Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manuel Vázquez | -- | 4392 | 2023-05-11 17:15:27 | | | |
| 2 | Beatrix Zheng | Meta information modification | 4392 | 2023-05-16 10:26:11 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 4392 | 2023-05-16 10:28:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Flórez, M.; Cazón, P.; Vázquez, M. Biopolymers’ Processing Methods. Encyclopedia. Available online: https://encyclopedia.pub/entry/44166 (accessed on 08 March 2026).
Flórez M, Cazón P, Vázquez M. Biopolymers’ Processing Methods. Encyclopedia. Available at: https://encyclopedia.pub/entry/44166. Accessed March 08, 2026.
Flórez, María, Patricia Cazón, Manuel Vázquez. "Biopolymers’ Processing Methods" Encyclopedia, https://encyclopedia.pub/entry/44166 (accessed March 08, 2026).
Flórez, M., Cazón, P., & Vázquez, M. (2023, May 11). Biopolymers’ Processing Methods. In Encyclopedia. https://encyclopedia.pub/entry/44166
Flórez, María, et al. "Biopolymers’ Processing Methods." Encyclopedia. Web. 11 May, 2023.
Copy Citation
The biopolymer functionality depends on several factors apart from their structure and composition, such as the type, quality, and quantity of the solvent used and the processing technique used to build the final structure that will determine the interaction of the materials. The main processing techniques for bio-based polymers from renewable sources are discussed in detail.
biopolymer
casting
coating
electrospinning
3D printing
1. Solvent Casting Method
The solvent casting method, also known as the solution function or wet processing method, consists of creating an aqueous or hydroalcoholic mixture with the presence of the biopolymer. This method is based on the use of a solvent that allows the polymer to be suspended in a film-forming solution followed by solvent evaporation and polymer chain reformulation [1]. Alcohol, water, or other organic solvents are commonly used to dissolve the selected polymer. Sometimes, for the best results, the suspension polymer solution is heated, or the pH adjusted. The polymer–solvent mixture is poured into a mold, drum, or flat surface, where it is left to dry for a specific time. Once the solvent is completely evaporated, the polymeric matrix is formed and can be peeled off from the mold [2][3]. Figure 1 shows an outline of the solvent casting method.
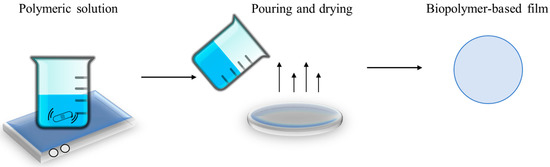
Figure 1. Solvent casting method scheme.
Casting is a simple method. However, there are a series of requirements to consider when applying the technique. One of the most important points is the solvent chosen to dissolve the polymer. If the polymer–polymer attraction forces in a solution are weaker than the polymer–solvent interactions, the chain segment will be stretched by solvent molecule diffusion. This results in swelling of the polymer matrix with the solvent. However, it should be noted that the dissolution capacity of a polymer varies depending on the solvent, hence the choice of solvent is an important point [3].
The molecular weight of the polymer to be used is another important point of this process. The molecular weight of the polymer affects the rate of solvent penetration. It was shown that higher molecular weight polymers dissolved more slowly compared to lower molecular weight polymers [4]. This is attributed to the fact that the high molecular weight polymer chain undergoes a slower rate of relaxation because it has greater entanglement in the chains. High molecular weight chains are unable to contract rapidly during environmental cooling, thus generating a larger free volume. For that reason, the higher the molecular weight, the greater the solvent penetration rate [5]. Moreover, this kind of biopolymer has sufficient cohesive strength and coalescence capacity. Another important requirement is that the polymer must be soluble in a volatile solvent or water. For best results, a stable solution with a suitable viscosity should be created [3]. The temperature and humidity of the environment are critical points for the process to develop correctly [6].
The main advantage of the casting method is the ease of developing the method without the need for special and expensive equipment [7]. As casting is a wet process, there is better particle–particle contact, which results in more homogeneous particle packaging and smaller and fewer defects [8]. On the other hand, a disadvantage of this method is the restrictions on the shape of the final product, which is usually simple sheets and shapes [9]. Perhaps the biggest challenge is to apply the solvent method on an industrial scale, since multiple variables, such as temperature and humidity, can cause variations in the quality of the final product [10].
Table 1 shows a compilation of the main applications of biopolymers according to the production method. For instance, the solvent casting method has been widely applied to the preparation of active or smart films for food applications. Examples include the development of carboxymethyl-cellulose/starch/purple sweet potato anthocyanin films that change color when fish begins to have pH/ammonia levels typical of contamination [11] or a chitosan-based film with the addition of sandalwood essential oil to retard lipid oxidation of butter [12]. Polymeric films based on cellulose nanocrystals and lignin nanoparticles were combined with poly(lactic acid) to provide antibacterial activity against a pathogen (Pseudomonas syringae) attacking tomatoes [13]. Film characteristics such as density, compactness, porosity, permeability, flexibility, and brittleness are all influenced by the polymer matrix’s degree of cohesion [1][14]. Cellulose derivatives and starch are the most commonly used polysaccharides for the manufacture of these films. Nevertheless, the low elasticity of the materials is a significant drawback that restricts their use. In order to facilitate the correct development of the process, tricks are used, such as cosolvent systems or the addition of additives such as wrecking agents, plasticizers, etc. [14]. The main non-volatile plasticizers used are glycerol, sorbitol, propylene glycol or polyethylene glycol [15][16]. However, due to its great availability, excellent plasticizing efficacy, and low exudation, glycerol is the plasticizer most commonly used [17].
Table 1. Methods of biopolymer processing and applications. CMC is carboxymethylcellulose, PLA is polylactic acid, PHA is polyhydroxyalkanoate, and PCL is polycaprolactone.
| Production Method | Biopolymer | Application | References |
|---|---|---|---|
| Solvent casting | CMC, starch | Detection of content of total volatile basic nitrogen value in contaminated fish | [11] |
| Chitosan | Retard lipid oxidation of butter | [12] | |
| Alginate | Wound dressing application | [18] | |
| Keratin, gelatin | Wound healing recipe for in vivo studies | [19] | |
| Chitosan PCL/PLA |
Controlled release patches for insulin Engineering of scaffold for tissue engineering |
[20] [21] |
|
| Coating | Alginate | Extend shelf life of fresh-cut watermelon | [22] |
| Chitosan | Delays ripening and reactive oxygen species in guava fruits after harvesting | [23] | |
| Gelatin | Bioactive packaging to prolong the shelf life of strawberries | [24] | |
| Cellulose | Bone tissue regeneration in vivo | [25] | |
| Chitosan PCL |
Antiosteomyelitis drug release and bone repair Bone healing and osteogenesis promoter |
[26] [27] |
|
| Electrospinning | Chitosan | Release of paclitaxel to kill prostate cancer cells | [28] |
| Gelatin | Scaffold for skin tissue engineering | [29] | |
| Alginate | Antioxidant/antimicrobial active packaging | [30] | |
| Chitosan, collagen | Guided bone regeneration | [31] | |
| Silk fibroin PCL |
Promoting bone cell growth Scaffolds promoting corneal keratocyte growth and proliferation |
[32] [33] |
|
| 3D printing | Collagen, alginate | Bioink for the creation of cartilaginous tissue | [34] |
| Chitosan | Cell adhesion and growth, tissue engineering | [35] | |
| Starch | Beads for bioactive compound release | [36] | |
| Starch PLA |
Structural enhancement for 3D printing of surimi Tubular scaffolds for bone tissue engineering |
[37] [38] |
|
| Injection molding | Chitosan Starch PLA PCL PLA/PHA |
Development of matrix biopolymer for agricultural/packaging applications Creation of cassava starch/sodium alginate composites Development of high-temperature-resistant stereocomplex PLA Creation of crayfish protein–PCL biocomposite material Development of PLA/PHA nanocomposites |
[39] |
| [40] | |||
| [41] | |||
| [42] | |||
| [43] | |||
| Compression molding | Chitosan | Film production | [44] |
| Wheat gluten | Film production | [45] | |
| Cassava starch | Film production for cold-stored pork meat slices | [46] | |
| Fish gelatin | Film production | [47] | |
| Extrusion | Starch/Gelatin PLA PLA PHA Starch/Chitosan |
Film production Film production Development of antioxidant film Development of PHA/thermoplastic starch film Development of corn starch/chitosan film |
[48] [49] [50] [51] [52] |
| Grafting copolymerization | Chitosan | Biomedical field | [53] |
| Chitosan | Water treatment for the removal of heavy metals | [54] | |
| Lignin | Wood composite manufacturing | [55] | |
| Cellulose | Green composite applications | [56] |
Although film formation for food applications is the most common application, this technique is used in other areas. Multiple studies related to the biomedical field apply the solvent casting method to develop novel films. An alginate-based film enriched with aloe vera gel and cellulose nanocrystals as a wound dressing was developed [18]. Additionally, a biopolymer film based on keratin, fibrin, gelatin, and mupirocin that served as a substitute for wound healing was evaluated [19]. In the field of drug delivery, the solvent casting technique was used to create transdermal patches containing insulin–chitosan nanoparticles, achieving a controlled release of the insulin [20]. The casting method was also applied to biopolyesters such as PCL/PLA, where once dissolved in chloroform they gave rise to scaffolds for tissue engineering. These scaffolds showed a better biological behavior, with a reduced pore size that favors cell growth [21].
2. Coating Method
Coating consists of the application of a solution-based coating made of a polymer or a mixture of components. This solution is applied on a surface, usually called a substrate. The coating method differs from the solvent casting method in the way of application. The purpose of the coating can be technical, decorative, or both [57][58]. Within the coating method, varieties can be found: the dipping process is the oldest technique and consists of immersing the product in the coating solution. It is commonly used in the food industry, for example, for coating vegetables and fruits to act as barrier against moisture and gases [59][60]. The brushing process is when the coating is applied directly to the surface of the product with a brush or brushing equipment [61][62]. In the spraying process, the final product is sprayed with many drops of the coating solution. Hydraulic spray nozzles, high-pressure spray guns, or air atomization systems are often used to facilitate the process [63]. In electrical spraying, the coating solution passes through the atomizer nozzle connected to a source of high electrical potential [64]. Figure 2 shows the coating method and its different modalities.
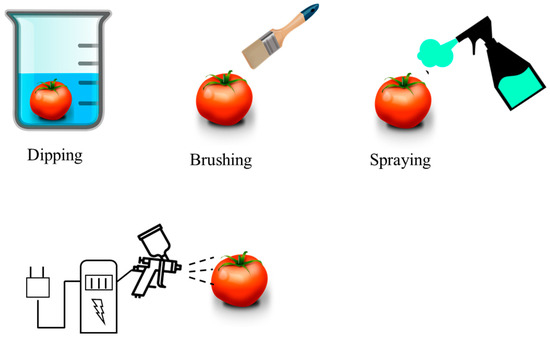
Figure 2. Diagram of the different coating methods.
Generally, the coating method has three important stages [65]:
-
immersion or permanence time: the substrate is plunged into the precursor solution at a constant speed to give the substrate enough interaction time with the coating solution (in the dipping method). After this, it is left to stand for a certain time.
-
Deposition and drainage: a thin layer of the precursor solution is entrained by drawing the substrate upward at a constant rate. Water that is in excess will drain off the surface.
-
Evaporation: the solvent evaporates, creating a thin film. This process can be accelerated by hot drying.
Coatings can be made from biological materials such as lipids, polysaccharides, and proteins. Polysaccharides and proteins are interesting materials since they can form cohesive molecular networks through strong interactions between molecules, such as hydrogen bond, van de Waals interactions, or crystallization. These molecular cohesions give the coating a good barrier property to gases (mainly O2 and CO2), and good mechanical properties [66][67][68]. Polyols that act as plasticizers (e.g., glycerol or polyethylene glycol) or acid/base substances (e.g., acetic or lactic acid) that control pH are typically included as minor components. The biopolymers applied in the coating method are usually prepared in aqueous solutions. However, there is a lack of studies on the influence of the solvent applied in the coating process.
The solubility of the biopolymers together with the viscosity of the final solution depends on the molecular weight of the polymer. A high polymer molecular weight results in a more viscous solution, but also decreases the solubility of the polymer [69]. On the other hand, creating a coating that adheres properly to the surface of the final product requires an estimation of the interfacial tension between the coating solution and the product surface [70]. For compatibility between the surface and the solution, an interesting action is to reduce the surface tension of the coating solution. This reduces the interfacial tension and improves adhesion between the product and the coating [71].
A positive aspect of the coating method is that this is a process where it is not necessary to spend large amounts of substrate since the layers that are created rarely exceed micrometers of thickness. It is also a simple and inexpensive method compared to other polymer production methods, in addition to having a high adhesion capacity in the coating of complex geometries [72]. Although it is a method that brings great benefits, it also suffers from certain disadvantages that diminish its reliability. For instance, there are negative thermal effects such as cracking or delamination, effects due to lack of atmospheric protection (e.g., penetration of contaminants into the substrate), and limitations of the coating materials, such as different melting points, availability in various forms such as sheets/powders, biocompatibility between the components of the coating solution, among others [72].
There are multiple studies where the biopolymer coating technique is applied. The main field of application is the food industry. In this field, edible coatings are applied directly to food and consumed as part of it. They have been used to prevent moisture migration or create shiny surfaces to make food more appealing to consumers. Most of the studies conducted on foods are on fruits and vegetables. For instance, an edible alginate-based coating for application on fresh-cut watermelon was used [22]. The coating maintained quality and sensory acceptability, as well as extended the shelf life of the food product. Chitosan-based coatings have also been developed to delay the ripening of fruits such as guava and prolong their quality [23]. Gelatin-based coatings incorporated with Mentha pulegium essential oil significantly inhibited the presence of molds and yeasts in strawberries stored at 4 °C [24].
On the other hand, materials engineering based on polymer coating for drug loading/release, cell immobilizations/protection, or antibacterial/antiviral applications is an area of great interest. For instance, a cellulose nanofibril-based coating was applied on a 3D scaffold obtained from bone marrow, thus promoting adhesion, proliferation, and osteogenesis of bone tissue cells [25]. Likewise, TiO2-SiO2/chitosan–lysine-based coatings were loaded with a drug to combat osteomyelitis. The research showed that the drug was released correctly and could be used as a drug delivery vehicle [26]. PCL dissolved in dichloromethane was used as a coating to improve the strength of a bone implant screw. The research in rats concluded that thicker, denser bone had formed around the PCL-coated screw in the animals’ femurs [27].
3. Electrospinning Method
Electrospinning is a technique in which a high electrical voltage created between two electrodes is used to generate charged strands of polymer at a specific flow rate. These fibers typically range in diameter from 2 nm to several micrometers [73]. The polymer solution is drawn through a nozzle with a high-voltage electric field using mechanical pressure combined or by gravity alone. This is followed by solvent evaporation, which causes the production of solid fiber [74][75]. Figure 3 shows a schematic of the electrospinning process. Generally, ordinary basic electrospinning equipment consists of four main elements:
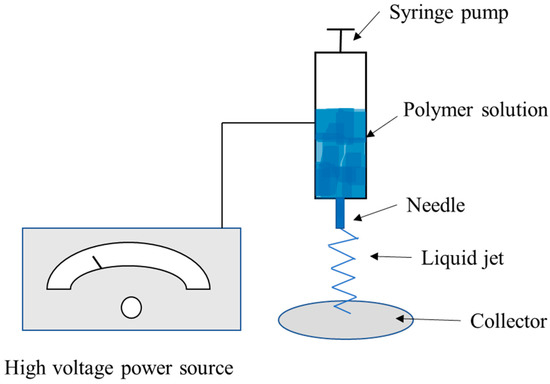
Figure 3. Diagram of the electrospinning method.
-
an electric field, which is applied on the needle containing the solution with which the spinning is performed, and the collector connected through two electrodes.
-
A flow-controlling syringe pump for supplying the rotating solution.
-
A syringe with a capillary tube or spinneret to hold the spinning fluid and a metal needle with a flat end.
-
An electrically conductive collector (target) present in various shapes.
Although it is a relatively simple method, there are several parameters that influence the subsequent characteristics of the fibers generated. These parameters to consider could be classified into three areas: solution, process, and environment. The cleanliness and safety of electrospinning are closely related to the solvent used in the solution, which can be water or organic solvents. The application of water as a solvent is becoming more and more frequent. However, this greatly restricts the choice of polymeric materials due to the low presence of water-soluble polymers [76]. In addition, the final product should undergo crosslinking to provide stability to the water present in the nanofibers [76]. Low-solubility solvents are also recommended, since solutions with better electrospinning capacity are obtained [77]. In addition, the surface tension of the solvents is important. By lessening the surface tension of the solution, bead-free fibers can be produced. For instance, cellulose fibers using two different solvents, acetone and dimethylacetamide, were manufactured [78]. The authors observed that when the solvents were used individually the fibers had beads. However, when applied together, the fibers were produced without beads. The viscosity of the solution is another key point that affects the subsequent behavior of the process and the quality of the product obtained. The viscosity of the solution is related to the molecular weight of the solute and the concentration of the polymer used [79]. Low molecular weight polymer solutions form beads instead of fibers, therefore it is interesting to use high molecular weight solutions with a suitable viscosity to create the fibers correctly [73].
Regarding the electrospinning process, the voltage applied directly affects the fiber diameter. If the applied voltage is high, a large volume of polymer solution is expelled, resulting in larger diameter fibers [80]. Additionally, the flow rate through the syringe nozzle will influence the final morphology of the fibers. If the flow velocity is high, the solvent evaporation rate is lower, and this means inadequate drying with the presence of beads on the fibers. On the other hand, if the speed is low, the solvent will have more time to evaporate. It is important to find the right speed point to obtain the best result [73][81].
Temperature and humidity are among the parameters to be considered in relation to the environment while fabricating electrospun fibers. Studies showed that at higher temperature the viscosity of the solution decreases, generating fibers of smaller diameter. On the other hand, the higher the humidity in the environment, the smaller the pores in the fiber structure [82][83]. In addition, humidity influences the evaporation of the solvent as well. A relatively high humidity is recommended because if the humidity is too low, the solvent can cause clogging at the needle tip of the equipment [73][84][85].
The polymeric fibers generated from this process have a large specific surface area, high porosity, and high absorbency capacity, which makes them interesting for applications that it will be mentioned below [82]. Electrospinning can be used with both synthetic and natural polymers, however, the latter have received a lot of attention in recent years [86][87]. Natural polymer nanofibers have properties such as low antigenicity or antimicrobial capacity, that make them interesting for many applications. However, the main limitations of natural polymers are that they are more difficult to process and have poorer mechanical properties. Therefore, synthetic and natural polymers are usually used together to improve the properties of the fibers.
Compared to other methods, electrospinning is easy to handle, cost effective, with a high loading capacity, and applicable at room temperature. Additionally, the micro/nanofibers produced by the electrospinning method have shown distinctive characteristics, such as a high surface/volume ratio, significant porosity, adjustable mechanical characteristics, and changeable morphologies [79][88]. Although successful fabrication and manufacturing of electrospun nanofibers have been carried out continuously, some critical drawbacks are associated with the processing parameters such as the viscosity of the solution and surface tension, among others [89].
Originally, this technique was used in the textile industry [90], but nowadays it is an interesting method for the economical and efficient manufacture of food packaging. According to the literature, polysaccharide and protein-derived polymers are the most widely used polymers in the electrospinning method. Specifically, collagen, gelatin, chitosan, cellulose, and alginate are the most used. Biomedical applications and wound healing are the most common fields where this technique is applied. For example, electrospinning has developed collagen-based fibers that showed improved healing progression [91]. The high porosity of biopolymer nanofibers favors gas exchange through the wound, preventing desiccation and dehydration [92]. Chitosan-coated collagen nanofibers enabling bone regeneration in a mesenchymal stem cell study were developed [31]. Additionally, nanofibers created from polycaprolactone and natural silk fibroins were shown to have high cellular biocompatibility and to promote cell growth of damaged bone tissue [32]. Gelatin/polyvinyl alcohol/chondroitin sulfate nanofibrous scaffolds for skin tissue engineering were also developed [29]. PCL was used as a material in the electrospinning technique for the creation of scaffolds that favored the growth and proliferation of corneal keratocytes. This use is interesting for corneal wound healing [33]. More studies are needed on the use of polymeric fibers made by electrospinning in the food industry. For instance, zein/sodium alginate nanofibers were developed [30] that were shown to exhibit antioxidant/antimicrobial effects and could be applied as an active inner layer of laminated films for food applications.
4. Three-Dimensional Printing Method
Three-dimensional printing makes it possible to manufacture objects by layering a material with the help of a print head and a nozzle. The selected material is applied on a substrate with a specific geometry that has been pre-designed [93]. Then, during the construction process, the different layers of the selected material are poured in. Finally, the structures are removed from the support. Depending on the type of printing, a curing phase may be necessary [94]. Figure 4 shows the process carried out by the 3D printer.
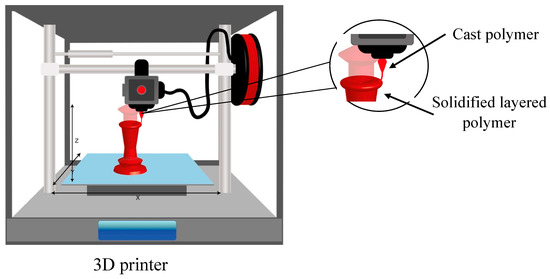
Figure 4. Schematic diagram of the 3D printing method.
Several essential requirements must be met for the equipment to be properly used in 3D printing procedures. The pressure-driven extrusion flow rate depends on the viscosity of the biopolymer used and, if it reaches the required volume flow rate under conventional system pressure, it is a suitable material. The formation of a bead with a table geometry is another key point, which depends on the superficial energy of the system. However, it is normal for certain residual stresses to appear within a component that is formed layer by layer [95].
Assuming these requirements are met, it is important to know the physicochemical characteristics of the biopolymer to be used as printing ink. First of all, the thermal property is the ability of the material to melt or solidify and, consequently, the transition temperature of the biopolymer from the fluid to the solid state must be known [96]. Rheological properties refer to the ability of the biopolymer to flow. For instance, it is sometimes of interest that they exhibit plastic-like behavior, with the fluid flowing through the nozzle but solidifying rapidly when the nozzle pressure is removed [97]. Likewise, the ability of the material to flow through the nozzle when a force is applied, also known as slip or surface property, is a point to be considered. The lower the surface tension of the material, the more likely it is to spread when it comes into contact with a surface [98].
Currently, the adoption of this technique remains complex due to the scarcity of printable materials that are environmentally friendly and meet performance and manufacturing requirements [99]. Moreover, biopolymers cannot be used in their natural form, and it is an effort to convert them into raw materials for this technique. Certain biopolymers have inherently poor physicochemical properties, such as insolubility in common solvents, which complicates the process [100]. However, 3D technology has interesting advantages, such as the fabrication of complex structures and the fabrication of customized designs composed of different components [101]. In addition, material waste is reduced, making manufacturing more cost effective [93][100][102]. Among the most important limitations are the time required to perform the technique and the cost of the equipment [103].
This technique has important applications that can revolutionize sectors such as medical applications. Complex constructions can be made using 3D printing technology to replace or repair worn-out bone and cartilage tissues. For the repair of these, biopolymers such as alginate are frequently utilized [34][104][105]. A collagen–alginate combination was tested as bioink for the creation of cartilage [34]. This cartilage had favorable mechanical strength and biological functionality [34]. Another biopolymer commonly used in this technique is chitosan. A chitosan hydrogel scaffold through 3D printing was developed [35]. A biopolymer-based scaffold with remarkable adhesion and proliferation capacity of human fibroblasts was achieved. Additionally, starch is made up of heat- and pressure-sensitive molecules, which facilitates the depolymerization process produced in 3D printing. This depolymerization modifies the structure and the physical and chemical properties of the biopolymer, making it interesting for applications such as functional food [99]. As a biopolyester, PLA was the material used for the bioprinting of tubular scaffolds for application in bone tissue engineering. The application of this material showed stable mechanical and thermal properties over time [38]. Three-dimensionally printed starch beads for the release of bioactive compounds in foodstuffs were manufactured [36]. The porosity feature could be attractive for application in bioactive compound/drug delivery in the food or pharmaceutical industry. In addition, this biopolymer has potential applications in the food industry. Starch is frequently used as a thickening/gelling agent or a rheological modifier in the field of 3D food printing. Sweet potato-derived starch as a structural enhancer of three-dimensional printing was used [37]. Its application even extends to the manufacture of customized foods, where packaging based on cellulose was developed for use with foods with low moisture content [106].
5. Injection Molding Method
Injection molding is one of the most widely used methods for manufacturing biopolymer-based products. Figure 5 shows the injection molding process. This process consists of three steps [107]:
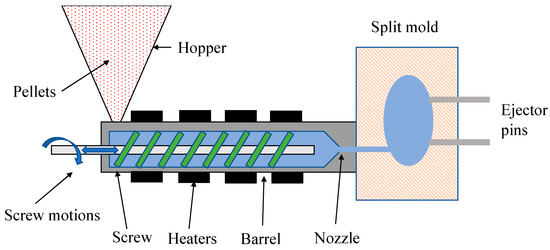
Figure 5. Schematic diagram of the injection molding method.
-
filling: the polymer is melted at high temperature and quickly fills a cold mold to form a cavity with the desired shape of the product.
-
Packing/holding: the pressure is increased, and additional material is pressed into the mold to offset the effects of temperature decline and crystallinity growth on density during the solidification.
-
Cooling: this stage begins when solidification occurs at the entrance of the cavity. From this point on, no more material will enter or leave the mold, and the retaining pressure can be released. The time to eject the mold is when the solid layer of the surface reaches a sufficient thickness to provide rigidity.
Like the other methods, this process causes important changes in the properties related to the rheology and thermomechanics of the biopolymers due to stress variations during the process, high temperature, and cooling rate of the final product [108]. Therefore, it is important to thoroughly analyze the factors that affect injection molding before deciding to manufacture a product. These factors can be divided into two categories [109]:
-
machine parameters: these include the barrel, nozzle, and coolant temperatures. Pressure is influenced by packaging pressure, back pressure, and injection pressure. It is also important to take into account the movement, switching point, injection speed, and shot volume.
-
Process parameters: these include the mold temperature and the melting and cooling temperature in addition to injection, holding, and cooling time, mold opening speed, injection, and heat/cooling dissipation.
Part dimensions, sink marks, resistance to weld lines, and other aesthetic defects such as irregular textures or marks are parameters used to determine part quality indexes [109].
The application of biopolymers in this technique is limited since the melting temperature used in this method is many times higher than the decomposition temperature of biopolymers such as chitosan. However, a chitosan/thermoplastic starch-based polymeric matrix was developed and subjected to injection molding followed by molding compression [39]. Starch is another biopolymer characterized by low mechanical properties and high water absorption. Sodium alginate in cassava starch was applied to injection molding, creating a composite that showed good compatibility between the two phases and improved mechanical properties [40]. On the other hand, the PLA biopolyester has favorable mechanical properties compared to other biopolymers, making it interesting to use it in this technique. Stereocomplex PLA formulations subjected to injection molding at different mold temperatures were produced. PCL combined with crayfish meal was used to create biocomposites. An improvement in the mechanical properties of the systems with the presence of PCL was observed [42]. This process is also carried out for the development of PLA/PHA nanocomposites. Although PHA has a similar chemical composition and flow temperature to PLA, it is not processable. However, when combined with PLA, it acts as a nucleating agent, which means that it improves the mechanical properties and the barrier behavior of the material [43].
References
- Rhim, J.W.; Ng, P.K.W. Natural Biopolymer-Based Nanocomposite Films for Packaging Applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433.
- Pechová, V.; Gajdziok, J.; Muselík, J.; Vetchý, D. Development of Orodispersible Films Containing Benzydamine Hydrochloride Using a Modified Solvent Casting Method. AAPS PharmSciTech 2018, 19, 2509–2518.
- Salit, M.S.; Jawaid, M.; Yusoff, N.B.; Hoque, M.E. Manufacturing of Natural Fibre Reinforced Polymer Composites; Springer: Cham, Switzerland, 2015; pp. 1–383.
- Cooper, W.J.; Krasicky, P.D. Dissolution Rates of Poly(Methyl Methacrylate) Films in Mixed Solvents. J. Appl. Polym. Sci. 1986, 31, 65–73.
- Papanu, J.S.; Hess, D.W.; Soane (Soong), D.S.; Bell, A.T. Swelling of Poly(Methyl Methacrylate) Thin Films in Low Molecular Weight Alcohols. J. Appl. Polym. Sci. 1990, 39, 803–823.
- Carolina Visser, J.; Weggemans, O.A.F.; Boosman, R.J.; Loos, K.U.; Frijlink, H.W.; Woerdenbag, H.J. Increased Drug Load and Polymer Compatibility of Bilayered Orodispersible Films. Eur. J. Pharm. Sci. 2017, 107, 183–190.
- Chen, Q.; Roethe, J.A.; Boccaccini, A.R. Tissue Engineering Scaffolds from Bioactive Glass and Composite Materials. Top. Tissue Eng. 2008, 4, 1–27.
- Yang, J.; Yu, J.; Huang, Y. Recent Developments in Gelcasting of Ceramics. J. Eur. Ceram. Soc. 2011, 31, 2569–2591.
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 1–16.
- Karki, S.; Kim, H.; Na, S.; Shin, D.; Jo, K.; Lee, J. Thin Films as an Emerging Platform For. Asian J. Pharm. Sci. 2016, 11, 559–574.
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and Characterization of Indicator Films from Carboxymethyl-Cellulose/Starch and Purple Sweet Potato (Ipomoea Batatas (L.) Lam) Anthocyanins for Monitoring Fish Freshness. Int. J. Biol. Macromol. 2020, 143, 359–372.
- Flórez, M.; Cazón, P.; Vázquez, M. Active Packaging Film of Chitosan and Santalum Album Essential Oil: Characterization and Application as Butter Sachet to Retard Lipid Oxidation. Food Packag. Shelf Life 2022, 34, 100938.
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic Effect of Cellulose and Lignin Nanostructures in PLA Based Systems for Food Antibacterial Packaging. Eur. Polym. J. 2016, 79, 1–12.
- Siemann, U. Progress in Colloid & Polymer Science; Springer: Berlin, Germany, 2005; ISBN 9783540253235.
- Kolhe, P.; Kannan, R.M. Improvement in Ductility of Chitosan through Blending and Copolymerization with PEG: FTIR Investigation of Molecular Interactions. Biomacromolecules 2003, 4, 173–180.
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the Presence of Glycerol and Tween 20 on the Chemical and Physical Properties of Films Based on Chitosan with Different Degree of Deacetylation. LWT 2008, 41, 2159–2165.
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and Properties of Glycerol-Plasticized Chitosan Obtained by Mechanical Kneading. Carbohydr. Polym. 2011, 83, 947–952.
- Thomas, D.; Nath, M.S.; Mathew, N.; Reshmy, R.; Philip, E.; Latha, M.S. Alginate Film Modified with Aloevera Gel and Cellulose Nanocrystals for Wound Dressing Application: Preparation, Characterization and in Vitro Evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101894.
- Sellappan, L.K.; Anandhavelu, S.; Doble, M.; Perumal, G.; Jeon, J.H.; Vikraman, D.; Kim, H.S. Biopolymer Film Fabrication for Skin Mimetic Tissue Regenerative Wound Dressing Applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 196–207.
- Sadhasivam, L.; Dey, N.; Francis, A.P.; Devasena, T. Transdermal Patches of Chitosan Nanoparticles for Insulin Delivery. Int. J. Pharm. Pharm. Sci. 2015, 7, 84–88.
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP 2013, 5, 110–114.
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved Multilayered Antimicrobial Alginate-Based Edible Coating Extends the Shelf Life of Fresh-Cut Watermelon (Citrullus Lanatus). LWT 2013, 51, 9–15.
- Batista Silva, W.; Cosme Silva, G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan Delays Ripening and ROS Production in Guava (Psidium Guajava L.) Fruit. Food Chem. 2018, 242, 232–238.
- Aitboulahsen, M.; Zantar, S.; Laglaoui, A.; Chairi, H.; Arakrak, A.; Bakkali, M.; Zerrouk, M.H. Gelatin-Based Edible Coating Combined with Mentha Pulegium Essential Oil as Bioactive Packaging for Strawberries. J. Food Qual. 2018, 2018, 1–7.
- Rashad, A.; Mohamed-Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.A.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2018, 19, 4307–4319.
- Mohan Raj, R.; Priya, P.; Raj, V. Gentamicin-Loaded Ceramic-Biopolymer Dual Layer Coatings on the Ti with Improved Bioactive and Corrosion Resistance Properties for Orthopedic Applications. J. Mech. Behav. Biomed. Mater. 2018, 82, 299–309.
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Jang, Y.S.; Kim, J.H.; Lee, M.H. Improvement of Osteogenesis by a Uniform PCL Coating on a Magnesium Screw for Biodegradable Applications. Sci. Rep. 2018, 8, 1–11.
- Faraji Dizaji, B.; Hasani Azerbaijan, M.; Sheisi, N.; Goleij, P.; Mirmajidi, T.; Chogan, F.; Irani, M.; Sharafian, F. Synthesis of PLGA/Chitosan/Zeolites and PLGA/Chitosan/Metal Organic Frameworks Nanofibers for Targeted Delivery of Paclitaxel toward Prostate Cancer Cells Death. Int. J. Biol. Macromol. 2020, 164, 1461–1474.
- Sadeghi, A.; Zandi, M.; Pezeshki-Modaress, M.; Rajabi, S. Tough, Hybrid Chondroitin Sulfate Nanofibers as a Promising Scaffold for Skin Tissue Engineering. Int. J. Biol. Macromol. 2019, 132, 63–75.
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and Characterization of TiO2NPs and Betanin Loaded Zein/Sodium Alginate Nanofibers. Food Packag. Shelf Life 2020, 24, 100504.
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological Evaluation (In Vitro and In Vivo) of Bilayered Collagenous Coated (Nano Electrospun and Solid Wall) Chitosan Membrane for Periodontal Guided Bone Regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144.
- Roy, T.; Maity, P.P.; Rameshbabu, A.P.; Das, B.; John, A.; Dutta, A.; Ghorai, S.K.; Chattopadhyay, S.; Dhara, S. Core-Shell Nanofibrous Scaffold Based on Polycaprolactone-Silk Fibroin Emulsion Electrospinning for Tissue Engineering Applications. Bioengineering 2018, 5, 68.
- Azari, P.; Luan, N.S.; Gan, S.N.; Yahya, R.; Wong, C.S.; Chua, K.H.; Pingguan-Murphy, B. Electrospun Biopolyesters as Drug Screening Platforms for Corneal Keratocytes. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 785–791.
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-Alginate as Bioink for Three-Dimensional (3D) Cell Printing Based Cartilage Tissue Engineering. Mater. Sci. Eng. C 2018, 83, 195–201.
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly Defined 3D Printed Chitosan Scaffolds Featuring Improved Cell Growth. Biomed. Mater. 2017, 12, 045009.
- Ahmadzadeh, S.; Ubeyitogullari, A. Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System. Foods 2022, 11, 913.
- Dong, X.; Huang, Y.; Pan, Y.; Wang, K.; Prakash, S.; Zhu, B. Investigation of Sweet Potato Starch as a Structural Enhancer for Three-Dimensional Printing of Scomberomorus Niphonius Surimi. J. Texture Stud. 2019, 50, 316–324.
- Vazquez-Vazquez, F.C.; Chavarria-Bolaños, D.; Ortiz-Magdaleno, M.; Guarino, V.; Alvarez-Perez, M.A. 3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications. Bioengineering 2022, 9, 189.
- Balla, B.; Bartos, A.; Kun, D.; Csiszár, E.; Móczó, J.; Fekete, E. Improving Mechanical and Water Sorption Properties of Thermoplastic Starch by Incorporating Chitosan Filler. Polym. Test. 2021, 101, 107278.
- Weerapoprasit, C.; Prachayawarakorn, J. Properties of Biodegradable Thermoplastic Cassava Starch/Sodium Alginate Composites Prepared From Injection Molding. Polym. Polym. Compos. 2016, 12, 3365–3372.
- Körber, S.; Moser, K.; Diemert, J. Development of High Temperature Resistant Stereocomplex PLA for Injection Moulding. Polymers 2022, 14, 384.
- Félix, M.; Romero, A.; Martín-Alfonso, J.E.; Guerrero, A. Development of Crayfish Protein-PCL Biocomposite Material Processed by Injection Moulding. Compos. Part B Eng. 2015, 78, 291–297.
- Relinque, J.J.; de León, A.S.; Hernández-Saz, J.; García-Romero, M.G.; Navas-Martos, F.J.; Morales-Cid, G.; Molina, S.I. Development of Surface-Coated Polylactic Acid/Polyhydroxyalkanoate (PLA/PHA) Nanocomposites. Polymers 2019, 11, 400.
- Guerrero, P.; Muxika, A.; Zarandona, I.; de la Caba, K. Crosslinking of Chitosan Films Processed by Compression Molding. Carbohydr. Polym. 2019, 206, 820–826.
- Zubeldía, F.; Ansorena, M.R.; Marcovich, N.E. Wheat Gluten Films Obtained by Compression Molding. Polym. Test. 2015, 43, 68–77.
- Valencia-Sullca, C.; Atarés, L.; Vargas, M.; Chiralt, A. Physical and Antimicrobial Properties of Compression-Molded Cassava Starch-Chitosan Films for Meat Preservation. Food Bioprocess Technol. 2018, 11, 1339–1349.
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of Fish Gelatin Edible Films Using Extrusion and Compression Molding. J. Food Eng. 2012, 108, 337–344.
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative Study of Processing Methods for Starch/Gelatin Films. Carbohydr. Polym. 2013, 95, 681–689.
- Rhim, J.W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the Processing Methods on the Performance of Polylactide Films: Thermocompression versus Solvent Casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742.
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an Antioxidant Polylactic Acid (PLA) Film Prepared with α-Tocopherol, BHT and Polyethylene Glycol Using Film Cast Extruder. J. Food Eng. 2010, 100, 239–244.
- Parulekar, Y.; Mohanty, A.K. Extruded Biodegradable Cast Films from Polyhydroxyalkanoate and Thermoplastic Starch Blends: Fabrication and Characterization. Macromol. Mater. Eng. 2007, 292, 1218–1228.
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable Polymer Blends Based on Corn Starch and Thermoplastic Chitosan Processed by Extrusion. Carbohydr. Polym. 2016, 137, 452–458.
- Bao, H.; Li, L.; Gan, L.H.; Ping, Y.; Li, J.; Ravi, P. Thermo-and PH-Responsive Association Behavior of Dual Hydrophilic Graft Chitosan Terpolymer Synthesized via ATRP and Click Chemistry. Macromolecules 2010, 43, 5679–5687.
- Zhang, L.; Zhao, Y.H.; Bai, R. Development of a Multifunctional Membrane for Chromatic Warning and Enhanced Adsorptive Removal of Heavy Metal Ions: Application to Cadmium. J. Memb. Sci. 2011, 379, 69–79.
- Hüttermann, A.; Mai, C.; Kharazipour, A. Modification of Lignin for the Production of New Compounded Materials. Appl. Microbiol. Biotechnol. 2001, 55, 387–394.
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Development of Functionalized Cellulosic Biopolymers by Graft Copolymerization. Int. J. Biol. Macromol. 2013, 62, 44–51.
- Kausar, A. Polymer Coating Technology for High Performance Applications: Fundamentals and Advances. J. Macromol. Sci. Part A 2018, 55, 440–448.
- Singh, R.S.; Kaur, N. Microbial Biopolymers for Edible Film and Coating Applications. Adv. Ind. Biotechnol. 2015, 12, 187–216.
- Parreidt, T.S.; Schott, M.; Schmid, M.; Müller, K. Effect of Presence and Concentration of Plasticizers, Vegetable Oils, and Surfactants on the Properties of Sodium-Alginate-Based Edible Coatings. Int. J. Mol. Sci. 2018, 19, 742.
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of Coating Thickness on Viscosity of Coating Solution Applied to Fruits and Vegetables by Dipping Method. J. Food Sci. 2003, 68, 503–510.
- Fritz, A.R.M.; Fonseca, J.D.M. Polymers for Agri-Food Applications; Springer: Switzerland, 2019; ISBN 9783030194161.
- Md Nor, S.; Ding, P. Trends and Advances in Edible Biopolymer Coating for Tropical Fruit: A Review. Food Res. Int. 2020, 134, 109208.
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337.
- Khan, M.K.I.; Nazir, A.; Maan, A.A. Electrospraying: A Novel Technique for Efficient Coating of Foods. Food Eng. Rev. 2017, 9, 112–119.
- Brinker, C.J. Dip Coating. In Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Vienna, Austria, 2013; pp. 233–261. ISBN 9783211993118.
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent Advances in Edible Coatings for Fresh and Minimally Processed Fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511.
- Cha, D.S.U.; Chinnan, M.S. Biopolymer-Based Antimicrobial Packaging: A Review Biopolymer-Based Antimicrobial Packaging: A Review. Food Sci. Nutr. 2004, 44, 223–237.
- Wu, Y.; Weller, C.L.; Hamouz, F.; Cuppett, S.L.; Schnepf, M. Development and Application of Multicomponent Edible Coatings and Films: A Review. Adv. Food Nutr. Res. 2002, 44, 347–394.
- Ilyina, A.V.; Tikhonov, V.E.; Albulov, A.I.; Varlamov, V.P. Enzymic Preparation of Acid-Free-Water-Soluble Chitosan. Process Biochem. 2000, 35, 563–568.
- Nussinovitch, A. Biopolymer Films and Composite Coatings; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123741950.
- Oliver, J.F.; Mason, S.G. Electron Microscopy. J. Colloid Interface Sci. 1977, 60, 480–487.
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347.
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.-S. Fabrication of Scaffolds in Tissue Engineering: A Review. Front. Mech. Eng. 2018, 13, 107–119.
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of Nanofiber Matrix as Tissue-Engineering Scaffolds. Tissue Eng. 2005, 11, 101–109.
- Agarwal, S.; Greiner, A. On the Way to Clean and Safe Electrospinning-Green Electrospinning: Emulsion and Suspension Electrospinning. Polym. Adv. Technol. 2011, 22, 372–378.
- Luo, C.J.; Nangrejo, M.; Edirisinghe, M. A Novel Method of Selecting Solvents for Polymer Electrospinning. Polymer (Guildf). 2010, 51, 1654–1662.
- Liu, H.; Hsieh, Y. Lo Ultrafine Fibrous Cellulose Membranes from Electrospinning of Cellulose Acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129.
- Rostamabadi, H.; Assadpour, E.; Tabarestani, H.S.; Falsafi, S.R. Trends in Food Science & Technology Electrospinning Approach for Nanoencapsulation of Bioactive Compounds; Recent Advances and Innovations. Trends Food Sci. Technol. 2020, 100, 190–209.
- Deitzel, J.M.; Kleinmeyer, D.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer (Guildf). 2001, 42, 261–272.
- Yuan, X.Y.; Zhang, Y.Y.; Dong, C.; Sheng, J. Morphology of Ultrafine Polysulfone Fibers Prepared by Electrospinning. Polym. Int. 2004, 53, 1704–1710.
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The Effect of Temperature and Humidity on Electrospinning. J. Mater. Sci. 2009, 44, 1357–1362.
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling Surface Morphology of Electrospun Polystyrene Fibers: Effect of Humidity and Molecular Weight in the Electrospinning Process. Macromolecules 2004, 37, 573–578.
- Almetwally, A.A.; El-Sakhawy, M.; Elshakankery, M.H.; Kasem, M.H. Technology of Nano-Fibers: Production Techniques and Properties-Critical Review. J. Text. Assoc. 2017, 78, 5–14.
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The Impact of Relative Humidity during Electrospinning on the Morphology and Mechanical Properties of Nanofibers. Int. J. Pharm. 2013, 456, 125–134.
- Beachley, V.; Wen, X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668.
- Pierschbacher, M.D.; Ruoslahti, E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature 1984, 309, 30–33.
- Garavand, F.; Rahaee, S.; Vahedikia, N.; Mahdi, S. Different Techniques for Extraction and Micro/Nanoencapsulation of Saffron Bioactive Ingredients. Trends Food Sci. Technol. 2019, 89, 26–44.
- Kumar, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45.
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569.
- Huang, R.; Li, W.; Lv, X.; Lei, Z.; Bian, Y.; Deng, H.; Wang, H.; Li, J.; Li, X. Biomimetic LBL Structured Nanofibrous Matrices Assembled by Chitosan/Collagen for Promoting Wound Healing. Biomaterials 2015, 53, 58–75.
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994.
- Valino, A.D.; Ryan, J.; Dizon, C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Progress in Polymer Science Advances in 3D Printing of Thermoplastic Polymer Composites and Nanocomposites. Prog. Polym. Sci. 2019, 98, 101162.
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with Polymers: Challenges among Expanding Options and Opportunities. Dent. Mater. 2016, 32, 54–64.
- Duty, C.; Ajinjeru, C.; Kishore, V.; Compton, B.; Hmeidat, N.; Chen, X.; Liu, P.; Hassen, A.A.; Lindahl, J.; Kunc, V. What Makes a Material Printable? A Viscoelastic Model for Extrusion-Based 3D Printing of Polymers. J. Manuf. Process. 2018, 35, 526–537.
- Hsueh, M.-H.; Lai, C.-J.; Liu, K.-Y.; Chung, C.-F.; Wang, S.-H.; Pan, C.-Y.; Huang, W.-C.; Hsieh, C.-H.; Zeng, Y.-S.; Grochowicz, M.; et al. Effects of Printing Temperature and Filling Percentage on the Mechanical Behavior of Fused Deposition Molding Technology Components for 3D Printing. Polymers 2021, 13, 2910.
- Jiang, H.; Zheng, L.; Zou, Y.; Tong, Z.; Han, S.; Wang, S. 3D Food Printing: Main Components Selection by Considering Rheological Properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 2335–2347.
- Chen, Y.; McClements, D.J.; Peng, X.; Chen, L.; Xu, Z.; Meng, M.; Zhou, X.; Zhao, J.; Jin, Z. Starch as Edible Ink in 3D Printing for Food Applications: A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–16.
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current Advances and Future Perspectives of 3D Printing Natural-Derived Biopolymers. Carbohydr. Polym. 2019, 207, 297–316.
- Pradhan, R.A.; Rahman, S.S.; Qureshi, A.; Ullah, A. Biopolymers: Opportunities and Challenges for 3D Printing; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-819240-5.
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D Printing to Innovate Biopolymer Materials for Demanding Applications: A Review. Mater. Today Chem. 2021, 20, 100459.
- González-henríquez, C.M.; Sarabia-vallejos, M.A.; Rodriguez-hernandez, J. Polymers for Additive Manufacturing and 4D-Printing: Materials, Methodologies, and Biomedical Applications. Prog. Polym. Sci. 2019, 94, 57–116.
- Martelli, N.; Serrano, C.; Van Den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and Disadvantages of 3-Dimensional Printing in Surgery: A Systematic Review. Surg. (United States) 2016, 159, 1485–1500.
- Fermani, M.; Platania, V.; Kavasi, R.; Karavasili, C.; Zgouro, P.; Fatouros, D.; Chatzinikolaidou, M.; Bouropoulos, N. Applied Sciences 3D-Printed Scaffolds from Alginate/Methyl Cellulose/Trimethyl Chitosan/Silicate Glasses for Bone Tissue Engineering. Appl. Sci. 2021, 11, 8677.
- Shi, P.; Laude, A.; Yeong, W.Y. Investigation of Cell Viability and Morphology in 3D Bio-Printed Alginate Constructs with Tunable Stiffness. J. Biomed. Mater. Res.-Part A 2017, 105, 1009–1018.
- Nida, S.; Moses, J.A.; Anandharamakrishnan, C. 3D Printed Food Package Casings from Sugarcane Bagasse: A Waste Valorization Study. Biomass Convers. Biorefinery 2021, 1, 1–11.
- Pantani, R.; Coccorullo, I.; Speranza, V.; Titomanlio, G. Modeling of Morphology Evolution in the Injection Molding Process of Thermoplastic Polymers. Prog. Polym. Sci. 2005, 30, 1185–1222.
- Kashyap, S.; Datta, D. Process Parameter Optimization of Plastic Injection Molding: A Review. Int. J. Plast. Technol. 2015, 19, 1–18.
- Chen, Z.; Turng, L.S. A Review of Current Developments in Process and Quality Control for Injection Molding. Adv. Polym. Technol. 2005, 24, 165–182.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.4K
Revisions:
3 times
(View History)
Update Date:
16 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





