
Cranberries, blueberries, plums, cherries, grapes, purple corn |
|
Petunidin |
-OH |
-OH |
-OCH3 |
Grapes, red berries |
3. Main Sources
4. Antidiabetic Potential
|
Source |
Anthocyanin Type |
Main Outcomes |
Reference |
|---|---|---|---|
|
Sweet Cherries (Prunus avium L.) |
Anthocyanins-enriched fraction |
α-glucosidase inhibition |
[5] |
|
Cinnamomum camphora L. fruit |
Cyanidin |
α-glucosidase inhibition |
[38] |
|
Blueberry, blackcurrant and blue honeysuckle fruits |
Anthocyanins-enriched fraction |
α-glucosidase inhibition |
[39] |
|
Blueberries (Vaccinium corymbosum) and blackberries (Rubus spp.) |
Anthocyanins-enriched fraction |
α-glucosidase inhibition |
[40] |
|
Vaccinium oxycoccos L. and Vaccinium myrtillus L. |
Anthocyanins-enriched fraction |
α-glucosidase inhibition |
[41] |
|
n.d. |
Cyanidin 3-rutinoside |
α-amylase inhibition ↓ postprandial glycemia |
[42] |
|
Blackcurrant extract (11 g per kg) |
Anthocyanins-enriched fraction |
↓ blood glucose ↑ glucose tolerance |
[43] |
|
n.d. |
Cyanidin 3-O-glucoside |
↓ fasting blood glucose levels ↓ accumulation of liver lipids ↑ glycogen synthesis |
[44] |
|
Blueberry anthocyanin extract (100.4 mg per kg) |
Anthocyanins-enriched fraction |
↓ fasting blood glucose levels ↓ insulin levels ↑ liver antioxidants |
[45] |
|
Purple sweet potato |
Anthocyanins-enriched fraction |
↓ blood glucose levels ↑ glucose tolerance ↓ liver damage ↑ antioxidant capacity |
[46] |
|
Whortleberry fruit hydroalcoholic extract (1.0 g per day) |
Anthocyanins-enriched fraction |
↓ blood glucose of fasting glucose |
[47] |
|
Freeze-dried strawberry (100 g per day) |
Anthocyanins-enriched fraction |
↓ lipid peroxidation ↓ HbA1c and total antioxidant status |
[48] |
|
n.d. |
Anthocyanins (320 mg per day) |
↑ adiponectin ↓ fasting glucose ↓ basal glycemia and insulinemia |
[49] |
References
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211.
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and Blueberries-Based Beverages: Functional Foods with Antidiabetic and Immune Booster Properties. Molecules 2022, 27, 3294.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892.
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; García-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185.
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688.
- Fang, Y.; Cao, W.; Liang, F.; Xia, M.; Pan, S.; Xu, X. Structure affinity relationship and docking studies of flavonoids as substrates of multidrug-resistant associated protein 2 (MRP2) in MDCK/MRP2 cells. Food Chem. 2019, 291, 101–109.
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495.
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631.
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and Their Nanoformulations: Protective Effects against Human Diseases. Life 2022, 12, 1639.
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690.
- Farias, P.F.; Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383.
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123.
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516.
- Burton-Freeman, B.; Brzeziński, M.; Park, E.; Sandhu, A.; Xiao, D.; Edirisinghe, I. A Selective Role of Dietary Anthocyanins and Flavan-3-ols in Reducing the Risk of Type 2 Diabetes Mellitus: A Review of Recent Evidence. Nutrients 2019, 11, 841.
- Liu, Y.-J.; Zhan, J.; Liu, X.-L.; Wang, Y.; Ji, J.; He, Q.-Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63.
- Guo, X.-F.; Ruan, Y.; Li, Z.-H.; Li, D. Flavonoid subclasses and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 2850–2862.
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809.
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Lopes, J.A.; Silva, L.R. Employ of Anthocyanins in Nanocarriers for Nano Delivery: In Vitro and In Vivo Experimental Approaches for Chronic Diseases. Pharmaceutics 2022, 14, 2272.
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500.
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 54–560.
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400.
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077.
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028.
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075.
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024.
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368.
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Álvarez, J.A.; Ayuso, J.; Barbero, G.F. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 1: Pressurized Liquid Extraction. Agronomy 2020, 10, 183.
- Martinotti, S.; Bonsignore, G.; Patrone, M.; Ranzato, E. Mediterranean Diet Polyphenols: Anthocyanins and Their Implications for Health. Mini Rev. Med. Chem. 2021, 21, 1692–1700.
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011, 106, 1090–1099.
- Liu, Y.; Wang, Q.; Wu, K.; Sun, Z.; Tang, Z.; Li, X.; Zhang, B. Anthocyanins’ effects on diabetes mellitus and islet transplantation. Crit. Rev. Food Sci. Nutr. 2022, 13, 1–24.
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013, 79, 1599–1604.
- Moein, S.; Moein, M.; Javid, H. Inhibition of α-Amylase and α-Glucosidase of Anthocyanin Isolated from Berberis integerrima Bunge Fruits: A Model of Antidiabetic Compounds. Evid. Based Complement. Altern. Med. 2022, 2022, 6529590.
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025.
- Ji, Y.; Liu, D.; Jin, Y.; Zhao, J.; Zhao, J.; Li, H.; Li, L.; Zhang, H.; Wang, H. In vitro and in vivo inhibitory effect of anthocyanin-rich bilberry extract on α-glucosidase and α-amylase. LWT 2021, 145, 111484.
- Poovitha, S.; Parani, M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16 (Suppl. 1), 185.
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal alpha-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41.
- Chen, J.-G.; Wu, S.-F.; Zhang, Q.-F.; Yin, Z.-P.; Zhang, L. α-Glucosidase inhibitory effect of anthocyanins from Cinnamomum camphora fruit: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 143, 696–703.
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.-C.; Guo, Y. Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem. 2019, 299, 125102.
- Johnson, M.H.; de Mejia, E.G.; Fan, J.; Lila, M.A.; Yousef, G.G. Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol. Nutr. Food Res. 2013, 57, 1182–1197.
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of Anthocyanins from Four Kinds of Berries and Their Inhibition Activity to α-Glycosidase and Protein Tyrosine Phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221.
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In vitro inhibitory effects of cyandin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules 2011, 16, 2075.
- Iizuka, Y.; Ozeki, A.; Tani, T.; Tsuda, T. Blackcurrant Extract Ameliorates Hyperglycemia in Type 2 Diabetic Mice in Association with Increased Basal Secretion of Glucagon-Like Peptide-1 and Activation of AMP-Activated Protein Kinase. J. Nutr. Sci. Vitaminol. 2018, 64, 258–264.
- Ye, X.; Chen, W.; Tu, P.; Jia, R.; Liu, Y.; Tang, Q.; Chen, C.; Yang, C.; Zheng, X.; Chu, Q. Antihyperglycemic effect of an anthocyanin{,} cyanidin-3-O-glucoside{,} is achieved by regulating GLUT-1 via the Wnt/β-catenin-WISP1 signaling pathway. Food Funct. 2022, 13, 4612–4623.
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100.
- Jiang, T.; Shuai, X.; Li, J.; Yang, N.; Deng, L.; Li, S.; He, Y.; Guo, H.; Li, Y.; He, J. Protein-Bound Anthocyanin Compounds of Purple Sweet Potato Ameliorate Hyperglycemia by Regulating Hepatic Glucose Metabolism in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2020, 68, 1596–1608.
- Kianbakht, S.; Abasi, B.; Dabaghian, F.H. Anti-hyperglycemic effect of Vaccinium arctostaphylos in type 2 diabetic patients: A randomized controlled trial. Forsch. Komplementmed. 2013, 20, 17–22.
- Moazen, S.; Amani, R.; Homayouni Rad, A.; Shahbazian, H.; Ahmadi, K.; Jalali, M.T. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2013, 63, 256–264.
- Yang, L.; Ling, W.; Qiu, Y.; Liu, Y.; Wang, L.; Yang, J.; Wang, C.; Ma, J. Anthocyanins increase serum adiponectin in newly diagnosed diabetes but not in prediabetes: A randomized controlled trial. Nutr. Metab. 2020, 17, 78.




