Liposome-based drug delivery systems are nanosized spherical lipid bilayer carriers that can encapsulate a broad range of small drug molecules (hydrophilic and hydrophobic drugs) and large drug molecules (peptides, proteins, and nucleic acids). They have unique characteristics, such as a self-assembling bilayer vesicular structure. There are various methods used in the preparation of lipid-based nanocarriers such as liposomes. The method of preparation affects critical parameters such as size of vesicle and size distribution, permeability, lamellarity, and entrapment efficiency. Entrapment of compounds is performed by two main techniques; passive loading where drug entrapment occurs during the liposome formation, and active loading where drug entrapment is after the liposome formation.
1. Thin Film Hydration
Thin film hydration (
Figure 1) is one of the oldest and commonly used methods for formulating liposomes in small batch sizes
[1]. The main steps for this technique include dissolving the lipids in organic solvent (such as chloroform and ethanol) in a flask, followed by the evaporation of the organic solvent, under vacuum or by using nitrogen stream, to form a dry film of lipids on the inner wall of the flask. The thin film will then be hydrated using a suitable aqueous media while heating the lipids above the phase transition temperature (Tm) and agitating/stirring the formulation. As a result of heating and agitation, the lipid film will get hydrated, swell, and detach from the inner flask wall to form multilamellar vesicles (MLVs). These vesicles tend to be highly heterogenous in lamellarity and size
[1]. Moreover, it is difficult to completely remove all toxic organic solvent.
Figure 1. Schematic diagram of thin film hydration method.
The MLVs can be further processed to control and reduce their size by using downsizing methods such as extrusion, sonication, or high-pressure homogenization
[1]. French pressure cell is a method of extrusion that involves applying high pressure and passing the material through a small orifice that transforms MLV into SUVs. It is considered a gentler size reduction technique and only allows for small volume processing
[2]. Membrane extrusion is a method that uses a polycarbonate membrane with a defined pore size. Liposomes are passed through this membrane which results in the reduction of the liposome size. Product losses and difficulty to scale up are the main drawbacks. Ultrasonication can be performed by using a bath sonicator or a probe sonicator. This method provides a homogenous suspension of liposomes as well as reducing the size of liposomes by ultrasonic irradiation. However, sonication generates heat, and metal (titanium) particles may be leached off the probe tip to contaminate products and degrade sensitive actives and lipids
[3]. Although it reduces size of MLVs, SUVs generated tend to have wide size distribution with lower entrapment efficiency.
2. Ethanol and Ether Injections
This method involves dissolving the phospholipid in ethanol (
Figure 2), and an aqueous medium is prepared and pre-heated. The ethanol solution containing the dissolved phospholipid is rapidly injected using a needle into the aqueous media containing the material to be entrapped. The mixture requires stirring at high temperature (55–65 °C) to ensure the formation of liposomes. Ethanol will evaporate
[4]. Ethanol injection technique is simple and can rapidly form liposomes
[5]. This method can form large unilamellar vesicles (LUVs) and small unilamellar vesicles (SUVs) depending on the rate of ethanol injection. Homogenous SUVs are formed when the ethanol volume does not exceed 7.5% of the total formulation volume. Otherwise, heterogenous MLVs are formed. Ethanol is a class 3 solvent which is less harmful, but is volatile and flammable. The presence of residual amount of ethanol in the liposomal dispersion can risk denaturing the entrapped biologically-active macromolecules
[1].
Figure 2. “Schematic representation of the main stages of the ethanol injection method”
[1] [Reuse permitted by MDPI].
Ether injection involves dissolving the phospholipid in ether. A solution containing phospholipids dissolved in ether is slowly injected into the aqueous media containing the desired material to be encapsulated. In order to ensure effective evaporation of ether, the mixture is heated to 55–65 °C
[4]. SUVs bear similar properties to those fabricated by the ethanol injection. As ether evaporates at a lower temperature than ethanol, it can be efficiently removed in a short time, forming concentrated liposome solutions with relatively good entrapment efficiency
[1].
3. Reverse Phase Evaporation
This method involves dissolving the lipids in an organic solvent such as chloroform/methanol (2:1
v/
v) which favours the inverted micelles formation. This is followed by the addition of aqueous buffer to create a water-in-oil microemulsion. Then, the organic solvent is evaporated using a rotary evaporator to form a viscous gel. The gel will then collapse forming liposomes
[6]. The presence of large aqueous core of the microemulsions promotes entrapment of especially hydrophilic molecules where the liposomal gels showed a controlled release with a good permeation profile
[7]. The technique employs a large amount of organic solvent and solvent extraction process is slow and time consuming
[1].
4. Detergent Removal
This method involves adding a detergent, such as sodium cholate and alkyl glycoside, to phospholipids to solubilise and hydrate the lipids by preventing the hydrophobic portions of the lipids from interacting with the aqueous media forming micelles containing lipid and detergent. Then, the detergent is removed progressively allowing the formation of lipid-rich micelles which spontaneously give rise to unilamellar vesicle formation
[1]. The easiest method to remove the detergent is by diluting the suspension using a buffer which also increases the micellar size and polydispersity. However, this technique produces low liposomal concentration and low EE of hydrophobic drugs, mainly due to the dilution step. Alternatively, dialysis technique can be used to remove the detergent. The detergent can also be removed using resin beads, centrifugation, and gel chromatography techniques
[1].
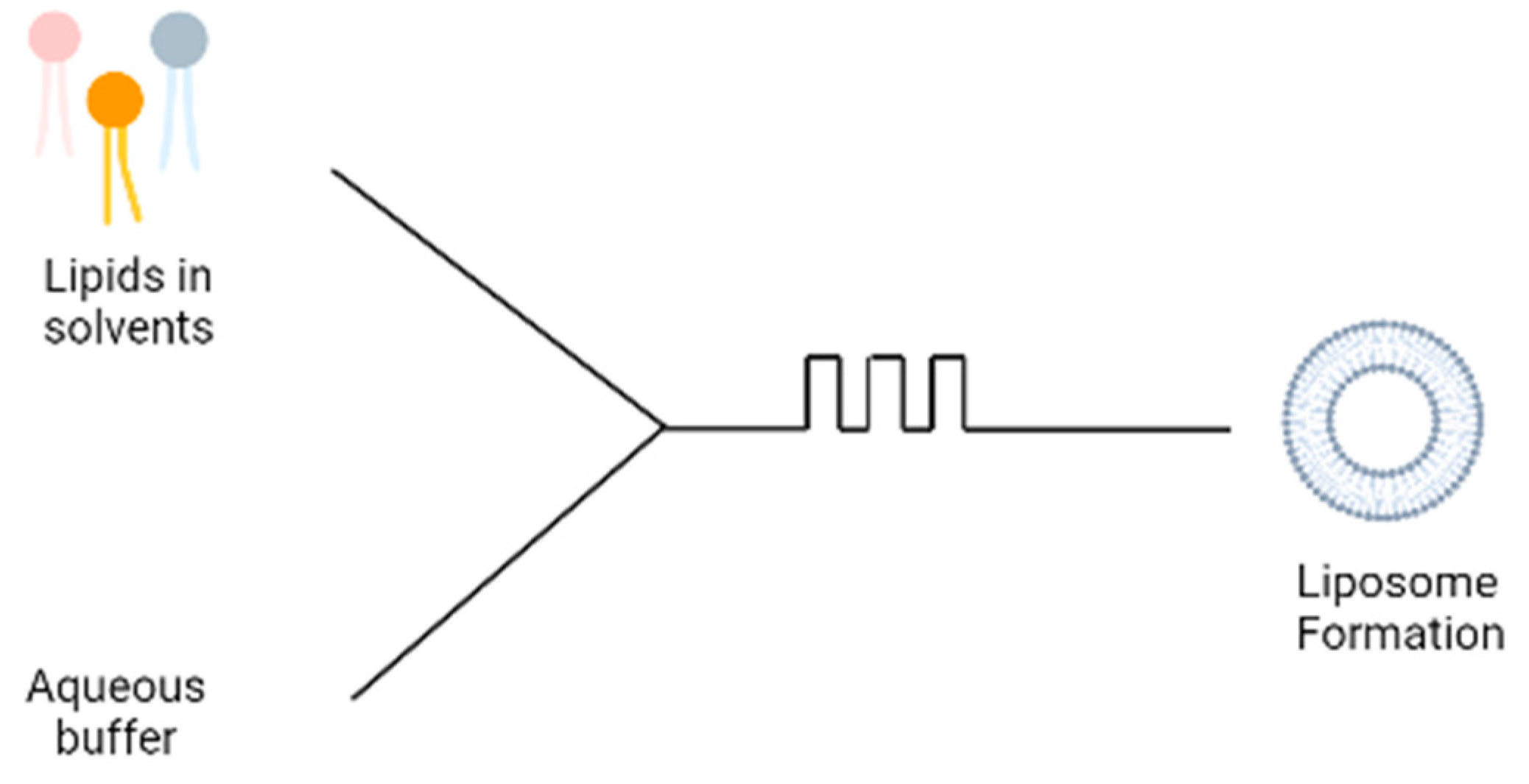
5. Microfluidic Devices
A more recent technique involves the use of microfluidic devices for the formation of liposomes; the microfluidic device contains two inlets; the aqueous buffer is added to one inlet and phospholipids dissolved in ethanol is added to the second inlet (
Figure 3). The two solutions are mixed through a micromixer, leading to the spontaneous self-assembly of the liposomes due to the change in polarity of the solution
[8]. The microfluidics device produces liposomes under ambient process temperature without heating the lipid above its transition temperature as is required in the lipid hydration technique. It also generates a laminar flow pattern for liposome formation in a controlled manner. There are different designs of the micromixers that provide an efficient mixing within short retention time in the mixing chamber, which has been extensively reviewed
[9]. Some drawbacks are the needs to remove residual organic solvent and cost of renewing microfluidic cartridges. This method can be made into a ‘lab-on-chip’ system and an adopted continuous flow process for potentially the large-scale liposome production.
Figure 3. Schematic diagram of microfluidic technique.
Although the methods described above are commonly-used methods for the effective formation of liposomes, each method has certain challenges (
Table 1). The main drawback of these methods is that they are only able to formulate symmetrical vesicles, meaning that the liposomes contain the same lipid composition in the outer and inner leaflets
[10]. This is considered a limitation in the formation of liposomal carriers as artificial bilayer carriers are mostly designed for the aim of mimicking biological membranes. However, biological membranes are highly asymmetrical with different lipid compositions in each leaflet. Thus, to improve the mimicking of biological membranes, liposomes need to be formulated with an asymmetrical nature
[11].
Table 1. Advantages and disadvantages of symmetric liposomes formulation techniques.
| Formulation Techniques |
Advantages |
Disadvantages |
| Thin film hydration |
|
|
| Ethanol injection |
|
|
| Ether injection |
|
|
| Reverse Phase Evaporation |
|
|
| Detergent removal |
|
|
| Microfluidic |
|
|