Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hisashi Kato-Noguchi | -- | 2463 | 2023-05-16 10:58:15 | | | |
| 2 | Catherine Yang | Meta information modification | 2463 | 2023-05-17 02:44:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kato-Noguchi, H. Invasive Mechanisms of Mimosa pigra. Encyclopedia. Available online: https://encyclopedia.pub/entry/44355 (accessed on 02 March 2026).
Kato-Noguchi H. Invasive Mechanisms of Mimosa pigra. Encyclopedia. Available at: https://encyclopedia.pub/entry/44355. Accessed March 02, 2026.
Kato-Noguchi, Hisashi. "Invasive Mechanisms of Mimosa pigra" Encyclopedia, https://encyclopedia.pub/entry/44355 (accessed March 02, 2026).
Kato-Noguchi, H. (2023, May 16). Invasive Mechanisms of Mimosa pigra. In Encyclopedia. https://encyclopedia.pub/entry/44355
Kato-Noguchi, Hisashi. "Invasive Mechanisms of Mimosa pigra." Encyclopedia. Web. 16 May, 2023.
Copy Citation
Mimosa pigra is native to Tropical America, and it has naturalized in many other countries especially in Australia, Eastern and Southern Africa and South Asia. The species is listed in the top 100 of the world’s worst invasive alien species and is listed as Least Concern in the IUCN Red List of Threatened Species. M. pigra forms very large monospecific stands in a wet–dry tropical climate with conditions such as floodplains, riverbanks, grasslands, forests and agricultural fields.
allelopathy
biological control
monospecific stand
mutualism
1. Reproduction and Growth
M. pigra is a fast-growing species, and it is capable of reaching reproductive maturity within 6–8 months [1][2][3][4][5]. Round flower heads (1–2 cm in diameter; mauve or pink) arise from actively growing young shoots, which contain approximately 100 flowers. Each flower head generates 1–30 seed pods. Pods are 3–8 cm in length and are covered with dense stiff hair (Figure 1). Each pod contains oblong-shaped 8–20 seeds (4–5 mm long, 2 mm wide) [1][2][5]. The species flowers throughout the year in Sri Lanka and Queensland, Australia, and during spring to autumn in the Northern Territory of Australia, which may be dependent on climate conditions [2][3][4]. Flowers are pollinated by mostly self-fertilization and sometimes by bee or wind [1]. The seeds take about five to nine weeks for the maturation after the flower-bud formation [2][5] (Figure 1).

Figure 1. Mimosa pigra flowers and pods.
Annual seed production was estimated to be up to 220,000 seeds per plant [5][6], and 9000–12,000 per m2 [7]. Top soil under the canopy contained 2000 to 45,000 seeds per m2 [3][7][8]. The wind dispersion of the seeds occurs a relatively short distance from the plants. The long-distance dispersion of the seeds occurs through the adhesion of the pod’s stiff hair onto animals and agricultural vehicles and through the floating of the pods on water streams and flooded waters [8].
The seeds germinate when they are first wetted, and the rate of the germination is 75–94% [9]. The half-life of viable seeds in seed banks in field conditions is 9–99 weeks, which is dependent on the soil types and conditions [7][10]. The seed coats are very hard and impermeable and some of seeds have remained dormant in the soil for up to 26 years [5][11]. Ten years after the complete clearance of 250 ha of a M. pigra stand, its 109 seedlings per m2 still remained to emerge from the seed banks [11]. Sand scarification of the seeds increased the germination [3], indicating that the movements of the seeds by water stream and flooding may stimulate the germination.
The species grows at a rate of 1.1 cm in height per day during the first 90 days after germination and grows ca. 2.5 cm and ca. 7.5 cm in the stem diameter in the first year and the second year, respectively [1][2][12][13]. The species forms impenetrable dense monospecific stands (3–6 m in high) and the stands expand 76 m per year in wetlands [8]. It was recorded that the infested areas of the active stands doubled in 1 year and on average every 6 years in the Northern Territory of Australia [5][8]. The coverage of M. pigra in the monospecific stands was 96.3% and the biomass was estimated to be 35–45 tons dry weight per ha in the Adelaide River floodplain and the Finniss River catchment in Australia [14][15].
The species also regenerates from the remaining trumps after clearance of the above-ground parts of the trees [9]. Substantial numbers of the plants regrow from the base of stems after fire burning and the fire stimulates its germination in the seed banks [16]. The regrowth from the young stubble can reach 2.5 m in height and can cover 6.3 m2 within 12 weeks [13].
The characteristics of life history such as the high reproduction and high growth rate are important for the invasiveness and naturalization of invasive plants [17][18][19][20]. The observations described in this section suggest that M. pigra has the ability of rapid growth through its vegetative phase to flowering, self-compatibility, high seed output, high rate of germination, great longevity of seeds and regrowth from the stubbles (Table 1). These characteristics may contribute the invasion and naturalization of the species in invasive ranges.
2. Adaptivity and Plasticity
The species is found in tropical regions where annual rainfall level is between 750 mm and 2200 mm. It can grow around water bodies even when the annual rainfall is less than 750 mm [2]. M. pigra grows well in soil ranging from black cracking clays, sandy clays and siliceous river sand, although the species can grow in any type of soil [1][6]. It is found at an altitude of ca. 500 m above sea levels [12]. M. pigra has shown phenotypic plasticity in response to abiotic and biotic stress conditions such as available water level and intraspecific competition [21]. The genetic variation and structure of the M. pigra population in Thailand is high [22]. The characteristics of phenotypic plasticity of the plants are important for the naturalization of invasive plants into non-native ranges [17][18][19][20][21]. However, information is limited to discuss the phenotypic plasticity of M. pigra in different environmental conditions.
3. Natural Enemy
Long-term investigations from 1979 in the native ranges of M. pigra such as in Central and South America and in Mexico have shown that over 400 phytophagous insects, consisting of 61 families in 5 orders, are the natural enemies of M. pigra. The largest family is Coleoptera (59%), followed by Hemiptera (23%) and Lepidoptera (17%) [23][24]. Among them, for example, a stem-boring moth Carmenta mimosa Eichilin and Passoa (Lepidoptera) caused a 90% reduction in the seed production of M. pigra, and a weevil Coelocephalapion pigrae Kissinger (Coleoptera) rapidly colonized the M. pigra stands and fed on their leaves [25][26].
Pathogenic fungi: Mycosphaerella mimosae-pigrae H. C. Evans, G. Carrión and Ruiz-Belin; Sphaerulina mimosae-pigrae H. C. Evans and G. Carrión; Diabole cubensis Arthur and J. R. Johnst.; and Microstroma ruizii-belinii H. C. Evans, G. Carrión and Ruiz-Belin were found to infect M. pigra along the Pacific Coast of Mexico, and Sphaerulina mimosae-pigrae and Diabole cubensis occurred along the Caribbean Coast [27][28]. Phloeospora momosa-pigrae H. C. Evans and G. Carrión and Diabole cubensis selectively infected M. pigra in Mexico [5][27]. Some of those natural enemies were selected as the biological control agents for M. pigra, which were described in Section 3.3.
The interactions of the invasive plants with natural enemies are very critical for the naturalization of the invasive plants [17][18][19][20][21]. A great number of herbivore insects and fungal pathogens have been identified in M. pigra stands in the native ranges described above. However, very few natural enemies were found in Australia [29]. Having few natural enemies may contribute to the superior growth rate and naturalization of M. pigra in invasive ranges (Table 1).
4. Mutualism
Plant species belongs to Mimosa genus nodulate generally with the member of the Betaproteobacteria (β-rhizobia or β-proteobacteria), which includes the genera of Cupriavidus, Burkholderia, Paraburkholderia and Trinickia [30][31]. The species of Burkholderia was the main symbiosis rhizobia for M. pigra, followed by the species of Cupriavidus in South and Central America and in Taiwan [32][33][34]. Among 191 rhizobia isolated from the root nodules from three separated populations of M. pigra in Taiwan, 96% and 4% of rhizobia were members of Burkholderia and Cupriavidus, respectively [35].
Rhizobium nodulation enhances the host plant performance through the nitrogen and ammonium supply to host plants [36][37]. The nitrogen-fixing ability of Burkholderia species nodulated with M. pigra was also much greater than that of Cupriavidus species [35]. M. pigra nodulated vigorously even under flooded condition and fixed substantial quantities of nitrogen [38][39][40].
Rhizobium species, Burkholderia mimosarum sp. nov. was isolated from the root nodules of M. pigra population in Taiwan and Venezuela. However, the strains of Burkholderia mimosarum sp. nov. from Taiwan (invasive range of M. pigra) differed from the strain from Venezuela (native range of M. pigra) [33][35][41]. The strain LMG 23256T of Burkholderia mimosarum sp. nov., which was isolated from the root nodules of the M. pigra population in Taiwan, was highly effective for nitrogen fixing than the strains from Venezuela [42]. The Taiwan strains showed fast growing and fast colony-forming ability [43][44] and outcompeted other rhizobium species for nodulation with M. pigra under flooded conditions [34].
Ninety rhizobia isolated from the root nodules of M. pigra in an Australia population (i.e., an invasive range) were characterized as Burkholderia spp., which are also the main rhizobia in Tropical America (i.e., native ranges) [32][33][34]. The strains of Burkholderia in Australia showed divergent lineages, and all of them did not have a close relationship to the Burkholderia strains in the native ranges. Inoculation of M. pigra with the Australian Burkholderia strains showed equal or higher nodule nitrogenase activity than that of with the Tropical American Burkholderia strains, which resulted in its high plant growth rate. Therefore, the M. pigra population in Australia acquired more effective nitrogen-fixing symbionts compared to the M. pigra population in the native ranges [45].
A high level of arbuscular mycorrhizal fugus colonization was found in the flooded roots of M. pigra in wetlands [46][47]. The dominant mycorrhizal fungi in the M. pigra roots are the members of the Rhizophagus and Glomus genera, which belong to the Glomerales order and are considered to be generalist mycorrhizal fungi [48]. Arbuscular mycorrhizal fungi enhance their host plant performance through increasing water and nutrient acquisition, photosynthetic activity, and defense functions against the pathogen attacks and stress conditions [49][50][51]. Arbuscular mycorrhizal fungi also improve the host plant performance even in wetland conditions [52].
Those observations suggest that M. pigra associates actively with rhizobia and arbuscular mycorrhizal fungi even under flooded conditions. M. pigra in the invasive ranges may colonize with rhizobia, which possess high nitrogen-fixing activity compared to that of its native ranges (Table 1). The mutualism with rhizobia with high nitrogen-fixing activity in the invasive ranges may contribute to the invasiveness of the species.
5. Allelopathy
Many secondary metabolites in the invasive plants exhibit the function of allelopathy [53][54][55][56]. Allelopathy is the interaction between donor plants and their neighboring plants through certain secondary metabolites that are defined as allelochemicals [57][58][59][60]. The allelochemicals are released into the vicinity of the donor plants either by volatilization, rainfall leachates, root exudation and decomposition processes of donor plant residues, and they suppress the germination, growth and establishment of neighboring plants, as well as exhibiting mutualism with rhizobia and arbuscular mycorrhizal fungi [61][62][63][64][65][66][67]. Since allelochemicals are synthesized and stored in certain plant tissues until releasing into the vicinity of donor plants [57][58][59][60], several researchers determined the allelopathic activity in the residues of the leaves and extracts from different plant parts of M. pigra.
The leaf powder of M. pigra was mixed with soil and then the seeds of Ruellia tuberosa L. were sown into the mixture. The mixture suppressed the germination and growth of Ruellia tuberosa [68]. Ruellia tuberosa is also invasive species from Central and South America [69]. Aqueous extracts of M. pigra leaf powder inhibited the germination and growth of Vigna radiata (L.) R. Wilczek in an extract concentration-dependent manner [70]. The leaves and stems of M. pigra were soaked in boiling water for 10 min and the obtained solutions inhibited the root growth of Allium cepa L. and disturbed the cell division of its meristematic root cells, such as reducing the mitotic index and increasing chromosomal aberrations [71]. Methanol leaf extracts of M. pigra suppressed the root and shoot growth of Ruellia tuberosa, Echinochloa crus-galli (L.) P. Beauv. and Lactuca sativa L. The extracts showed the reduction of cell viability of their roots and also disturbed the mitosis of their root cells in a concentration-dependent manner. The extracts also increased lipid peroxidation in their roots and shoots [72][73].
Those observations suggest that the leaf residues and leaf and stem extracts of M. pigra exhibit allelopathic activity that influences the germination and growth of some plant species, as well as probably contains water and methanol extractable allelochemicals. Some of these allelochemicals would be liberated into the soil during their decomposition processes of the residues. Total annual litterfall of M. pigra was estimated to be 758 g m2 [74], and such a litterfall may be one of the sources of allelochemicals of the species. Allelochemicals of the invasive plant species suppressed the regeneration process of the native plant species in their invasive ranges [53][54][55][56][57][58][59][60][61][62][63][64][65][66][67]. Allelochemicals of M. pigra may also suppress the regeneration process of the native plant species through the inhibition of their germination and growth. Total concentrations of flavonoids, tannins and saponins were estimated in M. pigra leaves [73]. However, there has been no information available on the isolation and identification of the allelochemicals from M. pigra.
Mimosine (synonym; leucenol) was first isolated from Mimosa pudica L. [75] and found in some other species of the Mimosa and Leucaena genera [76][77][78]. Mimosine has shown a wide range of biological properties such as allelopathic, anti-tumor, apoptotic, anti-inflammation, anti-viral, and cell cycle blocking activity [79]. However, mimosine has not yet been identified in M. pigra.
6. Secondary Metabolites
Pharmacological investigations showed that M. pigra contains several secondary metabolites, which have pharmacological activity such as analgesic, antipyretic, anti-inflammatory, anti-diabetic, anticancer and antioxidant activity [80][81][82]. The methanol extracts of M. pigra leaves showed antioxidant and anti-inflammatory actions in the Wister rat, and quercitrin (quercetin 3-O-rhamnoside) and myricitrin (myricetin 3-O-rhamnoside) were isolated from the extracts [80] (Figure 2). The methanol extracts of M. pigra leaves also showed anti-dermatophyte activity, and astragalin, luteolin and quercitrin were isolated from the extracts [81].
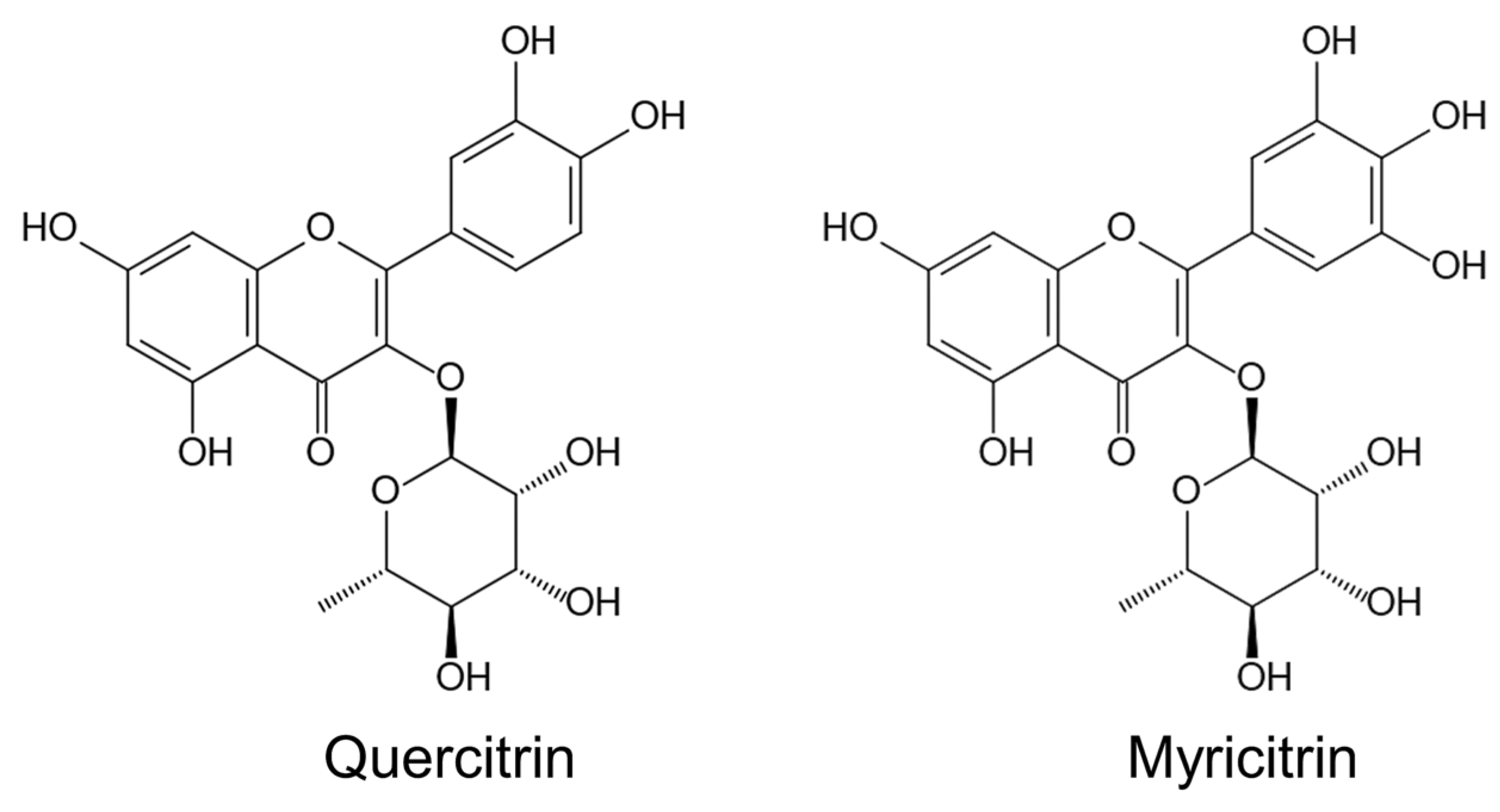
Figure 2. Possible allelochemicals of Mimosa pigra.
Several flavonol glycosides, quercetin (2″-O-galloyl)-3-O-α-L-rhamnopyranoside, quercetin-3-O-α-L-rhamnopyranoside, quercetin-3-O-α-L-arabinopyranoside, myricetin (2″-O-galloyl)-3-O-α-L-rhamnopyranoside and myricetin-3-O-α-L-rhamnopyranoside [83], and quercetin-3-O-α-L-rhamnopyranoside, quercetin-3-O-β-D-galactopyranoside, quercetin-3-O-α-L-arabinopyranoside, myricetin-3-O-α-L-rhamnopyranoside, kaempferol-3-O-α-L-rhamnopyranoside and kaempferol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside, and a flavonoid; 3′,4′,5,7-tetrahydroxyflavone [84], were isolated and identified in the leaf extracts. Terpenoid saponin: machaerinic acid was isolated from stems of M. pigra [85]. A furanochromone, 6,8-dihydroxy-2-methyl-9H-furo(3,2-b)choromen-9-one, was isolated and identified in the leaf extracts of M. pigra [86]. Pharmacological active compounds identified in the plant species of Mimosa genus were also reviewed by Rizwan et al. [82].
Table 1. Possible mechanisms for the Mimosa pigra invasion.
| Characteristic | Reference |
|---|---|
| Rapid growth through the vegetative phase to flowering | [1][2][3][4][5] |
| Self-compatible | [1] |
| High seed output | [3][5][6][7][8][9] |
| High germination rate | [2][3][9] |
| Longevity of seeds | [5][11] |
| Regeneration from remaining plant parts | [9][16] |
| Vigorous mutualism with rhizobia and arbuscular mycorrhizal fungi | [38][39][40][46][47] |
| Colonizing with rhizobia with high nitrogen-fixing activity in the invasive ranges | [34][42][43][44][45] |
| Very few natural enemies in the invasive ranges | [29] |
| Allelopathy | [68][69][70][71][72][73] |
| Secondary metabolites | [80][81][82][83][84][85][86] |
Many flavonoids have shown anti-herbivore, anti-fungal and anti-bacterial activity [87][88][89]. In addition, quercitrin and myricitrin have also been isolated from Ludwigia hexapetala Hook. (i.e., water primrose), and they displayed allelopathic activity [90]. Ludwigia hexapetala is native to Central and South America, it is a noxious invasive species in Western Europe and the United States and it grows well in swampy lands such as the margins of lakes and streams [91]. Quercitrin was reported to work as an allelopathic agent for the other territorial plant kiwifruit (Actinidia deliciosa (A. Chev.) C. F. Liang et A. R. Ferguson), and it has inhibited the growth of several other plant species [92][93].
Although most of the identified compounds in M. pigra have not yet been related to the invasiveness of the plant species, some of them may be involved in allelopathy and defense functions against herbivores and pathogenic fungi. Therefore, these compounds may contribute to the invasiveness and naturalization of M. pigra in the invasive ranges.
References
- Beilfuss, R. Adaptive Management of the Invasive Shrub Mimosa pigra at Gorongosa National Park; Department of Scientific Services: Gorongoza, Mozanbique, 2007; pp. 1–19.
- Lonsdale, W.M.; Miller, I.L.; Forno, I.W. The biology of Australian weeds. 20. Mimosa pigra. Plant Prod. Q. 1989, 4, 119–131.
- Marambe, B.; Amarasinghe, L.; Silva, K.; Gamage, G.; Dissanayake, S.; Seneviratne, A. Distribution, biology and management of Mimosa pigra in Sri Lanka. In Research and Management of Mimosa pigra; Julien, M., Flanagan, G., Heard, T., Hennecke, B., Paynter, Q., Wilson, C., Eds.; CSIRO Entomology: Canberra, Australia, 2004; pp. 85–90.
- Vitelli, J.S.; Madigan, B.A.; Worsley, K.J. Mimosa pigra in Queensland. In Proceedings of the 15th Australian Weeds Conference, Adelaide, Australia, 24–28 September 2006; Weed Management Society of South Australia: Adelaide, Australia, 2006; pp. 251–254.
- Lonsdale, W.M.; Miller, I.L.; Forno, I.W. Mimosa pigra L. In The Biology of Australian Weeds; Groves, R.H., Shepherd, R.C.H., Richardson, R.G., Eds.; RG and FJ Richardson Publisher: Melbourne, Australia, 1995; pp. 169–188.
- Lonsdale, W.M. The biology of Mimosa pigra. In A Guide to the Management of Mimosa pigra; Harley, K.L.S., Ed.; CSIRO: Canberra, Australia, 1992; pp. 8–32.
- Lonsdale, W.; Braithwaite, R.W. The shrub that conquered the bush. New Sci. 1988, 15, 52–55.
- Lonsdale, W.M. Rates of spread of an invading species—Mimosa pigra in northern Australia. J. Ecol. 1993, 81, 513–521.
- Creager, R.A. Seed germination, physical and chemical control of catclaw mimosa (Mimosa pigra var. pigra). Weed Technol. 1992, 6, 884–891.
- van Klinken, R.D.; Goulier, J.B. Habitat-specific seed dormancy-release mechanisms in four legume species. Seed Sci. Res. 2013, 23, 181–188.
- Lukitsch, B.; Elliott, L. Mimosa pigra seed bank remains significant 10 years after stand removal: Further investigation on a floodplain in northern Australia. In Proceedings of the 18th Australasian Weeds Conference, Melbourne, Australia, 8–11 October 2012; Weed Society of Victoria Inc.: Melbourne, Australia, 2012; pp. 364–366.
- Marambe, B.; Amarasinghe, L.; Dissanayake, S. Growth and development of Mimosa pigra: An alien invasive plant in Sri Lanka. In Plant Invasions: Species Ecology and Ecosystem Manageme; Brundu, G., Brock, J., Camarda, I., Child, L., Wade, M., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2001; pp. 115–122.
- Facts Sheet: Restricted Invasive Plant: Mimosa pigra: Queensland Department of Agriculture and Fisheries. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0007/65149/mimosa-pigra.pdf (accessed on 4 April 2023).
- Presnell, K. The potential use of mimosa as fuel for power generation. In Proceedings of the 3rd International Symposium on the Management of of Mimosa pigra, Darwin, Australia, 22–25 September 2002; CSIRO Entomology: Canberra, Australia, 2004; pp. 68–72.
- Paynter, Q.; Flanagan, G.J. Integrating herbicide and mechanical control treatments with fire and biological control to manage an invasive wetland shrub, Mimosa pigra. J. Appl. Ecol. 2004, 41, 615–629.
- Lonsdale, W.M.; Miller, I.L. Fire as a management tool for a tropical woody weed: Mimosa pigra in northern Australia. J. Environ. Manag. 1993, 30, 77–87.
- Thompson, J.D.; McNeilly, T.; Gray, A.J. Population variation in Spartina anglica C.E. Hubbard. I. Evidence from a common garden experiment. New Phytol. 1991, 117, 115–128.
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121.
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia. Engin. 2011, 18, 240–246.
- Warren, R.J.; Matt Candeias, M.; Labatore, A.; Olejniczak, M.; Yang, L. Multiple mechanisms in woodland plant species invasion. J. Plant Ecol. 2019, 12, 201–209.
- NurZhafarina, A.; Asyraf, M. Effects of biotic and abiotic environmental stimuli on the morphology and biomass allocation of Mimosa pigra L. Sains Malays 2017, 46, 1241–1248.
- Pramual, P.; Khumkratok, S.; Wongpakam, K. Population genetics of invasive weed Mimosa pigra L. (Mimosoideae) in Thailand. Pak. J. Bot. 2011, 43, 2721–2726.
- Harley, K.L.S.; Gillett, J.D.; Winder, J.; Forno, I.W.; Segura, R.; Miranda, H.; Kassulke, R.C. Natural enemies of Mimosa pigra and M. berlandieri (Mimosaceae) and prospects for biological control of M. pigra. Environ. Entomol. 1995, 24, 1664–1678.
- Heard, T.A.; Pettit, W. Review and analysis of the surveys for natural enemies of Mimosa pigra: What does it tell us about surveys for broadly distributed hosts? Biol. Control. 2005, 34, 247–254.
- Heard, T.A.; Segura, R. Agents for biological control of Mimosa pigra in Australia: Review and future prospects. In Research and Management of Mimosa pigra; Julien, M., Flanagan, G., Heard, T., Hennecke, B., Paynter, Q., Wilson, C., Eds.; CSIRO Entomology: Canberra, Australia, 2004; pp. 126–140.
- Paynter, Q. Evaluating Mimosa pigra biological control in Australia. In Research and Management of Mimosa pigra; Julien, M., Flanagan, G., Heard, T., Hennecke, B., Paynter, Q., Wilson, C., Eds.; CSIRO Entomology: Canberra, Australia, 2004; pp. 141–148.
- Evans, H.C.; Carrión, G.; Guzman, G. A new species of Sphaerulina and its Phloeospora anamorph, with potential for biological control of Mimosa pigra. Mycol. Res. 1993, 97, 59–67.
- Evans, H.C.; Carrión, G.; Ruiz-Belin, F. Mycobiota of the giant sensitive plant, Mimosa pigra sensu lato in the Neotropics. Mycol. Res. 1995, 99, 420–428.
- Flanagan, G.J.; Wilson, C.G.; Gillett, J.D. The abundance of native insects on the introduced weed Mimosa pigra in Northern Australia. J. Trop. Ecol. 1990, 6, 219–230.
- Moulin, L.; Munive, A.; Dreyfus, B.; Boivin-Masson, C. Nodulation of legumes by members of the b-subclass of proteobacteria. Nature 2001, 411, 948–950.
- Vandamme, P.; Goris, J.; Chen, W.M.; de Vos, P.; Willems, A. Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov. nodulate the roots of tropical legumes. Syst. Appl. Microbiol. 2002, 25, 507–512.
- Barrett, C.F.; Parker, M.A. Coexistence of Burkholderia, Cupriavidus and Rhizobium sp. nodule bacteria on two Mimosa species in Costa Rica. Appl. Environ. Microbiol. 2006, 72, 1198–1206.
- Chen, W.M.; James, E.K.; Coenye, T.; Chou, J.H.; Barrios, E.; de Faria, S.M.; Ellio, N.; Sheu, S.Y.; Sprent, J.I.; Vandamme, P. Burkholderia mimosarum sp. nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. Int. J. Syst. Evol. Microbiol. 2006, 56, 1847–1851.
- Elliott, G.N.; Chou, J.H.; Chen, W.M.; Bloemberg, G.V.; Bontemps, C.; Martínez-Romero, E.; Velázquez, E.; Young, J.P.W.; Sprent, J.I.; James, E.K. Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ. Microbiol. 2009, 11, 762–778.
- Chen, W.M.; James, E.K.; Chou, J.H.; Sheu, S.Y.; Yang, S.Z.; Sprent, J.I. β-Rhizobia from Mimosa pigra, a newly discovered invasive plant in Taiwan. New Pytol. 2005, 168, 661–675.
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050.
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765.
- de Faria, S.M.; de Lima, H.C. Additional studies of the nodulation status of legume species in Brazil. Plant Soil 1998, 200, 185–192.
- Saur, E.; Carcelle, S.; Guezennec, S.; Rousteau, A. Nodulation of legume species in wetlands of Guadeloupe (Lesser Antilles). Wetlands 2000, 20, 730–734.
- James, E.K.; de Fatima Loureiro, M.; Pott, A.; Pott, V.J.; Martins, C.M.; Franco, A.A.; Sprent, J.I. Flooding-tolerant legume symbioses from the Brazilian Pantanal. New Phytol. 2001, 150, 723–738.
- Chen, W.M.; de Faria, S.M.; Straliotto, R.; Pitard, R.M.; Simões-Araùjo, J.L.; Chou, J.H.; Chou, Y.J.; Barrios, E.; Prescott, A.R.; Elliott, G.N.; et al. Proof that Burkholderia forms effective symbioses with legumes: A study of novel Mimosa-nodulating strains from South America. Appl. Environ. Microbiol. 2005, 71, 7461–7471.
- Willems, A.; Tian, R.; Bräu, L.; Goodwin, L.; Han, J.; Liolios, K.; Huntemann, M.; Pati, A.; Woyke, T.; Mavrommatis, K.; et al. Genome sequence of Burkholderia mimosarum strain LMG 23256T, a Mimosa pigra microsymbiont from Anso, Taiwan. Stand. Genom. Sci. 2014, 9, 484–494.
- Howieson, J.G.; Ewing, M.A.; D’antuono, M.F. Selection for acid tolerance in Rhizobium meliloti. Plant Soil. 1988, 105, 179–188.
- Terpolilli, J.J. Why Are the Symbioses between Some Genotypes of Sinorhizobium and Medicago suboptimal for N2 Fixation? Murdoch University: Perth, Australia, 2009; pp. 1–223. Available online: https://researchrepository.murdoch.edu.au/id/eprint/683/ (accessed on 4 April 2023).
- Parker, M.A.; Wurtz, A.K.; Paynter, Q. Nodule symbiosis of invasive Mimosa pigra in Australia and in ancestral habitats: A comparative analysis. Biol. Invasions 2007, 9, 127–138.
- Rodríguez, Y.; Dalpé, Y.; Séguin, S.; Fernández, K.; Fernández, F.; Rivera, R.A. Glomus cubense sp. nov., an arbuscular mycorrhizal fungus from Cuba. Mycotaxon 2011, 118, 337–347.
- Santillán-Manjarrez, J.; Solís-Hernández, A.P.; Castilla-Hernández, P.; Maldonado-Mendoza, I.E.; Vela-Correa, G.; Chimal-Hernández, A.; Hernández-Díaz, C.; Signoret-Poillon, M.; Diederik van Tuinen, D.; Rivera-Becerril, F. Exploring plant root-fungal interactions in a neotropical freshwater wetland. Bot. Sci. 2019, 97, 661–674.
- Davison, J.; Öpik, M.; Daniell, T.J.; Moora, M.; Zobel, M. Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol. Ecol. 2011, 78, 103–115.
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; pp. 1–815.
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370.
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 2022, 13, 919166.
- Ramírez-Viga, T.K.; Aguilar, R.; Castillo-Argüero, S.; Chiappa-Carrara, X.L.; Guadarrama, P.; Ramos-Zapata, J. Wetland plant species improve performance when inoculated with arbuscular mycorrhizal fungi: A meta-analysis of experimental pot studies. Mycorrhiza 2018, 28, 477–493.
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523.
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 419–426.
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193.
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant. Ecol. 2012, 213, 1861–1867.
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578.
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326.
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028.
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672.
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551.
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235.
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521.
- Koodkaew, I.; Rottasa, R. Allelopathic effects of giant sensitive plant (Mimosa pigra) leaf powder on germination and growth of popping pod and purslane. Int. J. Agric. Biol. 2017, 19, 1113–1118.
- Oso, O.A.; Ajayi, B.; Okanume, O.E. Descriptive anatomy of invasive weed, Ruellia tuberosa Linn. Asian J. Res. Bot. 2022, 7, 31–36.
- Prasangani, W.D.; Sinhalage, I.D.; Karunathilake, A.A.; Madusinghe, M. Phytotoxicity Effect of three invasive plant species: Mimosa pigra, Parthenium hysierophorus, Ulex europaeus. In Proceedings of the Research Symposium of Uva Wellassa University, Badulla, Sri Lanka, 22–23 November 2012.
- Araújo, M.S.; Santos, S.P.; Barros-Filho, B.A.; Lima, M.M.O.; Leite, A.S. Toxicogenetic potential of Mimosa pigra (Fabaceae) infusion in Allium cepa meristematic cells. Genet. Mol. Res. 2020, 19, gmr18588.
- Koodkaew, I.; Wannathong, R. Effects of Mimosa pigra L. leaf extract on growth behavior of Ruellia tuberosa L. and Echinochloa crus-galli (L.) P. Beauv. Asia-Pac. J. Sci. Technol. 2018, 23, APST-23-07.
- Koodkaew, I.; Senaphan, C.; Sengseang, N.; Suwanwong, S. Characterization of phytochemical profile and phytotoxic activity of Mimosa pigra L. Agric. Nat. Resour. 2018, 52, 162–168.
- Lonsdale, W.M. Litterfall in an Australian population of Mimosa pigra, an invasive tropical shrub. J. Trop. Ecol. 1988, 4, 381–392.
- Renz, J. Mimosine. Physiol. Chem. 1936, 244, 153–158.
- Brewbaker, J.L.; Hylin, J.W. Variations in mimosine content among Leucaena species and related mimosaceae. Crop Sci. 1965, 5, 348–349.
- Smith, I.K.; Fowden, L.A. Study of mimosine toxicity in plants. J. Exp. Bot. 1996, 17, 750–761.
- Soedarjo, M.; Borthakur, D. Mimosine produced by the tree-legume Leucaena provides growth advantages to some Rhizobium strains that utilize it as a source of carbon and nitrogen. Plant Soil 1996, 186, 87–92.
- Nguyen, B.C.; Tawata, S. The chemistry and biological activities of mimosine: A review. Phytother. Res. 2016, 30, 1230–1242.
- Rakotomalala, G.; Agard, C.; Tonnerre, P.; Tesse, A.; Derbré, S.; Michalet, S.; Hamzaoui, J.; Rio, M.; Cario-Toumaniantz, C.; Richomme, P.; et al. Extract from Mimosa pigra attenuates chronic experimental pulmonary hypertension. J. Ethnopharm. 2013, 148, 106–116.
- De Morais, C.B.; Scopel, M.; Pedrazza, G.P.R.; da Silva, F.K.; Dalla Lana, D.F.; Tonello, M.L.; Miotto, S.T.S.; Machado, M.M.; De Oliveira, L.F.S.; Fuentefria, A.M.; et al. Anti-dermatophyte activity of Leguminosae plants from Southern Brazil with emphasis on Mimosa pigra (Leguminosae). J. Mycol. Med. 2017, 27, 530–538.
- Rizwan, K.; Majeed, I.; Bilal, M.; Rasheed, T.; Shakeel, A.; Iqbal, S. Phytochemistry and diverse pharmacology of Genus Mimosa: A review. Biomolecules 2022, 12, 83.
- Okonkwo, C.J.; Njoku, O.U.; Okonkwo, T.J.; Afieroho, O.E.; Proksch, P. Two new acylated flavonol glycosides from Mimosa pigra L. leaves sub-family Mimosoideae. Future J. Pharm. Sci. 2016, 2, 71–75.
- Hawwal, M.F.; Ali, Z.; Fantoukh, O.I.; Chittiboyina, A.G.; Khan, I.A. Phytochemical investigation of Mimosa pigra leaves, a sensitive species. Biochem. Syst. Ecol. 2021, 99, 104354.
- Englert, J.; Weniger, B.; Lobstein, A.; Anton, R.; Krempp, E.; Guillaume, D.; Leroy, Y. Triterpenoid saponins from Mimosa pigra. J. Nat. Prod. 1995, 58, 1265–1269.
- Nguyen, L.N.P.; Huu, D.M.N.; Dang, H.P.; Huynh, N.V.; Dang, P.H. A new furanochromone from the leaves of Mimosa pigra. Nat. Prod. Res. 2021, 35, 3963–3969.
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147.
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297.
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265.
- Santonja, M.; Le Rouzic, B.; Thiébaut, G. Seasonal dependence and functional implications of macrophyte-phytoplankton allelopathic interactions. Freshw. Biol. 2018, 63, 1161–1172.
- Thouvenot, L.; Haury, J.; Thiébaut, G. A success story: Water primroses, aquatic plant pests. Aquatic Conservation Mar. Freshw. Ecosyst. 2013, 23, 790–803.
- Okada, S.; Iwasaki, A.; Kataoka, I.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of a phytotoxic substance in kiwifruit leaves. Acta Hortic. 2018, 1218, 207–212.
- Parvez, M.M.K.; Yokotani, T.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635.
More
Information
Subjects:
Plant Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
17 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





