
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zoltan Molnar | -- | 2120 | 2023-05-15 17:46:17 | | | |
| 2 | Dean Liu | Meta information modification | 2120 | 2023-05-16 02:39:11 | | |
Video Upload Options
One of the most significant constraints on agricultural productivity is the low availability of iron (Fe) in soil, which is directly related to biological, physical, and chemical activities in the rhizosphere. The rhizosphere has a high iron requirement due to plant absorption and microorganism density. Plant roots and microbes in the rhizosphere play a significant role in promoting plant iron (Fe) uptake, which impacts plant development and physiology by influencing nutritional, biochemical, and soil components. The concentration of iron accessible to these live organisms in most cultivated soil is quite low due to its solubility being limited by stable oxyhydroxide, hydroxide, and oxides. The dissolution and solubility rates of iron are also significantly affected by soil pH, microbial population, organic matter content, redox processes, and particle size of the soil. In Fe-limiting situations, plants and soil microbes have used active strategies such as acidification, chelation, and reduction, which have an important role to play in enhancing soil iron availability to plants. In response to iron deficiency, plant and soil organisms produce organic (carbohydrates, amino acids, organic acids, phytosiderophores, microbial siderophores, and phenolics) and inorganic (protons) chemicals in the rhizosphere to improve the solubility of poorly accessible Fe pools. The investigation of iron-mediated associations among plants and microorganisms influences plant development and health, providing a distinctive prospect to further our understanding of rhizosphere ecology and iron dynamics.
1. Introduction
2. Dynamics of Iron in the Rhizosphere
2.1. Status of Fe in the Rhizosphere and Soil
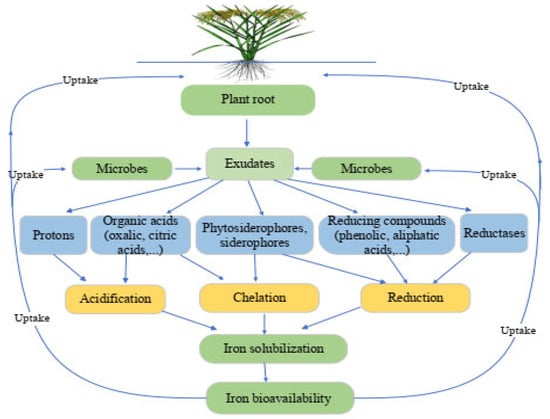
2.2. Iron Interaction with Plant and Rhizospheric Microorganisms
2.3. Impact of Plants and Microorganisms on the Iron Status
References
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M.; et al. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Environ. Sci. 2018, 5, 86.
- Balk, J.; Schaedler, T.A. Iron cofactor assembly in plants. Annu. Rev. Plant Biol. 2014, 65, 125–153.
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99.
- Mahender, A.; Swamy, B.P.M.; Anandan, A.; Ali, J. Tolerance of iron-deficient and -toxic soil conditions in rice. Plants 2019, 8, 31.
- Kim, S.A.; Guerinot, M.L. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007, 581, 2273–2280.
- Kanwar, P.; Baby, D.; Bauer, P. Interconnection of iron and osmotic stress signalling in plants: Is fit a regulatory hub to cross-connect abscisic acid responses? Plant Biol. 2021, 23 (Suppl. S1), 31–38.
- Colombo, C.; Palumbo, G.; He, J.-Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548.
- Schmidt, W.; Thomine, S.; Buckhout, T.J. Editorial: Iron nutrition and interactions in plants. Front. Plant Sci. 2019, 10, 1670.
- Zuo, Y.; Zhang, F. Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil 2011, 339, 83–95.
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Leeuwenhoek 2013, 104, 321–330.
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40.
- Ivanov, R.; Brumbarova, T.; Bauer, P. Fitting into the harsh reality: Regulation of iron-deficiency responses in dicotyledonous plants. Mol. Plant 2012, 5, 27–42.
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152.
- Robin, A.; Vansuyt, G.; Hinsinger, P.; Meyer, J.M.; Briat, J.F.; Lemanceau, P. Chapter 4 iron dynamics in the rhizosphere: Consequences for plant health and nutrition. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2008; pp. 183–225.
- Lemanceau, P.; Bauer, P.; Kraemer, S.; Briat, J.-F. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 2009, 321, 513–535.
- Naranjo-Arcos, M.A.; Maurer, F.; Meiser, J.; Pateyron, S.; Fink-Straube, C.; Bauer, P. Dissection of iron signaling and iron accumulation by overexpression of subgroup ib bhlh039 protein. Sci. Rep. 2017, 7, 10911.
- Carrillo-González, R.; Šimůnek, J.; Sauvé, S.; Adriano, D. Mechanisms and pathways of trace element mobility in soils. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2006; pp. 111–178.
- Mueller, C.W.; Carminati, A.; Kaiser, C.; Subke, J.-A.; Gutjahr, C. Rhizosphere functioning and structural development as complex interplay between plants, microorganisms and soil minerals. Front. Environ. Sci. 2019, 7, 130.
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512.
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642.
- Winkelmann, G.s. Ecology of siderophores with special reference to the fungi. Biometals 2007, 20, 379–392.
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488.
- Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Astolfi, S.; Lehto, N.; Robinson, B.; Brunetto, G.; Terzano, R.; Cesco, S. Nutrient Availability in the Rhizosphere: A Review; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2018.
- Gorka, S.; Dietrich, M.; Mayerhofer, W.; Gabriel, R.; Wiesenbauer, J.; Martin, V.; Zheng, Q.; Imai, B.; Prommer, J.; Weidinger, M.; et al. Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front. Microbiol. 2019, 10, 168.
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Iron. In Principles of Plant Nutrition; Mengel, K., Kirkby, E.A., Kosegarten, H., Appel, T., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 553–571.
- Johnson, D.B.; Kanao, T.; Hedrich, S. Redox transformations of iron at extremely low ph: Fundamental and applied aspects. Front. Microbiol. 2012, 3, 96.
- Rengel, Z.s. Availability of mn, zn and fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409.
- Morrissey, J.; Guerinot, M.L. Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567.
- Onaga, G.; Edema, R.; Aseas, G. Tolerance of rice germplasm to iron toxicity stress and the relationship between tolerance, Fe2+, P and K content in the leaves and roots. Arch. Agron. Soil Sci. 2013, 59, 213–229.
- Hawkes, C.V.; DeAngelis, K.M.; Firestone, M.K. Chapter 1—Root interactions with soil microbial communities and processes. In The Rhizosphere; Cardon, Z.G., Whitbeck, J.L., Eds.; Academic Press: Burlington, VT, USA, 2007; pp. 1–29.
- Van Hees, P.a.W.; Lundströms, U.S. Equilibrium models of aluminium and iron complexation with different organic acids in soil solution. Geoderma 2000, 94, 201–221.
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front. Plant Sci. 2019, 10, 675.
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Genet. 2006, 4, 752–764.
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol. Biochem. 2011, 43, 883–894.
- Xue, Y.; Xia, H.; Christie, P.; Zhang, Z.; Li, L.; Tang, C. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: A critical review. Ann. Bot. 2016, 117, 363–377.
- Gunes, A.; Inal, A.; Adak, M.S.; Alpaslan, M.; Bagci, E.G.; Erol, T.; Pilbeam, D.J. Mineral nutrition of wheat, chickpea and lentil as affected by mixed cropping and soil moisture. Nutr. Cycl. Agroecosystems 2007, 78, 83–96.
- Zuo, Y.; Liu, Y.; Zhang, F.; Christie, P. A study on the improvement iron nutrition of peanut intercropping with maize on nitrogen fixation at early stages of growth of peanut on a calcareous soil. Soil Sci. Plant Nutr. 2004, 50, 1071–1078.
- Lemanceau, P.; Expert, D.; Gaymard, F.; Bakker, P.; Briat, J.F. Chapter 12 role of iron in plant–microbe interactions. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; pp. 491–549.
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 2016, 99, 39–48.
- Jin, C.W.; Li, G.X.; Yu, X.H.; Zheng, S.J. Plant fe status affects the composition of siderophore-secreting microbes in the rhizosphere. Ann. Bot. 2010, 105, 835–841.
- Lurthy, T.; Pivato, B.; Lemanceau, P.; Mazurier, S. Importance of the rhizosphere microbiota in iron biofortification of plants. Front. Plant Sci. 2021, 12, 744445.
- Fujii, K. Soil acidification and adaptations of plants and microorganisms in bornean tropical forests. Ecol. Res. 2014, 29, 371–381.
- Wang, Y.; Kang, Y.; Zhong, M.; Zhang, L.; Chai, X.; Jiang, X.; Yang, X. Effects of iron deficiency stress on plant growth and quality in flowering chinese cabbage and its adaptive response. Agronomy 2022, 12, 875.
- M’sehli, W.; Youssfi, S.; Donnini, S.; Dell’orto, M.; De Nisi, P.; Zocchi, G.; Abdelly, C.; Gharsalli, M. Root exudation and rhizosphere acidification by two lines of medicago ciliaris in response to lime-induced iron deficiency. Plant Soil 2008, 312, 151–162.




