
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | LATIFAH OMAR | -- | 2346 | 2023-05-12 04:43:29 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2346 | 2023-05-16 10:45:31 | | |
Video Upload Options
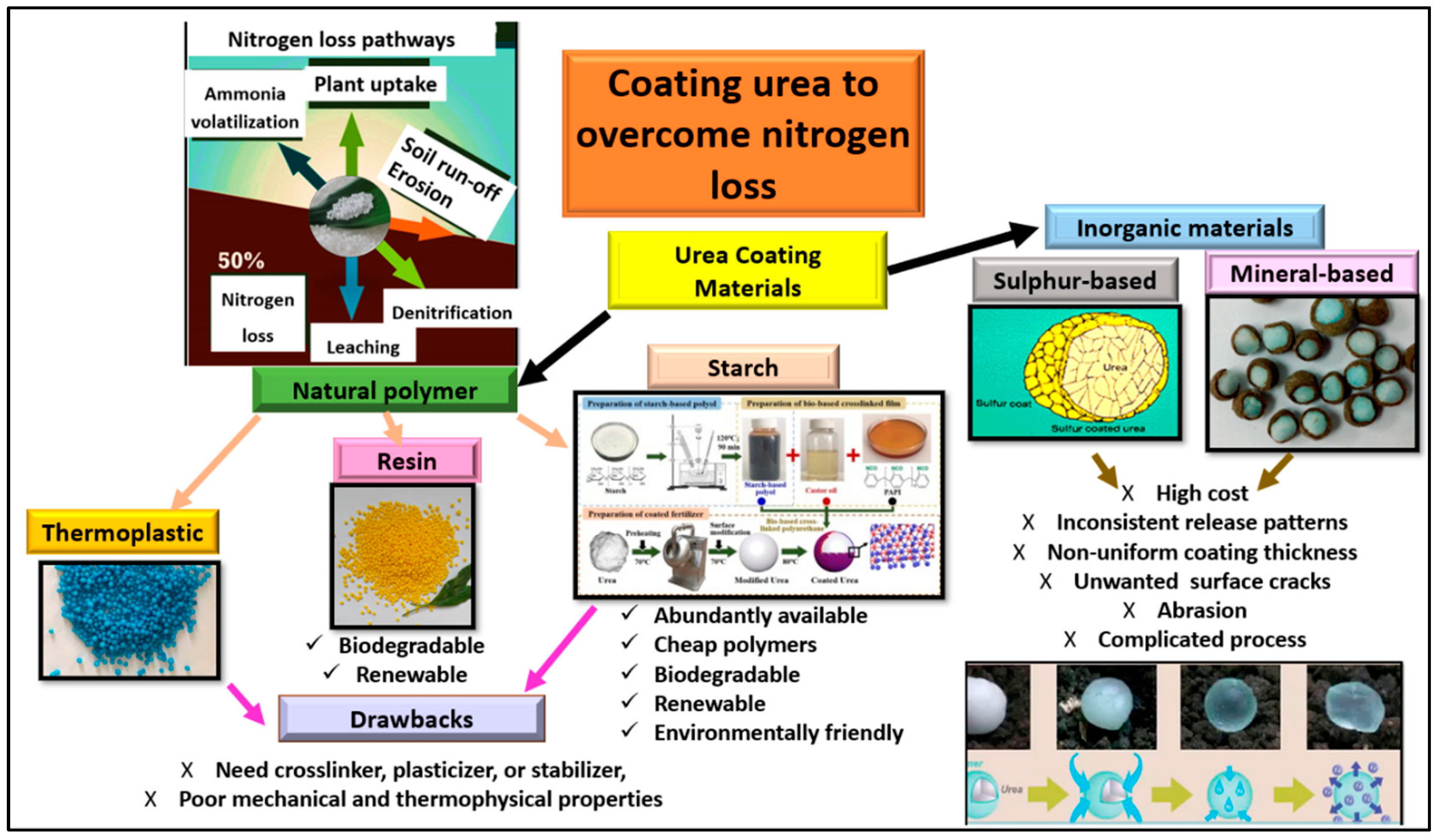
Increases in food production to meet global food requirements lead to an increase in the demand for nitrogen (N) fertilizers, especially urea, for soil productivity, crop yield, and food security improvement. To achieve a high yield of food crops, the excessive use of urea has resulted in low urea-N use efficiency and environmental pollution. One promising alternative to increase urea-N use efficiency, improve soil N availability, and lessen the potential environmental effects of the excessive use of urea is to encapsulate urea granules with appropriate coating materials to synchronize the N release with crop assimilation. Chemical additives, such as sulfur-based coatings, mineral-based coatings, and several polymers with different action principles, have been explored and used for coating the urea granule. However, their high material cost, limited resources, and adverse effects on the soil ecosystem limit the widespread application of urea coated with these materials.
1. Introduction
2. Natural Polymers for Encapsulating Urea
| 1. Thermoplastic-Based | |||
|---|---|---|---|
| Material | Modifier/Binder/ Sealant |
Research Findings | References |
| Poly (butylene succinate) (PBS) | Empty fruit bunch | Irradiation of PBS prior to mixing improved the adhesion and increased the biodegradation rate compared with the non-irradiated SRF composites. This is because EFB fibres are hydrophilic in nature, while the matrix polymer is hydrophobic. The mixture of these two materials causes weak adhesion and poor fibres. | [19] |
| Poly (lactic) acid | - | Utilizing the higher molecular weight poly (lactic acid) resulted in a slower urea release due to the decreasing permeability of the PLA. This slow-release fertilizer is promising because it does not leave residues that damage the soil structure or the nutrient balance in the soil. | [20] |
| Polyester | - | The increasing size of the controlled-release fertilizer while using smaller urea crystals slows down the degradability and release rate. | [21] |
| Polyurethane | - | The application of at least 50% total N as coated urea strongly reduced N leaching and improved N agronomic efficiency in comparison with traditional fertilizers, ensuring a similar fruit production in the same time. | [22] |
| Mesoporous silica | Filler morphology affects the release rate. | [23] | |
| Hydroxypropyl-terminated polydimethylsiloxane (HP-PDMS) | Implementation of hydrophobic gradient layer increases urea diffusion resistance. | [24] | |
| Polystyrene | Wax, polyurethane | Wax is brittle and cannot prevent water penetrating the coating. Increasing the size slows down the release and reduces the amount of coating material required. | [17] |
| Polyether sulfone | Fe2O3 nanoparticles (NPs) | A new class of controlled-release fertilizer. Fe2O3 NPs increase the coating thickness and reduce the release rate. They also allow the carrier to be recovered and recycled. | [25] |
| 2. Resin-Based | |||
| Acrylic resin | N-(n-butyl) thiophosphric triamide(NBPT) and dicyandiamde(DCD) | The film is integrated on the urea core surface, the coated material is uniformly distributed with the coating and closely combined with the urea core, and the surface is smooth and able to control the urea release. | [26] |
| Bio-based epoxy | - | Urea coating with epoxy is environmentally friendly and has a superior controlled release property. | [27] |
| 3. Natural-Based | |||
| Cellulose | Silica NP, bentonite, montmorillonite (MMT) | Incorporation of filler into cellulose-based coating material promotes tortuous path and compactness which slows down diffusion. | [28] |
| Ethyl cellulose (EC) as inner coating and cellulose-based superabsorbent polymer (cellulose-SAP) adsorbing biochemical inhibitors dicyandiamide (DCD) and thiourea as outer coating. | It has the functions of water retention and slow release, but it also inhibits the conversion of NH4+-N to NO3−N. | [29] | |
| Lignin | Alkenyl succinic anhydride | Film-forming properties show great potential to retard nutrient release. | [30] |
| - | Lignin indicated as an eco-friendly material with good controlled-release capacities that potentially could be applied in agriculture and horticulture. | [31] | |
| Phenol-formaldehyde resin | Phosphorus is partly retained inside the slightly soluble calcium sulphate matrix. | [32] | |
| Acetic acid/sodium metabisulfite | Lignin increases the efficiency of the delivery of the bioactive material for a longer period and prevents the pollution of surface and underground water with NH4+. | [33] | |
| Chitosan | Magnesium, rice-husk-ash, liquid natural rubber, Epsom salt | Increases the yield of rice. | [34] |
| - | The chitosan’s resistance slows down the release rate of N, P, and K. | [35] | |
| Poly (acrylic acid-co-acrylamide) (P(AA-co-AM) | Properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention features releases the nutrient slowly. | [36] | |
| Starch | The presence of chitosan on the coating system is responsible for producing a porous matrix surface, while the availability of starch tends to reduce the number of pores in the surface. The swelling analysis shows that the presence of chitosan–starch coatings increases the water absorption ability. | [14] | |
| Biochar | Bentonite | Water retention and controlled-release properties improve the N utilisation efficiency and reduce the environmental impact. | [37] |
| - | Biochar with a high specific surface area, hydrophilic oxygen-containing functional groups, and a low pH slow down the release. | [38] | |
| - | Porous nature and surface functional groups of biochar minimize NO3− leaching and improve the NUE. | [39] | |
References
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils, 3rd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 396–420. ISBN 978-01-3501-433-2.
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO A J. Hum. Environ. 2002, 31, 132–140.
- Dobermann, A. Nitrogen Use Efficiency—State of the Art. IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, International Fertilizer Industry Association (IFA), Paris. 2005, Paper 316. Available online: https://digitalcommons.unl.edu/agronomyfacpub/316 (accessed on 5 April 2022).
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Paddy husk compost addition for improving nitrogen availability. Indian J. Agric. Res. 2019, 387, 165–171.
- Mustafa, A.; Athar, F.; Khan, I. Improving crop productivity and nitrogen use efficiency using sulfur and zinc-coated urea: A Review. Front. Plant Sci. 2022, 13, 942384.
- Tufa, T.; Abera, T.; Midega, T. Nitrogen use efficiency and maize performance through application of urea stable and urea in Highland Nitisol of Midakegn and Toke Kutaye districts. Int. J. Plant Soil Sci. 2022, 22, 61–70.
- Bortoletto-Santos, R.; Guimarães, G.G.; Roncato Junior, V. Biodegradable oil-based polymeric coatings on urea fertilizer: N release kinetic transformations of urea in soil. Sci. Agric. 2020, 77, e20180033.
- Guo, M.; Liu, M.; Zhan, F.; Wu, L. Preparation and properties of a slow-release membrane-encapsulated urea fertilizer with superabsorbent and moisture preservation. Ind. Eng. Chem. Res. 2005, 44, 4206–4211.
- Thind, H.S.; Pannu, R.P.; Gupta, R.K.; Vashistha, M.; Singh, J.; Kumar, A. Relative performance of neem (Azadirachta indica) coated urea vis-à-vis ordinary urea applied to rice on the basis of soil test or following need-based nitrogen management using Leaf Colour Chart. Nutr. Cycl. Agroecosystems 2009, 87, 1–8.
- Mulder, W.J.; Gosselink, R.J.A.; Vingerhoeds, M.H.; Harmsen, P.F.H.; Eastham, D. Lignin based controlled release coatings. Ind. Crop. Prod. 2011, 34, 915–920.
- Beig, B.; Niazi, M.B.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating materials for slow release of nitrogen from urea fertilizer: A review. J. Plant Nutr. 2020, 43, 1510–1533.
- Micro Plastics. Fertilizers Europe. 2022. Available online: https://www.fertilizerseurope.com/circular-economy/micro-plastics/ (accessed on 15 March 2023).
- Rychter, P.; Kot, M.; Bajer, K.; Rogacz, D.; Šišková, A.; Kapuśniak, J. Utilization of starch films plasticized with urea as fertilizer for improvement of plant growth. Carbohydr. Polym. 2016, 137, 127–138.
- Savitri, E.; Purwanto, E.; Kodrat, A.N.; Yonathan, E. Controlled release fertilizer based on starch chitosan encapsulation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 703, 012019.
- Zafar, N.; Niazi, M.B.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and polyvinyl alcohol encapsulated biodegradable nanocomposites for environment friendly slow release of Urea Fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123.
- Ibrahim, K.A.; Naz, M.Y.; Shukrullah, S.; Sulaiman, S.A.; Ghaffar, A.; AbdEl-Salam, N.M. Controlling nitrogen pollution via encapsulation of urea fertilizer in cross-linked corn starch. BioResources 2019, 14, 7775–7789.
- Yang, Y.-C.; Zhang, M.; Li, Y.; Fan, X.-H.; Geng, Y.-Q. Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets. J. Agric. Food. Chem. 2012, 60, 11229–11237.
- Tian, H.; Liu, Z.; Zhang, M.; Guo, Y.; Zheng, L.; Li, Y.C. Biobased polyurethane, epoxy resin, and polyolefin wax composite coating for controlled-release fertilizer. ACS Appl. Mater. Interfaces 2019, 11, 5380–5392.
- Ahmad Saffian, H.; Abdan, K.; Hassan, M.A.; Ibrahim, N.A.; Lee, S.H.; Abdul Rahman, M.F. Properties of Slow Release Fertilizer Composites Made from Electron Beam-irradiated Poly(Butylene Succinate) Compounded with Oil Palm Biomass and Fertilizer. BioResources 2018, 13, 8677–8689.
- Kaavessina, M.; Distantina, S.; Shohih, E.N. A Slow-Release Fertilizer of Urea Prepared via Melt Blending with Degradable Poly(lactic acid): Formulation and Release Mechanisms. Polymers 2021, 13, 1856.
- Ye, H.-M.; Li, H.-F.; Wang, C.-S.; Yang, J.; Huang, G.; Meng, X.; Zhou, Q. Degradable polyester/urea inclusion complex applied as a facile and environment-friendly strategy for slow-release fertilizer: Performance and mechanism. Chem. Eng. J. 2020, 381, 122704.
- Incrocci, L.; Maggini, R.; Cei, T.; Carmassi, G.; Botrini, L.; Filippi, F.; Clemens, R.; Terrones, C.; Pardossi, A. Innovative Controlled-Release Polyurethane-Coated Urea Could Reduce N Leaching in Tomato Crop in Comparison to Conventional and Stabilized Fertilizers. Agronomy 2020, 10, 1827.
- Li, L.; Sun, Y.; Cao, B.; Song, H.; Xiao, Q.; Yi, W. Preparation and performance of polyurethane/mesoporous silica composites for coated urea. Mater. Des. 2016, 99, 21–25.
- Dai, C.; Yang, L.; Xie, J.; Wang, T.-J. Nutrient diffusion control of fertilizer granules coated with a gradient hydrophobic film. Colloids Surf. A 2020, 588, 124361.
- Emami, N.; Razmjou, A.; Noorisafa, F.; Korayem, A.H.; Zarrabi, A.; Ji, C. Fabrication of smart magnetic nanocomposite asymmetric membrane capsules for the controlled release of nitrate. Environ. Nanotechnol. Monit. Manag. 2017, 8, 233–243.
- Li, D.P.; Wu, Z.; Liang, C.; Chen, L.; Zhang, S.; Wang, J. Preparation of acrylic resin coated urea fertilizers and their controlled effects. Trans. CSAE 2007, 23, 218–224.
- Li, Y.; Jia, C.; Zhang, X.; Jiang, Y.; Zhang, M.; Lu, P.; Chen, H. Synthesis and performance of bio-based epoxy coated urea as controlled release fertilizer. Prog. Org. Coat. 2018, 119, 50–56.
- Li, X.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Su, Y. A novel wheat straw cellulose-based semi-IPNs superabsorbent with integration of water-retaining and controlled-release fertilizers. J. Taiwan Inst. Chem. Eng. 2015, 55, 170–179.
- Zhang, M.; Yang, J. Preparation and characterization of multifunctional slow release fertilizer coated with cellulose derivatives. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 774–781.
- Bortolin, A.; Aouada, F.A.; Mattoso, L.H.; Ribeiro, C. Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J. Agric. Food. Chem. 2013, 61, 7431–7439.
- Jiao, G.-J.; Xu, Q.; Cao, S.-L.; Peng, P.; She, D. Controlled-Release Fertilizer with Lignin Used to Trap Urea/Hydroxymethylurea/ Urea-Formaldehyde Polymers. BioResources 2018, 13, 1711–1728.
- Rotondo, F.; Coniglio, R.; Cantera, L.; Pascua, I.D.; Clavijo, L.; Dieste, A. Lignin-based coatings for controlled P-release fertilizer consisting of granulated simple superphosphate. Holzforschung 2018, 72, 637–643.
- Behin, J.; Sadeghi, N. Utilization of waste lignin to prepare controlled-slow release urea. Int. J. Recycl. Org. Waste Agric. 2016, 5, 289–299.
- Adlim, M.; Ramayani, R.F.I.; Khaldun, I.; Muzdalifah, F.; Sufardi, S.; Rahmaddiansyah, R. Fertilizing Properties of Urea-Magnesium Slowrelease Fertilizer Made of Rice-Husk-Ash Natural-Rubber Chitosan Composite. Rasayan J. Chem. 2021, 14, 1851–1859.
- Gumelar, M.D.; Hamzah, M.; Hidayat, A.S.; Saputra, D.A. Idvan Utilization of Chitosan as Coating Material in Making NPK Slow Release Fertilizer. Macromol. Symp. 2020, 391, 1900188.
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247.
- Liu, X.; Liao, J.; Song, H.; Yang, Y.; Guan, C.; Zhang, Z. A Biochar-Based Route for Environmentally Friendly Controlled Release of Nitrogen: Urea-Loaded Biochar and Bentonite Composite. Sci. Rep. 2019, 9, 9548.
- Jia, Y.; Hu, Z.; Mu, J.; Zhang, W.; Xie, Z.; Wang, G. Preparation of biochar as a coating material for biochar-coated urea. Sci. Total Environ. 2020, 731, 139063.
- Jia, Y.; Hu, Z.; Ba, Y.; Qi, W. Application of biochar-coated urea-controlled loss of fertilizer nitrogen and increased nitrogen use efficiency. Chem. Biol. Technol. Agric. 2021, 8, 3.
- Puoci, F.; Lemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review American. J. Agric. Biol. Sci. 2008, 3, 299–314.
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375.
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical properties of thermoplastic starch derived from natural resources and its blends: A Review. Polymers 2021, 13, 1396.
- Diwani, G.E.; Motawie, N.; Shaarewy, H.H.; Shalaby, M.S. Nitrogen Slow-Release Biodegradable Polymer Based on Oxidized Starch Prepared via Electrogenerated Mixed Oxidants. J. Appl. Sci. Res. 2013, 9, 1931–1939.
- Zou, H.; Ling, Y.; Dang, X.; Yu, N.; Zhang, Y.; Zhang, Y.; Dong, J. Solubility Characteristics and Slow-Release Mechanism of Nitrogen from Organic-Inorganic Compound Coated Urea. Int. J. Photoenergy 2015, 2015, 705471.




