
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Alexandri | -- | 1932 | 2023-05-12 17:17:02 | | | |
| 2 | Conner Chen | Meta information modification | 1932 | 2023-05-15 05:03:23 | | |
Video Upload Options
Biotechnologically produced carotenoids occupy an important place in the scientific research. Owing to their role as natural pigments and their high antioxidant properties, microbial carotenoids have been proposed as alternatives to their synthetic counterparts. Natural carotenoids can be obtained either by extraction from plants or via microbial production. To this end, many studies are focusing on their efficient and sustainable production from renewable substrates. Besides the development of an efficient upstream process, their separation and purification as well as their analysis from the microbial biomass confers another important aspect to be adressed.
1. Types and Chemistry of Carotenoids
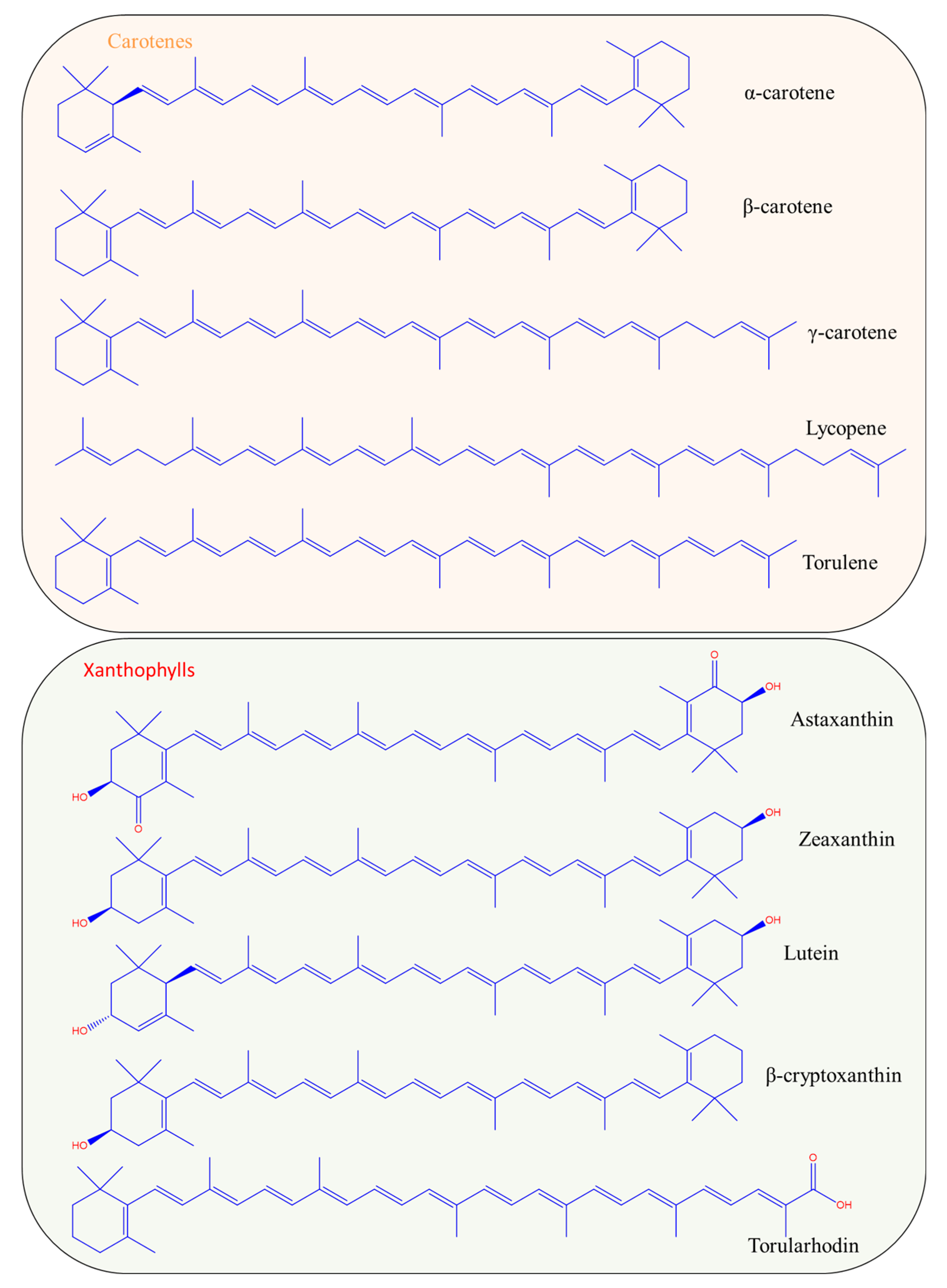

2. Sources of Natural Carotenoids
2.1. Plants
2.2. Microorganisms
3. Biotechnological Production of Carotenoids
| Main Carotenoid Produced | Microbial Strain | Ref |
|---|---|---|
| α-carotene | Rhodotorula mucilaginosa | [37] |
| β-carotene | Rhodotorula glutinis CCT-2186 | [38] |
| Xanthophyllomyces dendrorhous | [39] | |
| Phaffia rhodozyma | [40] | |
| Rhodotorula mucilaginosa | [37] | |
| Blakeslea trispora | [41] | |
| Dunaliella salina CCAP 19/41 | [42] | |
| Rhodosporidium kratochvilovae Y-42 and Y-43 | [21] | |
| γ-carotene | Rhodotorula mucilaginosa Blakeslea trispora |
[37] [41] |
| Lycopene | Blakeslea trispora | [41] |
| Torulene | Rhodotorula glutinis CCT-2186 | [38] |
| Rhodotorula mucilaginosa | [37] | |
| Astaxanthin | Xanthophyllomyces dendrorhous | [39][43][44] |
| Phaffia rhodozyma | [40] | |
| Zeaxanthin | Flavobacterium sp. P8 | [45] |
| Synechococcus sp. PCC7002, Synechocystis sp. PCC6803 and Rhodosorus sp. | [46] | |
| Lutein | Asterarcys quadricellulare PUMCC 5.1.1 | [47] |
| Auxenochlorella spp. LEU27 | [48] | |
| Chlorella minutissima | [49] | |
| Chlorella pyrenoidosa | [50] | |
| Chlorella sorokiniana AK-1 | [51] | |
| Chlorella sorokiniana FZU60 | [52][53] | |
| Chlorella sorokiniana MB-1-M12 | [54][55][56] | |
| Chlorella sorokiniana MUM002 | [57] | |
| Chlorella saccharophila UTEX247 | [58] | |
| Chlorella sp. GY-H4 | [59] | |
| Chlorella vulgaris | [60] | |
| Tetraselmis sp. CTP4 | [61] | |
| Scenedesmus sp. | [62] | |
| Torularhodin | Sporobolomyces ruberrimus | [63] |
| Rhodotorula glutinis CCT-2186 | [38] | |
| Rhodotorula mucilaginosa | [37] |
4. Conclusions
Νatural carotenoids demonstrate a plethora of advantages for human health, οwing tο their strοng antiοxidant and anti-inflammatory effects. These traits render carotenoids as compounds of scientific interest, and their biotechnological production seems to be the key for their cost-effective and sustainable production. As in many biotechnological products, recovery and chemical analysis are essential, considering the interrelation with the end applications. Modern extraction techniques ensure “greener” and safer processes to extract carotenoids, whereas parameters optimization is still an ongoing research. Current analytical tools provide an in-depth analysis that could also reveal new compounds and potential carotenoid producers. State-of-the-art research carried out indicates that each microbial strain capable of producing carotenoids necessitates different handling, and thus advantages and limitations of methods should be carefully and meticulously considered.
References
- Pfander, H. Carotenoids: An Overview. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 3–13.
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. In The Arabidopsis Book; The American Society of Plant Biologists: Rockville, MD, USA, 2012; Volume 10, p. e0158.
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological Production of Carotenoids by Yeasts: An Overview. Microb. Cell Fact. 2014, 13, 12.
- Zhang, C. Biosynthesis of Carotenoids and Apocarotenoids by Microorganisms and Their Industrial Potential. In Progress in Carotenoid Research; Queiroz Zepka, L., Jacob-Lopes, E., Vera De Rosso, V., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 85–105.
- Rodriguez-Amaya, D.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; HarvestPlus: Washington, DC, USA, 2004; ISBN 978-953-307-683-6.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16.
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Chapter 1 Structures, Nomenclature and General Chemistry of Carotenoids and Their Esters. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; The Royal Society of Chemistry: London, UK, 2019; pp. 1–50. ISBN 978-1-78801-242-3.
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of Commercially Important Carotenoids (Lycopene, β-Carotene, and Astaxanthin) by Natural Catalysts: Isothiocyanates and Polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237.
- Cheng, Y.; Zhang, L.; Tsao, R. Chemistry and Biochemistry of Dietary Carotenoids: Bioaccessibility, Bioavailability and Bioactivities Cheng. J. Food Bioact. 2020, 10, 32–46.
- Honda, M.; Takasu, S.; Nakagawa, K.; Tsuda, T. Differences in Bioavailability and Tissue Accumulation Efficiency of (All-E)- and (Z)-Carotenoids: A Comparative Study. Food Chem. 2021, 361, 130119.
- Liaaen-Jensen, S. Basic Carotenoid Chemistry. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 1–30.
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71.
- Stephen, N.; Ravichandran, G.; Niranjana, R.; Prasad, Y. Carotenoids: Types, Sources, and Biosynthesis. In Plant Secondary Metabolites: Volume 2 Stimulation, Extraction, and Utilization; Siddiqui, M., Bansal, V., Prasad, K., Eds.; Apple Academic Press: Waretown, NJ, USA, 2016; pp. 77–106. ISBN 978-1-77188-355-9.
- Cassani, L.; Marcovich, N.E.; Gomez-Zavaglia, A. Valorization of Fruit and Vegetables Agro-Wastes for the Sustainable Production of Carotenoid-Based Colorants with Enhanced Bioavailability. Food Res. Int. 2022, 152, 110924.
- Metličar, V.; Vovk, I.; Albreht, A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants 2019, 8, 384.
- Metličar, V.; Kranjc, K.; Albreht, A. Utilization of Plant-Based Wastes for a Sustainable Preparation of Xanthophyll Esters via Acid Anhydrides Using β-Pinene as a Bio-Derived Solvent. ACS Sustain. Chem. Eng. 2021, 9, 10651–10661.
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.; Rajesh, J.; Kumar, G. Technological Advances in the Production of Carotenoids and Their Applications—A Critical Review. Bioresour. Technol. 2023, 367, 128215.
- Böhm, V. (Ed.) Carotenoids; Antioxidants MDPI: Basel, Switzerland, 2019; ISBN 9783039218646.
- Demain, A.L.; Sánchez, S. Advancement of Biotechnology by Genetic Modifications. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2018; pp. 1–43. ISBN 978-1-4939-8742-9.
- Gupta, I.; Adin, S.N.; Panda, B.P.; Mujeeb, M. β-Carotene—Production Methods, Biosynthesis from Phaffia Rhodozyma, Factors Affecting Its Production during Fermentation, Pharmacological Properties: A Review. Biotechnol. Appl. Biochem. 2022, 69, 2517–2529.
- Sereti, F.; Papadaki, A.; Alexandri, M.; Kachrimanidou, V.; Kopsahelis, N. Exploring the Potential of Novel R. Kratochvilovae Red Yeasts towards the Sustainable Synthesis of Natural Carotenoids. Sustainability 2023, 31, 100927.
- Siziya, I.N.; Hwang, C.Y.; Seo, M. Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants 2022, 11, 1963.
- Kholany, M.; Coutinho, J.A.P.; Ventura, S.P.M. Carotenoid Production from Microalgae: The Portuguese Scenario. Molecules 2022, 27, 2540.
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422.
- Srivastava, A.; Kalwani, M.; Chakdar, H.; Pabbi, S.; Shukla, P. Biosynthesis and Biotechnological Interventions for Commercial Production of Microalgal Pigments: A Review. Bioresour. Technol. 2022, 352, 127071.
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microalgal Lutein Biosynthesis: Recent Trends and Challenges to Enhance the Lutein Content in Microalgal Cell Factories. Front. Mar. Sci. 2022, 9, 1015419.
- Assunção, J.; Malcata, F.X. Enclosed “Non-Conventional” Photobioreactors for Microalga Production: A Review. Algal Res. 2020, 52, 102107.
- Mannazzu, I.; Landolfo, S.; da Silva, T.L.; Buzzini, P. Red Yeasts and Carotenoid Production: Outlining a Future for Non-Conventional Yeasts of Biotechnological Interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673.
- Papadaki, A.; Kopsahelis, N.; Mallouchos, A.; Mandala, I.; Koutinas, A.A. Bioprocess Development for the Production of Novel Oleogels from Soybean and Microbial Oils. Food Res. Int. 2019, 126, 108684.
- Cipolatti Pereira, E.; Diaz Remedi, R.; do Santos Sá, S.; Bueno Rodrigues, A.; Markowiski Gonçalves, J.; Veiga Burkert, C.A.; Badiale Furlong, E.; Fernandes, J.; de Medeiros Burkert, J.F. Biocatalysis and Agricultural Biotechnology Use of Agroindustrial Byproducts as Substrate for Production of Carotenoids with Antioxidant Potential by Wild Yeasts. Biocatal. Agric. Biotechnol. 2019, 20, 101208.
- Li, W.; Luna-Flores, C.H.; Anangi, R.; Zhou, R.; Tan, X.; Jessen, M.; Liu, L.; Zhou, R.; Zhang, T.; Gissibl, A.; et al. Oxidative Stress Induced by Plasma-Activated Water Stimulates Astaxanthin Production in Phaffia rhodozyma. Bioresour. Technol. 2023, 369, 128370.
- Mantzouridou, F.; Tsimidou, M.Z. Lycopene Formation in Blakeslea Trispora. Chemical Aspects of a Bioprocess. Trends Food Sci. Technol. 2008, 19, 363–371.
- Liu, Y.; Shao, Y.; Li, X.; Wang, Z.M.; Yang, L.R.; Zhang, Y.Z.; Wu, M.; Yao, J.M. Analysis of Nicotine-Induced Metabolic Changes in Blakeslea Trispora by GC-MS. J. Zhejiang Univ. Sci. B 2020, 21, 172–177.
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a Protection Mechanism against Oxidative Stress in Haloferax Mediterranei. Antioxidants 2020, 9, 1060.
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution Uhplc-q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230.
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735.
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of Olive Mill Waste as Substrate for Carotenoid Production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 11.
- Mussagy, C.U.; Santos-Ebinuma, V.C.; Gonzalez-Miquel, M.; Coutinho, J.A.P.; Pereira, J.F.B. Protic Ionic Liquids as Cell-Disrupting Agents for the Recovery of Intracellular Carotenoids from Yeast Rhodotorula Glutinis CCT-2186. ACS Sustain. Chem. Eng. 2019, 7, 16765–16776.
- Gervasi, T.; Pellizzeri, V.; Benameur, Q.; Gervasi, C.; Santini, A.; Cicero, N.; Dugo, G. Valorization of Raw Materials from Agricultural Industry for Astaxanthin and β-Carotene Production by Xanthophyllomyces Dendrorhous. Nat. Prod. Res. 2018, 32, 1554–1561.
- Mussagy, C.U.; Santos-Ebinuma, V.C.; Herculano, R.D.; Coutinho, J.A.P.; Pereira, J.F.B.; Pessoa, A. Ionic Liquids or Eutectic Solvents? Identifying the Best Solvents for the Extraction of Astaxanthin and β-Carotene from Phaffia rhodozyma Yeast and Preparation of Biodegradable Films. Green Chem. 2022, 24, 118–123.
- Shariati, S.; Zare, D.; Mirdamadi, S. Screening of Carbon and Nitrogen Sources Using Mixture Analysis Designs for Carotenoid Production by Blakeslea trispora. Food Sci. Biotechnol. 2019, 28, 469–479.
- Xu, Y.; Harvey, P.J. Carotenoid Production by Dunaliella Salina under Red Light. Antioxidants 2019, 8, 123.
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.M.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; De Pasquale, P.; Dugo, G.; Cicero, N. Astaxanthin Production by Xanthophyllomyces Dendrorhous Growing on a Low Cost Substrate. Agrofor. Syst. 2019, 6, 1229–1234.
- Hara, K.Y.; Kageyama, Y.; Tanzawa, N.; Hirono-Hara, Y.; Kikukawa, H.; Wakabayashi, K. Development of Astaxanthin Production from Citrus Peel Extract Using Xanthophyllomyces Dendrorhous. Environ. Sci. Pollut. Res. 2021, 28, 12640–12647.
- Vila, E.; Hornero-Méndez, D.; Lareo, C.; Saravia, V. Biotechnological Production of Zeaxanthin by an Antarctic Flavobacterium: Evaluation of Culture Conditions. J. Biotechnol. 2020, 319, 54–60.
- Bourdon, L.; Jensen, A.A.; Kavanagh, J.M.; McClure, D.D. Microalgal Production of Zeaxanthin. Algal Res. 2021, 55, 102266.
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High Production of Carotenoids by the Green Microalga Asterarcys quadricellulare PUMCC. PLoS ONE 2019, 14, e0221930.
- Asker, D.; Awad, T.S. Isolation and Characterization of a Novel Lutein-Producing Marine Microalga Using High Throughput Screening. Food Res. Int. 2019, 116, 660–667.
- De Bhowmick, G.; Sen, R.; Sarmah, A.K. Consolidated Bioprocessing of Wastewater Cocktail in an Algal Biorefinery for Enhanced Biomass, Lipid and Lutein Production Coupled with Efficient CO2 Capture: An Advanced Optimization Approach. J. Environ. Manag. 2019, 252, 109696.
- Sampathkumar, S.J.; Gothandam, K.M. Sodium Bicarbonate Augmentation Enhances Lutein Biosynthesis in Green Microalgae Chlorella pyrenoidosa. Biocatal. Agric. Biotechnol. 2019, 22, 101406.
- Chen, C.Y.; Kuo, E.W.; Nagarajan, D.; Di Dong, C.; Lee, D.J.; Varjani, S.; Lam, S.S.; Chang, J.S. Semi-Batch Cultivation of Chlorella sorokiniana AK-1 with Dual Carriers for the Effective Treatment of Full Strength Piggery Wastewater Treatment. Bioresour. Technol. 2021, 326, 124773.
- Ma, R.; Zhang, Z.; Ho, S.H.; Ruan, C.; Li, J.; Xie, Y.; Shi, X.; Liu, L.; Chen, J. Two-Stage Bioprocess for Hyper-Production of Lutein from Microalga Chlorella sorokiniana FZU60: Effects of Temperature, Light Intensity, and Operation Strategies. Algal Res. 2020, 52, 102119.
- Xie, Y.; Li, J.; Ho, S.H.; Ma, R.; Shi, X.; Liu, L.; Chen, J. Pilot-Scale Cultivation of Chlorella sorokiniana FZU60 with a Mixotrophy/Photoautotrophy Two-Stage Strategy for Efficient Lutein Production. Bioresour. Technol. 2020, 314, 123767.
- Chen, J.H.; Kato, Y.; Matsuda, M.; Chen, C.Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Chang, J.S. Lutein Production with Chlorella sorokiniana MB-1-M12 Using Novel Two-Stage Cultivation Strategies—Metabolic Analysis and Process Improvement. Bioresour. Technol. 2021, 334, 125200.
- Chen, J.H.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, C.H.; Ng, I.S.; Chang, J.S. Enhancing Lutein Production with Mixotrophic Cultivation of Chlorella sorokiniana MB-1-M12 Using Different Bioprocess Operation Strategies. Bioresour. Technol. 2019, 278, 17–25.
- Chen, J.H.; Kato, Y.; Matsuda, M.; Chen, C.Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Di Dong, C.; Lee, D.J.; Chang, J.S. A Novel Process for the Mixotrophic Production of Lutein with Chlorella sorokiniana MB-1-M12 Using Aquaculture Wastewater. Bioresour. Technol. 2019, 290, 121786.
- Shiong, K.; Mun, Y.; Sing, W.; Min, J.; Chern, S. Permeabilization of Chlorella sorokiniana and Extraction of Lutein by Distillable CO 2 -Based Alkyl Carbamate Ionic Liquids. Sep. Purif. Technol. 2021, 256, 117471.
- Paliwal, C.; Rehmanji, M.; Mohd, K.; Uz, S.; Pavan, P. Green Extraction Processing of Lutein from Chlorella saccharophila in Water-Based Ionic Liquids as a Sustainable Innovation in Algal Biorefineries. Algal Res. 2022, 66, 102809.
- Wang, X.; Zhang, M.; Sun, Z.; Liu, S.; Qin, Z.; Mou, J.; Zhou, Z.; Sze, C.; Lin, K. Sustainable Lipid and Lutein Production from Chlorella Mixotrophic Fermentation by Food Waste Hydrolysate. J. Hazard. Mater. 2020, 400, 123258.
- McClure, D.D.; Nightingale, J.K.; Luiz, A.; Black, S.; Zhu, J.; Kavanagh, J.M. Pilot-Scale Production of Lutein Using Chlorella vulgaris. Algal Res. 2019, 44, 101707.
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved Production of Lutein and β-Carotene by Thermal and Light Intensity Upshifts in the Marine Microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732.
- Rajendran, L.; Nagarajan, N.G.; Karuppan, M. Enhanced Biomass and Lutein Production by Mixotrophic Cultivation of Scenedesmus sp. Using Crude Glycerol in an Airlift Photobioreactor. Biochem. Eng. J. 2020, 161, 107684.
- Kanno, K.Y.F.; Karp, S.G.; Rodrigues, C.; de Andrade Tanobe, V.O.; Soccol, C.R.; da Costa Cardoso, L.A. Influence of Organic Solvents in the Extraction and Purification of Torularhodin from Sporobolomyces ruberrimus. Biotechnol. Lett. 2021, 43, 89–98.





