Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arja Kullaa | -- | 1448 | 2023-05-11 12:15:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 1448 | 2023-05-15 06:59:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hyvärinen, E.; Solje, E.; Vepsäläinen, J.; Kullaa, A.; Tynkkynen, T. Salivary Metabolomics in Neurodegenerative Dementia Diagnosis and Monitoring. Encyclopedia. Available online: https://encyclopedia.pub/entry/44148 (accessed on 07 February 2026).
Hyvärinen E, Solje E, Vepsäläinen J, Kullaa A, Tynkkynen T. Salivary Metabolomics in Neurodegenerative Dementia Diagnosis and Monitoring. Encyclopedia. Available at: https://encyclopedia.pub/entry/44148. Accessed February 07, 2026.
Hyvärinen, Eelis, Eino Solje, Jouko Vepsäläinen, Arja Kullaa, Tuulia Tynkkynen. "Salivary Metabolomics in Neurodegenerative Dementia Diagnosis and Monitoring" Encyclopedia, https://encyclopedia.pub/entry/44148 (accessed February 07, 2026).
Hyvärinen, E., Solje, E., Vepsäläinen, J., Kullaa, A., & Tynkkynen, T. (2023, May 11). Salivary Metabolomics in Neurodegenerative Dementia Diagnosis and Monitoring. In Encyclopedia. https://encyclopedia.pub/entry/44148
Hyvärinen, Eelis, et al. "Salivary Metabolomics in Neurodegenerative Dementia Diagnosis and Monitoring." Encyclopedia. Web. 11 May, 2023.
Copy Citation
Spectroscopic methods (NMR, MS) give us a broad view of changes in salivary metabolites in neurodegenerative diseases and deepen the knowledge of the systemic communication between the oral cavity and the brain. Further studies with larger patient cohorts should be carried out to investigate the association between salivary metabolites and brain function and thus learn more about the complicated pathways in the human body.
neurodegenerative diseases
dementia
saliva
metabolites
1. Introduction
Approximately 55 million people suffer with dementia worldwide. Dementia is a syndrome affecting memory, thinking, orientation, comprehension, calculation, learning capacity, language and judgement [1]. Most commonly, dementia is caused by progressive diseases inducing neurodegeneration including Alzheimer’s disease (AD), frontotemporal dementia (FTD), vascular dementia (VaD) and alpha synucleinopathies: dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD). AD accounts for about 70% of all dementia cases, and the number of patients suffering from dementia is increasing due to increasing average lifetime [2].
Diagnosis of neurodegenerative diseases is difficult, especially in the pre-clinical stages [3][4]. Many biomarkers based on imaging and cerebrospinal fluid (CSF) have been suggested to be positively associated with early diagnosis, but disease specificity is lacking [5]. In cognitively asymptomatic individuals with positive biomarkers for AD, the lifetime dementia risk is estimated to be from 5% to 42% [6]. Blood neurofilament light chain (NfL) is suggested to be a biomarker for neurodegenerative disorders, but it is not disease-specific and rather reflects neuronal damage in general [7]. Hence, there is an urgent need for new diagnostic, prognostic and monitoring biomarker innovations.
Saliva, a complex biofluid with a high variety of molecules, mainly consists of water (99%) and inorganic and organic substances [8]. Saliva is secreted from three pairs of major salivary glands (i.e., parotid, submandibular, sublingual) and numerous minor salivary glands throughout the oral cavity and pharynx. The functions of salivary glands are controlled by the sympathetic/parasympathetic nervous system. Primary saliva is produced from blood components by the acinar cells via transcellular diffusion and via the tight cell junctions of these cells [9]. Before entering the mouth, saliva is modified by the ductal cells, including the intercalated, striated and excretory cells, via reabsorption to the bloodstream. Furthermore, saliva flow rate, oral microbiota, oral mucosal transudate, immune cells and other environmental factors have an impact on the final composition of whole mouth saliva [9][10][11]. Saliva contains several compounds that are involved in oral health maintenance. In addition to oral diseases, the origin of saliva enables salivary diagnostics of systemic diseases [12].
Salivary glands work as an exocrine (external secretions as saliva) and endocrine organ. Some of the salivary products are transferred into the bloodstream via endocrine mechanisms and communicate with other organs, including the brain (Figure 1) [13]. Hence, saliva is an accessible source of information as a ‘mirror of the body’ and a promising biofluid for the diagnosis and monitoring of human diseases because of its bidirectional mechanisms. Furthermore, in contrast to blood or CSF, the collection of saliva is non-invasive and safe.
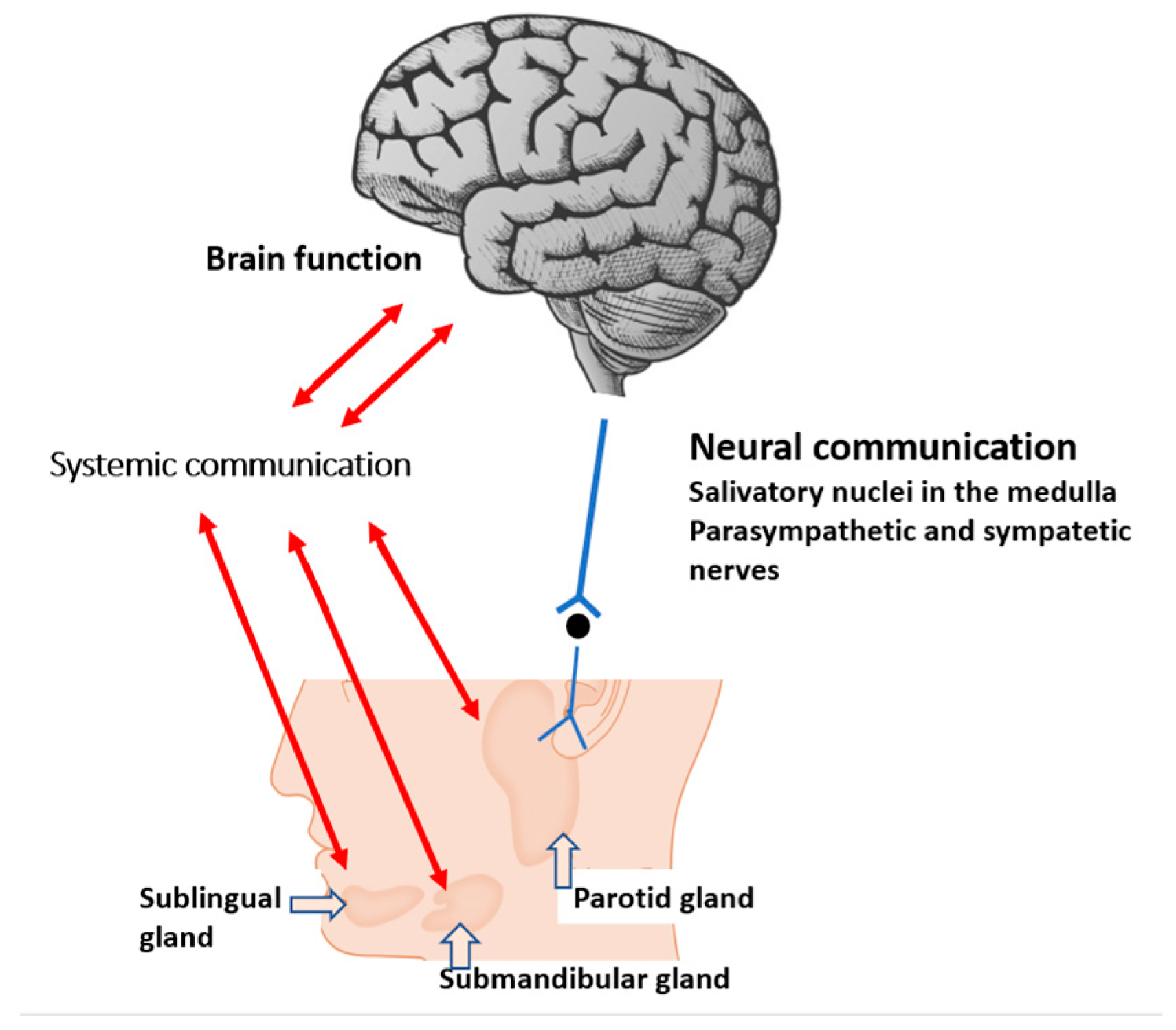
Figure 1. Systemic and neural pathways linking the salivary gland with brain function. Metabolites play a central role in systemic communication.
Salivary analysis requires precise methods due to the low concentration of salivary components. Metabolites provide comprehensive information about the cellular functions of oral tissues and changes in the phenotype of cells or tissues in response to genetic or environmental changes. The most common methods are enzyme-linked immunosorbent assays (ELISA) and different spectroscopic methods. Mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy are frequently used methods in saliva research [12]. Mass spectrometry is commonly used in conjunction with either two-dimensional gas chromatography (2DGC-MS) or high-performance liquid chromatography (HPLC-MS) [12]. NMR spectroscopy is based on the behaviour of magnetically active atomic nuclei, e.g., 1H or 13C, in an external magnetic field. Identification of small molecules is possible because most compounds have highly characteristic resonance frequencies [14]. Additionally, Raman spectroscopy, Fourier-transform infrared (FTIR) spectroscopy and photoacoustic spectroscopy (PAS) have been used in salivary research [15][16].
2. Salivary Metabolomics in Neurodegenerative Dementia Diagnosis and Monitoring
One study analysed AD and VaD [17] and only one analysed AD and FTD [18]. Two articles [19][20] handled PD, but did not differentiate patients according to cognitive symptoms (PDD). In future studies, the underlying neuropathology or pathophysiologial process in the research subjects should be established using neuropathological analysis or modern beyond-state-of-the-art methods. In particular, CSF RT-quIC [7] in the identification of the underlying proteinopathy and transcranial magnetic stimulation [21] in the recognition of the disease-specific neurotransmitter system deficit could increase the validity of saliva biomarker studies.
Some single salivary metabolites, including Aβ, t-tau and lactoferrin, are associated with AD. Increased salivary Aβ is shown in AD patients, but is not evident in studies with MS and NMR spectroscopy. Decreased salivary lactoferrin and increased t-tau are shown also with MS in some studies [22][23]. Lactoferrin, one component of the innate defence mechanism of saliva, is produced via salivary glands and also from gingival cervicular fluid, and it is active against oral microbes [9]. Hence, it can be a biomarker of gingivitis and periodontitis.
With spectroscopic methods, it can obtain a wide scale of different salivary metabolites and thus identify disease-associated changes in oral metabolism as a mirror of whole human body physiology. François et al. [24] discovered that serotonin is increased in patients with AD versus MCI and healthy controls. Tryptophan is a precursor for serotonin [25], and L-tryptophan has been discovered to be elevated in AD versus MCI [26]. Serotonin affects nearly all human behavioural processes, but a major amount of serotonin is found outside the central nervous system. Approximately 95% of total body serotonin is produced by the intestinal enterochromaffin cells [27] and therefore it may not be a promising salivary biomarker for AD. In addition, high levels of tryptophan-tyrosine dipeptide in the saliva of AD patients might indicate memory impairments due to altered dopaminergic activity [28]. In the future, studies of serotonin, tryptophan and dipeptides in the saliva might indicate pathway changes and episodic memory impairment in patients with AD.
Studied with NMR spectroscopy, salivary propionate has been found to be upregulated in patients with AD when compared to controls [17][29]. However, propionate is also increased in inflammatory oral diseases, including periodontal diseases and dental caries, therefore its effectiveness as a specific salivary biomarker for neurodegenerative diseases is questionable. On the other hand, periodontitis and tooth loss have been shown to increase the risk of dementia [30][31][32]. Gut microbiota and their metabolites, like propionate, have been mentioned in mediating brain function [33]. Salivary propionate is produced by oral bacteria [11], but the link between salivary propionate and the brain has not been studied. In addition to inflammatory diseases, oral dysbiosis together with salivary metabolomics could be one target to study further in patients with neurodegenerative dementia.
Salivary metabolites mainly reflect the oral microbiome. Concentrations of some metabolites, including short chain fatty acids (SCFAs: acetate, butyrate, propionate, formate), correlate with salivary bacterial load [11]. On the other hand, SCFAs, as immune-regulatory metabolites, can stimulate the autonomic nervous system [34][35]. These metabolites, produced by proteolytic bacteria, are associated with periodontitis [36][37] and some of these metabolites have also been found in patients with MCI and VaD vs. controls [17][29]. In this regard, scholars hypothesize that salivary SCFAs circulate in the blood and can cause low-level systemic inflammation and associate with brain function. The biological mechanisms and systemic communication between the brain and oral health are yet unknown. Hence, the association between inflammatory oral diseases and brain function presents a target for further study on salivary metabolites. The role of salivary SCFAs in the mouth–brain axis needs more investigation.
The level of salivary taurine was lower in patients with MCI [38] and AD/VaD [17] when compared to controls. Taurine has numerous functions in the nervous system, including neurotransmission, neuromodulation and osmoregulation, and it prevents the neurotoxicity of Aβ [39].
Salivary histamine was increased in patients with AD and VaD versus controls [17]. The central histaminergic system in the brain plays a major role in basic body functions, such as the sleep-waking cycle and learning, and has been reported to be involved in AD [40]. In addition to histaminergic neurons, histamine is primarily produced by mast cells, basophils, and enterochromaffin-like cells in the stomach [41].
Recent metabolomic studies have often been conducted with relatively small study populations. To verify these results, multi-centre investigations with larger cross-sectional populations are needed. Such projects would also enable longitudinal, long-term follow-up studies and include more background information on patient health. Furthermore, an important object of biomarker research in neurodegenerative dementia is to compare the validated metabolic biomarkers from multiple biofluids including blood, CSF and saliva. Standardized collection and storage methods and increasing interest in saliva research could make high-quality saliva research possible in the future.
Salivary metabolites have recently been investigated with spectroscopic methods in different diseases [12]. However, the collection methods vary considerably. Stimulation of salivary secretion is necessary with some patients with hyposalivation, e.g., elderly people.
References
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 9 December 2022).
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152.
- Bloudek, L.M.; Spackman, D.E.; Blankenburg, M.; Sullivan, S.D. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J. Alzheimers Dis. 2011, 26, 627–645.
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N.; Hansson, O. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat. Med. 2021, 27, 1034–1042.
- Brookmeyer, R.; Abdalla, N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018, 14, 981–988.
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the international working group. Lancet Neurol. 2021, 20, 484–496.
- Solje, E.; Benussi, A.; Buratti, E.; Remes, A.M.; Haapasalo, A.; Borroni, B. State-of-the-art methods and emerging fluid biomarkers in the diagnostics of dementia-a short review and diagnostic algorithm. Diagnostics 2021, 11, 788.
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77.
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320.
- Bardow, A.; Lynge Pedersen, A.M.; Nauntofte, B. Saliva. In Clinical Oral Physiology, 1st ed.; Miles, T.S., Nauntofte, B., Svensson, P., Eds.; Quintessence Publishing Co. Ltd.: Copenhagen, Denmark, 2004; pp. 17–51.
- Gardner, A.; Parkes, H.G.; So, P.W.; Carpenter, G.H. Determining bacterial and host contributions to the human salivary metabolome. J. Oral Microbiol. 2019, 11, 1617014.
- Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary metabolomics for diagnosis and monitoring diseases: Challenges and possibilities. Metabolites 2021, 11, 587.
- Isenman, L.; Liebow, C.; Rothman, S. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am. J. Physiol. 1999, 276, E223–E232.
- Mikkonen, J.J.W.; Singh, S.P.; Akhi, R.; Salo, T.; Lappalainen, R.; González-Arriagada, W.A.; Ajudarte Lopes, M.; Kullaa, A.M.; Myllymaa, S. Potential role of nuclear magnetic resonance spectroscopy to identify salivary metabolite alterations in patients with head and neck cancer. Oncol. Lett. 2018, 16, 6795–6800.
- Carlomagno, C.; Bertazioli, D.; Gualerzi, A.; Picciolini, S.; Andrico, M.; Rodà, F.; Meloni, M.; Banfi, P.I.; Verde, F.; Ticozzi, N.; et al. Identification of the raman salivary fingerprint of Parkinson’s disease through the spectroscopic-computational combinatory approach. Front. Neurosci. 2021, 15, 704963.
- Mikkonen, J.J.W.; Raittila, J.; Rieppo, L.; Lappalainen, R.; Kullaa, A.M.; Myllymaa, S. Fourier transform infrared spectroscopy and photoacoustic spectroscopy for saliva analysis. Appl. Spectrosc. 2016, 70, 1502–1510.
- Figueira, J.; Jonsson, P.; Nordin Adolfsson, A.; Adolfsson, R.; Nyberg, L.; Öhman, A. NMR analysis of the human saliva metabolome distinguishes dementia patients from matched controls. Mol. Biosyst. 2016, 12, 2562–2571.
- Tsuruoka, M.; Hara, J.; Hirayama, A.; Sugimoto, M.; Soga, T.; Shankle, W.R.; Tomita, M. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis 2013, 34, 2865–2872.
- Kumari, S.; Goyal, V.; Kumaran, S.S.; Dwivedi, S.N.; Srivastava, A.; Jagannathan, N.R. Quantitative metabolomics of saliva using proton NMR spectroscopy in patients with Parkinson’s disease and healthy controls. Neurol. Sci. 2020, 41, 1201–1210.
- Figura, M.; Sitkiewicz, E.; Świderska, B.; Milanowski, Ł.; Szlufik, S.; Koziorowski, D.; Friedman, A. Proteomic profile of saliva in Parkinson’s disease patients: A proof of concept study. Brain Sci. 2021, 11, 661.
- Padovani, A.; Benussi, A.; Cantoni, V.; Dell’Era, V.; Cotelli, M.S.; Caratozzolo, S.; Turrone, R.; Rozzini, L.; Alberici, A.; Altomare, D.; et al. Diagnosis of mild cognitive impairment due to Alzheimer’s disease with transcranial magnetic stimulation. J. Alzheimers Dis. 2018, 65, 221–230.
- Carro, E.; Bartolomé, F.; Bermejo-Pareja, F.; Villarejo-Galende, A.; Molina, J.A.; Ortiz, P.; Calero, M.; Rabano, A.; Cantero, J.L.; Orive, G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimers Dement (Amst). 2017, 8, 131–138.
- Shi, M.; Sui, Y.T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary tau species are potential biomarkers of Alzheimer’s disease. J. Alzheimers Dis. 2011, 27, 299–305.
- François, M.; Karpe, A.; Liu, J.W.; Beale, D.; Hor, M.; Hecker, J.; Faunt, J.; Maddison, J.; Johns, S.; Doecke, J.; et al. Salivaomics as a potential tool for predicting Alzheimer’s disease during the early stages of neurodegeneration. J. Alzheimers Dis. 2021, 82, 1301–1313.
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. (Eds.) Neurotransmitter systems. In Neuroscience: Exploring the Brain, 4th ed.; Wolters Kluwer Ltd.: Philadelphia, PA, USA, 2016; pp. 143–178.
- Liang, Q.; Liu, H.; Li, X.; Zhang, A.H. High-throughput metabolomics analysis discovers salivary biomarkers for predicting mild cognitive impairment and Alzheimer’s disease. RSC Adv. 2016, 6, 75499–75504.
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366.
- Cueno, M.E.; Ochiai, K. Gingival periodontal disease (PD) level-butyric acid affects the systemic blood and brain organ: Insights into the systemic inflammation of periodontal disease. Front Immunol. 2018, 9, 1158.
- Yilmaz, A.; Geddes, T.; Han, B.; Bahado-Singh, R.O.; Wilson, G.D.; Imam, K.; Maddens, M.; Graham, S.F. Diagnostic biomarkers of Alzheimer’s disease as identified in saliva using 1H NMR-based metabolomics. J. Alzheimers Dis. 2017, 58, 355–359.
- Leira, Y.; Domínguez, C.; Seoane, J.; Seoane-Romero, J.; Pías-Peleteiro, J.M.; Takkouche, B.; Blanco, J.; Aldrey, J.M. Is periodontal disease associated with Alzheimer’s disease? A systematic review with meta-analysis. Neuroepidemiology 2017, 48, 21–31.
- Holmer, J.; Eriksdotter, M.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Association between periodontitis and risk of Alzheimer’s disease, mild cognitive impairment and subjective cognitive decline: A case-control study. J. Clin. Periodontol. 2018, 45, 1287–1298.
- Asher, S.; Stephen, R.; Mäntylä, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709.
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748.
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72.
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020, 100, 171–210.
- Aimetti, M.; Romano, F.; Guzzi, N.; Carnevale, G. Full-mouth disinfection and systemic antimicrobial therapy in generalized aggressive periodontitis: A randomized, placebo-controlled trial. J. Clin. Periodontol. 2012, 39, 284–294.
- Rzeznik, M.; Triba, M.N.; Levy, P.; Jungo, S.; Botosoa, E.; Duchemann, B.; Le Moyec, L.; Bernaudin, J.F.; Savarin, P.; Guez, D. Identification of a discriminative metabolomic fingerprint of potential clinical relevance in saliva of patients with periodontitis using 1H nuclear magnetic resonance (NMR) spectroscopy. PLoS ONE 2017, 12, e0182767.
- Zheng, J.; Dixon, R.A.; Li, L. Development of isotope labeling LC-MS for human salivary metabolomics and application to profiling metabolome changes associated with mild cognitive impairment. Anal. Chem. 2012, 84, 10802–10811.
- Louzada, P.R.; Paula Lima, A.C.; Mendonca-Silva, D.L.; Noël, F.; De Mello, F.G.; Ferreira, S.T. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: Activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004, 18, 511–518.
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of neuroinflammation in autism spectrum disorder and the emergence of brain histaminergic system. Lessons also for BPSD? Front. Pharmacol. 2020, 11, 886.
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular regulation of histamine synthesis. Front. Immunol. 2018, 9, 1392.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
672
Revisions:
2 times
(View History)
Update Date:
15 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




