Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cyprine Neba Funeh | -- | 1298 | 2023-05-13 09:26:51 | | | |
| 2 | Sirius Huang | Meta information modification | 1298 | 2023-05-15 03:22:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Funeh, C.N.; Bridoux, J.; Ertveldt, T.; De Groof, T.W.M.; Chigoho, D.M.; Asiabi, P.; Covens, P.; D’huyvetter, M.; Devoogdt, N. Biological Vectors in Targeted Radionuclide Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/44230 (accessed on 11 January 2026).
Funeh CN, Bridoux J, Ertveldt T, De Groof TWM, Chigoho DM, Asiabi P, et al. Biological Vectors in Targeted Radionuclide Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/44230. Accessed January 11, 2026.
Funeh, Cyprine Neba, Jessica Bridoux, Thomas Ertveldt, Timo W. M. De Groof, Dora Mugoli Chigoho, Parinaz Asiabi, Peter Covens, Matthias D’huyvetter, Nick Devoogdt. "Biological Vectors in Targeted Radionuclide Therapy" Encyclopedia, https://encyclopedia.pub/entry/44230 (accessed January 11, 2026).
Funeh, C.N., Bridoux, J., Ertveldt, T., De Groof, T.W.M., Chigoho, D.M., Asiabi, P., Covens, P., D’huyvetter, M., & Devoogdt, N. (2023, May 13). Biological Vectors in Targeted Radionuclide Therapy. In Encyclopedia. https://encyclopedia.pub/entry/44230
Funeh, Cyprine Neba, et al. "Biological Vectors in Targeted Radionuclide Therapy." Encyclopedia. Web. 13 May, 2023.
Copy Citation
The precise delivery of cytotoxic radiation to cancer cells through the combination of a specific targeting vector with a radionuclide for targeted radionuclide therapy (TRT) has proven valuable for cancer care. TRT is increasingly being considered a relevant treatment method in fighting micro-metastases in the case of relapsed and disseminated disease. While antibodies were the first vectors applied in TRT, increasing research data has cited antibody fragments and peptides with superior properties and thus a growing interest in application.
antibody fragments
peptides
radionuclide
radiopharmaceutical
targeted radionuclide therapy
vectors
cancer
bio-vectors
1. Introduction
The goal of targeted radionuclide therapy (TRT) is to precisely deliver a toxic dose of ionizing radiation to disease sites by leveraging the specificity of biomolecules in targeting certain molecular patterns expressed by cancer cells. In the field of oncology, this is used for curative, suppressive, or palliative outcome. Surgery and external beam radiotherapy have been used successfully in treating cancer; however, drawbacks including residual disease, relapse, and disseminated disease remain a concern [1]. Chemotherapy proved to be useful in fighting disseminated and residual diseases but suffers from chemoresistance and toxicity due to the non-specific nature of the mechanism of action. Targeted therapies soon developed as an alternative to overcome the constraints of conventional therapies. Targeted therapies are an inventive treatment strategy that incorporates a diagnostic step to determine the presence of molecular patterns of interest that ensure a given patient will benefit from the drug.
TRT is an attractive concept developed about 4 decades ago that has proven very useful as an add-on to chemotherapeutic modalities or as a preferred alternative where other modalities fail. This is especially useful in disseminated disease and relapse. TRT uses high-affinity and specific targeting vectors that can be administered either compartmentally or systemically, giving it the advantage of targeting both primary and metastasized cancer cells. For a radiopharmaceutical to be effective, a molecular target (receptor or antigen) exclusively expressed or (over)expressed on cancer cells must be identified. A biomolecule (targeting vector) with high specificity and affinity against the molecular target is then generated. Subsequently, a suitable radionuclide is selected and linked to the targeting vector using a suitable linkage chemistry that yields a stable radiopharmaceutical construct. This construct circulates, traces, and binds to its target, leading to an in situ radioactive decay that is toxic to the cancer cells [2]. Figure 1 depicts a schematic representation of TRT with key characteristics to consider for each component that constitutes a therapeutic radiopharmaceutical. Acting as a guide for a successful outcome of TRT, a radiotracer with similar pharmacokinetic properties as the treatment compound is usually administered followed by imaging to picture the specific accumulation of the compound to the intended site. This diagnostic step allows for patient selection, dose estimation, approximation of adverse events, therapy monitoring, and treatment follow-up.
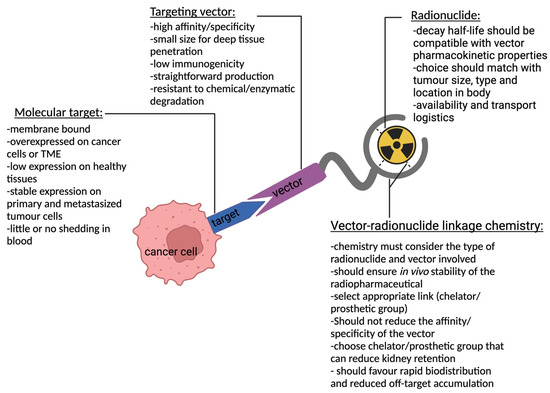
Figure 1. Schematic representation of TRT and key characteristics for each component depicting a targeting vector linked to a radionuclide using a chelator/prosthetic group that binds to a molecular target expressed on a cancer cell.
With remarkable clinical outcomes, the field of TRT improved over the past two decades with five approved compounds: yttrium-90 ibritumomab (Zevalin®), iodine-131 toxitumomab (BEXXAR®), iodine-131 metuximab F(ab’)2 (Licartin®), lutetium-177 oxodotreotide (Lutathera®), and lutetium-177 PSMA-617 (Pluvicto™). Dozens of other radiotherapeutic compounds are in clinical development [3][4][5]. Amongst a plethora of targeting vectors studied, monoclonal antibodies (mAbs) were the first vectors to be exploited, with two compounds (Zevalin® and BEXXAR®) approved by the FDA for the treatment of indolent B-cell lymphoma. However, a wide array of limitations has stalled the advancement and effective use of mAbs for TRT. This has opened the window for the development of more promising bio-vectors, peptides, and antibody fragments with superior properties as alternatives to mAbs.
2. Types of Biological Vectors in TRT
The high affinity, specificity, and professional capacity of mAbs as inherent self-proteins that bind to pathological molecules are astonishing properties that led to their application as vectors for transporting radioactivity to cancer cells. Despite the maturity of the science behind radiopharmaceuticals, particularly vector design and linkage chemistry, mAb-radioconjugates remain largely unsuccessful in treating solid tumors; however, they have recorded successes in treating hematological tumors. The inefficiency in treating solid tumors is due to their large sizes (~150 kDa), that precipitates low penetration of the radioconjugate in disease tissues. Moreso, the presence of the Fc region in immunoglobulins G (IgG) mediates FcRn recycling of mAbs, extending their availability in circulation. Consequently, the long biological half-life of 10–21 days (depending on the isotype) results in off-target accumulation of radioactivity in highly vascularized organs and thus causes myelotoxicities [6]. These factors and others have resulted in decreased use of mAbs as vectors in treating solid tumors, with two times fewer clinical trials using radiolabeled mAbs for TRT from phase I to III conducted within the past 10 years [6].
To overcome the intrinsic limitations of mAbs, bio-vectors (refered to here as peptides and antibody fragments) have been explored as alternatives. Focused on improved pharmacokinetics, better tissue penetration, and increased tumor-to-normal-tissue dose, these vectors proved to have superior properties over mAbs and virtually fit as perfect vectors for TRT [6]. Bio-vectors can be rapidly designed, synthesized, or expressed in microbial expression systems with high yields and low production costs. Their small sizes (peptides: 0.5–5 kDa; scaffold proteins: 2–20 kDa; antibody fragments: 12–110 kDa) enable rapid bio-distribution and rapid renal clearance (±65 kDa glomerular filtration threshold), resulting in a short biological half-life, access to cryptic and challenging epitopes, as well as deep penetration and homogenous distribution in tumors [7]. Bio-vectors lack a functional Fc fragment (Figure 2), resulting in the absence of immune cell activation (complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity), FcRn mediated recycling, and thus improved pharmacokinetics. The short half-life of bio-vectors in circulation allows the use of short-lived radionuclides (bismuth-213; t1/2 46 min, astatine-211; t1/2 7.2 h) in TRT, which are not commonly used with mAbs. This increases the spectrum of radionuclides that can be used in TRT. However, the rapid clearance and subsequent reabsorption of bio-vectors by the proximal tubules of the kidneys poses a risk of nephrotoxicity [8]. Moreso, rapid clearance may require higher or repeated dosing to ensure therapeutic efficacy. This has necessitated the development of different strategies that can overcome these downsides (as reviewed in [9]). Among many peptides investigated, somatostatin analogues such as octreotide and prostate-specific membrane antigen (PSMA) have been the most explored. Others include cholecystokinin, bombesin, and exendin analogues. Scaffold proteins under investigation include bicyclic peptides, cysteine knots, FN3 scaffolds, DARPins, ADAPTs, and affibodies [7][10]. Single-domain antibodies (SdAbs), F(ab’)2, Fab, scFv, minibodies, and diabodies (Figure 2) constitute the antibody fragments that have been studied at the pre-clinical and clinical levels for TRT with remarkable results.
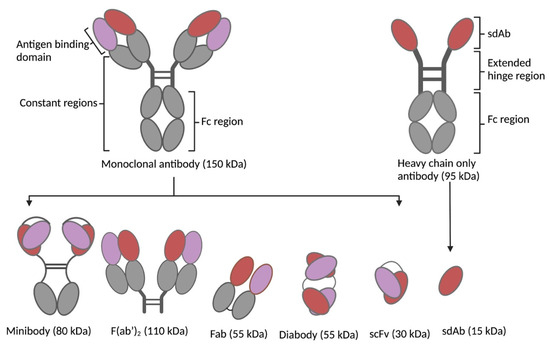
Figure 2. Antibodies and respective fragments investigated as targeting vectors for TRT: minibody, F(ab’)2, Fab, diabody, single-chain variable fragment (scFv), and SdAb.
3. Development Status of Biological Vectors in TRT
In the last decade, a surge in radiolabeled bio-vectors developed for TRT was observed. Strikingly, the approvals of [131I]I-metuximab-F(ab’)2 (Licartin®) by the Chinese FDA for the treatment of metastatic refractory hepatocellular carcinoma, [177Lu]Lu-DOTATATE (Lutathera®) by the EMA and FDA for the treatment of metastatic and inoperable gastroenteropancreatic neuroendocrine tumors (GEP-NET), and more recently [177Lu]Lu-PSMA-617 (Pluvito™) for the treatment of PSMA-positive metastatic castration-resistant metastatic prostate cancer have played a pivotal role. Several compounds have been pre-clinically developed or are in development, with dozens in early-stage or advanced clinical studies. As the use of bio-vectors has matured and shown increasing evidence of safety and efficacy compared to mAb approaches, an upsurge in early investment by pharma companies has become noticeable. This raises hope for the much-needed funding for randomized clinical trials that are required to establish the safety and efficacy of bio-radiopharmaceuticals. The field of bio-radiopharmaceuticals remains promising; however, as new compounds are introduced, major limitations linger and need to be tackled: from design to clinical application and improvements in the therapeutic index.
References
- Carter, L.; Poty, S.; Sharma, S.K.; Lewis, J.S. Preclinical optimization of antibody-based radiopharmaceuticals for cancer imaging and radionuclide therapy-Model, vector, and radionuclide selection. J. Label. Compd. Radiopharm. 2018, 61, 611–635.
- Dash, A.; Knapp, F.F.R.; Pillai, M.R.A. Targeted Radionuclide Therapy—An Overview. Curr. Radiopharm. 2013, 6, 152–180.
- Chen, Z.-N.; Mi, L.; Xu, J.; Song, F.; Zhang, Q.; Zhang, Z.; Xing, J.-L.; Bian, H.-J.; Jiang, J.-L.; Wang, X.-H.; et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical Phase I/II trials. Int. J. Radiat. Oncol. 2006, 65, 435–444.
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium177PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60.
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114.
- Rondon, A.; Rouanet, J.; Degoul, F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers 2021, 13, 5570.
- Krasniqi, A.; D’Huyvetter, M.; Devoogdt, N.; Frejd, F.Y.; Sörensen, J.; Orlova, A.; Keyaerts, M.; Tolmachev, V. Same-day imaging using small proteins: Clinical experience and translational prospects in oncology. J. Nucl. Med. 2018, 59, 885–891.
- Vegt, E.; de Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and an-tibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058.
- Chigoho, D.M.; Bridoux, J.; Hernot, S. Reducing the renal retention of low- to moderate-molecular-weight radiopharmaceuticals. Curr. Opin. Chem. Biol. 2021, 63, 219–228.
- Garousi, J.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Imaging using radiolabelled targeted proteins: Radioimmunodetection and beyond. EJNMMI Radiopharm. Chem. 2020, 5, 16.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
15 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




