Hybrid nanostructured materials composed of transition metal oxides/hydroxides, metal chalcogenides, metal carbides, metal–organic frameworks, carbonaceous compounds and polymer-based porous materials have been used as electrodes for designing energy storage systems such as batteries, supercapacitors (SCs), and so on. Available energy storage devices can be classified into various types; for example, based on storage duration, there are three types: short-, mid-, and long-term. Depending on their reaction time, they are considered to be either rapid or slow. According to their storage capacity, they can be classified as small-, medium-, or large-scale. There are many techniques for the storage of various types of energies, including electrical, mechanical, chemical, and thermal. Moreover, based on the precise needs and applications, storage technologies have different technical and economic criteria.
1. Types of Energy Storage Systems
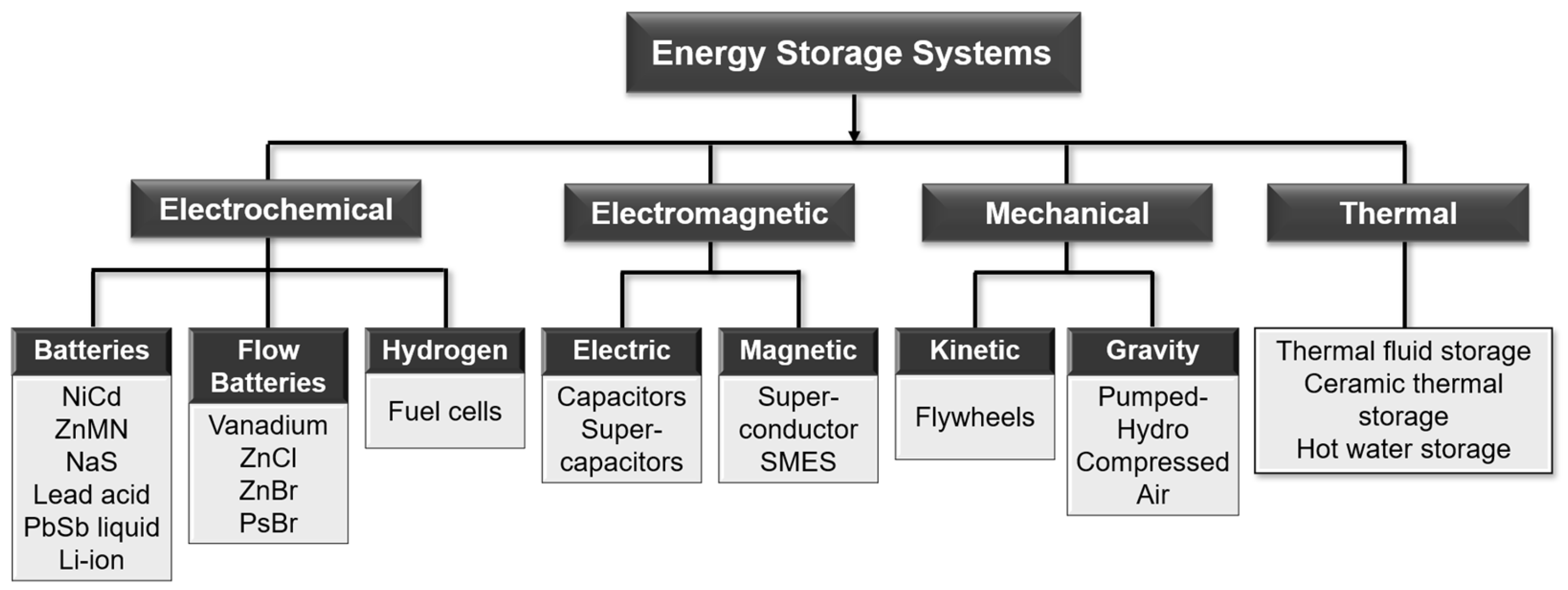
The classification of energy storage systems is consistent with the various associated shapes; energy is often stored within a mold, which can be electrochemical, electromagnetic, mechanical, and thermal one (
Figure 1). Hydrogen batteries and fuel cells are electrochemical systems, while electromagnetic systems include SCs and superconductors. Mechanical systems are often divided into kinetic energy storage (e.g., flywheels) and potential energy storage (e.g., pumped hydraulic and compressed gas systems)
[1][2].
Figure 1. Classification of energy storage systems based on their natural construction.
Techniques and devices are constantly changing and improving to meet the increasing demands for energy storage devices. The battery, as the primary systematic energy memory device, remains the leading and most widely used technique, with a general efficiency of over 90%. A battery may be a chemical device, storing electricity within chemicals and converting the stored energy into a direct current (DC) through an electrochemical reaction. A battery usually consists of three main components: two electrodes and an electrolyte. It also consists of terminals, a separator, and a container. There are two types of electrodes: anode (negative electrode) and cathode (positive electrode)
[3]. The Italian physicist Alessandro Volta invented the first battery (in the modern sense) in 1800, which consisted of disks of zinc and copper, while a concentrated salt solution (brine) was used as an electrolyte. To date, many different types of batteries have been reported, such as Ni-Cd, Li-O
2, and Li-S batteries
[4][5].
2. Batteries for Energy Storage
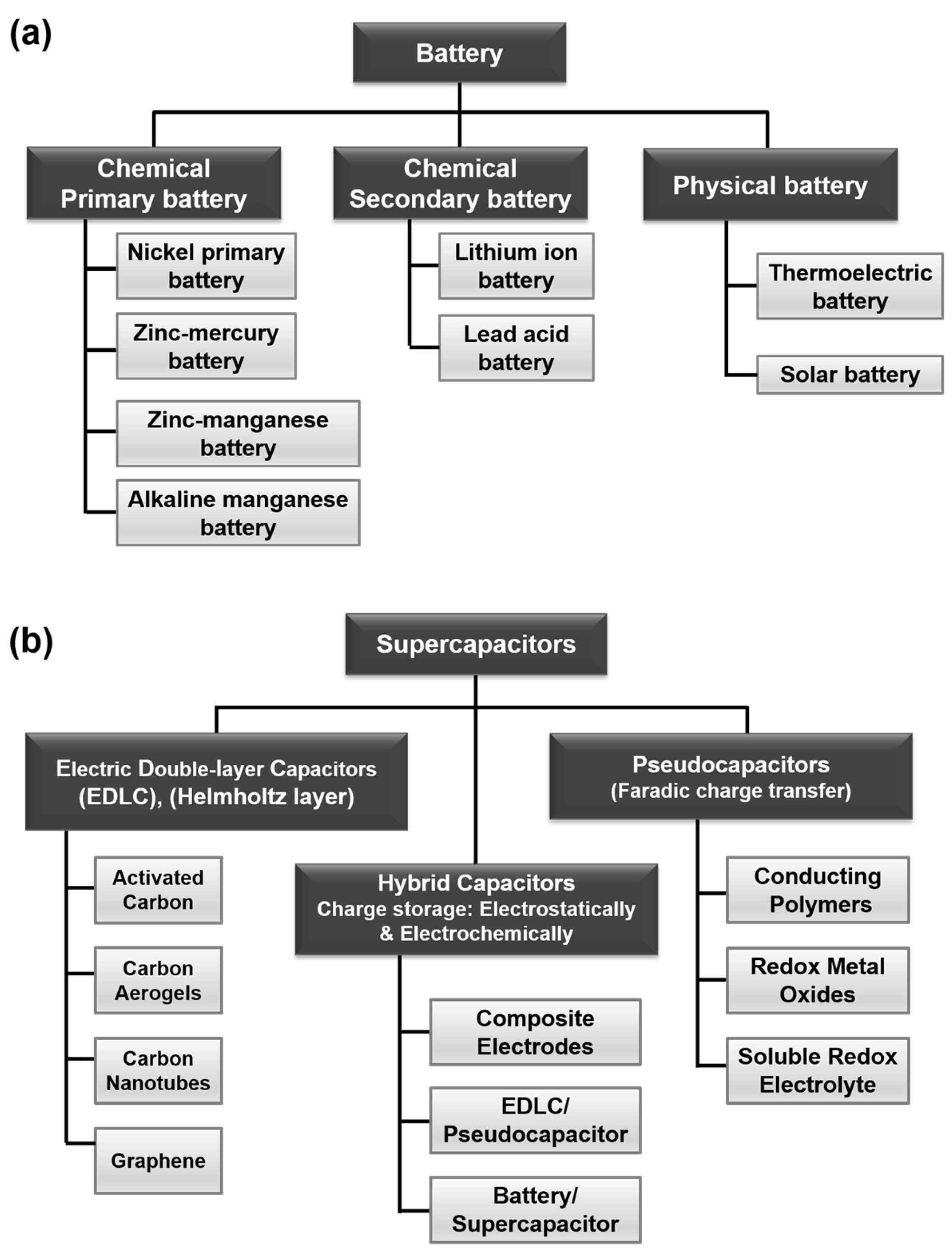
There are two main types of batteries: physical and chemical batteries (Figure 2a). A physical battery is a device that directly converts solar power, thermal energy, or atomic energy into DC electricity using physical effects, such as solar cells, thermoelectric generators, core batteries, and so on. A chemical battery is a device that converts energy directly into DC electricity. Chemical batteries are the most common type of batteries, and can be classified into two categories: primary (non-rechargeable) and secondary (rechargeable). A primary battery is a straightforward and convenient power source, such as a zinc–manganese or alkaline–manganese battery, which cannot be charged electrically. They are utilized in household applications for various portable electronic and electrical appliances, such as lamps, cameras, watches, toys, radios, and so on. A secondary battery is commonly referred to as a rechargeable battery, which may be recharged after being discharged to its original state by a current through the cells during discharge. For instance, lead–acid and lithium-ion batteries are well-reported rechargeable batteries. It should be noted that the energy storage capacity of an electrochemical system is restricted by the electrochemical properties of the electrode materials. Therefore, storage abilities need to be increased through the use of coupling (or hybridizing) with materials having very low equivalent weights. At present, the world is entering a new era of digital technologies featuring updated energy storage devices.
Figure 2. Classifications of: (a) batteries; and (b) supercapacitors based on electrode materials and charge storage mechanism.
3. Supercapacitors for Energy Storage
After the invention of rechargeable batteries, another storage device, known as a capacitor, has been widely used for this purpose. A supercapacitor (SC) is an upgraded version of a capacitor. In this line, the hybridization of a battery and capacitor can be accomplished to achieve high energy storage efficiency. The general performance of an SC is mainly governed by the nature and structure of the electrodes, separator, current collector, and electrolytes. SCs provide many advantages over batteries, such as a high power density (>10 kW kg
−1), quicker charging/discharging speed (within seconds), cyclic stability (>100,000 cycles), and being more cost-effective and safer
[6][7].
Figure 2b exhibits the different types of SCs. Based on their charge storage behavior and electrode materials, there are three kinds of supercapacitors: electrochemical double-layer capacitors (EDLCs), pseudo-capacitors, and hybrid capacitors
[8]. The most well-known type of energy storage technology now employed in industrial applications is the EDLC. Here, electricity is stored at the interface between the electrolyte and electrode through Helmholtz double layers
[9]. As this process does not allow for a Faradaic redox reaction, the swelling of the active material, EDLCs typically present high stability and rapid charge/discharge rates. Due to their environmental friendliness, large specific surface area, good electrical conductivity, high chemical stability, and wide working temperature range, carbon-based materials such as graphene, activated charcoal, CNTs are used to make the majority of EDLC electrodes. Furthermore, depending on the type of electrolyte utilized, EDLC performance might be altered. However, the energy density of EDLC equipment is constrained as a result of the electrostatic surface charging mechanism, which severely limits the use of EDLCs.
On the other hand, pseudocapacitor electrodes are constructed of some redox components, such as polymers, metal oxides, or hydroxides
[10]. Moreover, their charge storage mechanism follows a fast Faradaic reaction. The formation of such an electrical double layer near the surface provides larger capacitance with high energy density, compared to other capacitors. As pseudocapacitors can store charge through electroporation, redox reactions, or intercalation, they have higher capacitance and energy density than EDLCs. In contrast to EDLCs, pseudocapacitance can be created both on the electrode surface and throughout the entire electrode, resulting in a higher energy density and larger capacitance.
Another type is called hybrid capacitors, which are a combination of EDLCs and pseudocapacitors, with higher specific capacitance compared to each individual type. A hybrid capacitor accumulates charges either electrostatically or electrochemically, using electrochemical or electrostatic absorption-desorption or oxidation-reduction reactions. The hybrid supercapacitor is further divided into three categories: battery-type hybrid, composite hybrid, and asymmetric hybrid. With its extensive power and energy density, as well as improved cycling stability, the hybrid supercapacitor can perform well
[11]. In addition, SCs may have various geometric properties, such as flexible SCs including a skinny film, sandwich-type, and planar structure (e.g., as integrated micro-SCs). Flexible SCs are ultrafast rechargeable energy storage devices. It is necessary to utilize a material with very good conductivity, high mechanical rigidity, compact structure, and lightweight properties for the design of flexible SC devices. A planar structure design enhances the rapid ion transfer within the 2D-direction of flexible SCs
[12][13][14].
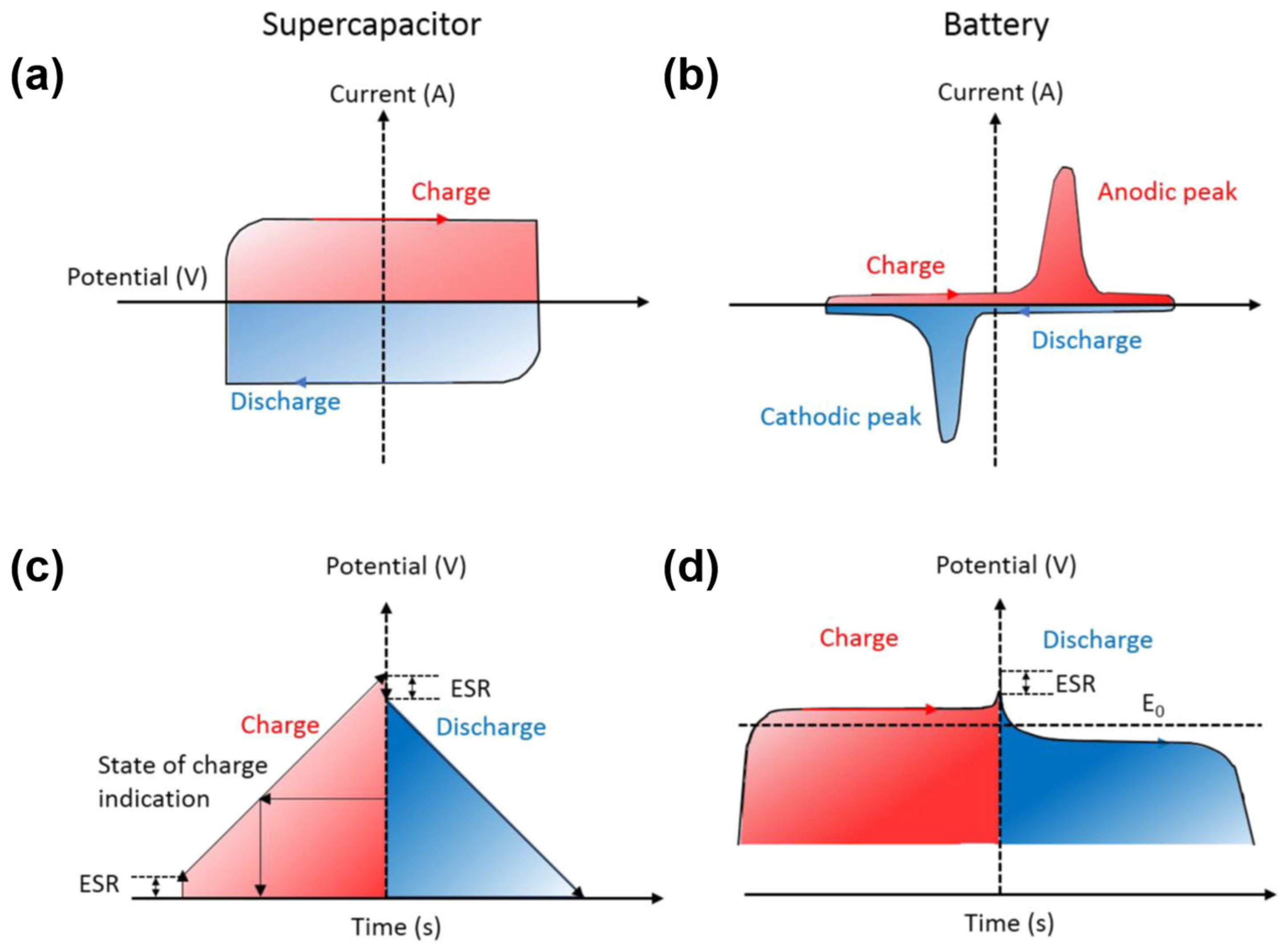
In terms of the storage and release of electricity, batteries and capacitors are very similar; however, they perform in a completely different manner. In general, batteries offer better energy density for storage, whereas capacitors may charge and discharge more quickly
[15]. The supercapacitor’s cyclic voltammetry (CV) curve (
Figure 3a) is rectangular during the charge and discharge operation, yet the current is nearly constant. Additionally, the galvanostatic charge/discharge (GCD) curve (
Figure 3c) of this device is typically inclined with a constant slope. Generally, a battery keeps its voltage steady except when it is close to 100% charged/discharged (TOC/EOD), during which it exhibits Faradaic reactions and its CV curve displays a clear redox peak (
Figure 3b,d). In addition, the GCD curve shows a relatively flat charge-discharge platform. At the same time, supercapacitors must be integrated with a DC-DC converter for applications requiring a consistent output voltage to control and maintain the output voltage. The energy stored in these two types of electrodes is measured differently (in terms of capacitance vs. capacity) due to the difference in the charge-storage process
[16].
Figure 3. (
a,
b) Cyclic voltammetry (CV) curves and (
c,
d) galvanostatic charge-discharge (GCD) curves of supercapacitors and batteries. Reproduced with permission from
[16]. Copyright 2018 American Chemical Society.
Based on the power density equation, the energy density of a capacitor is determined by the specific capacitance of the electrode material and the potential difference between the positive and negative electrodes, developing porous nanoelectrode materials is one of the best strategies to raise the energy density of supercapacitors. Porous nanoelectrode materials can boost energy density by increasing the specific surface area, which in turn raises the specific capacitance. Constructing hybrid or asymmetric supercapacitors is a further approach that can improve the performance of the entire device.