You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Katarzyna Wójtowicz | -- | 1221 | 2023-05-11 11:09:55 | | | |
| 2 | Camila Xu | + 3 word(s) | 1224 | 2023-05-12 03:09:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Łach, A.; Wnuk, A.; Wójtowicz, A.K. Transport through the Blood–Brain Barrier. Encyclopedia. Available online: https://encyclopedia.pub/entry/44140 (accessed on 22 December 2025).
Łach A, Wnuk A, Wójtowicz AK. Transport through the Blood–Brain Barrier. Encyclopedia. Available at: https://encyclopedia.pub/entry/44140. Accessed December 22, 2025.
Łach, Andrzej, Agnieszka Wnuk, Anna Katarzyna Wójtowicz. "Transport through the Blood–Brain Barrier" Encyclopedia, https://encyclopedia.pub/entry/44140 (accessed December 22, 2025).
Łach, A., Wnuk, A., & Wójtowicz, A.K. (2023, May 11). Transport through the Blood–Brain Barrier. In Encyclopedia. https://encyclopedia.pub/entry/44140
Łach, Andrzej, et al. "Transport through the Blood–Brain Barrier." Encyclopedia. Web. 11 May, 2023.
Copy Citation
The blood–brain barrier (BBB) is a complex structure present in mammalian organisms and is responsible for maintaining the parameters of the internal environment of the central nervous system (CNS).
blood–brain barrier
in vitro modeling
permeability
1. Introduction
The blood–brain barrier (BBB) is a complex structure present in mammalian organisms and is responsible for maintaining the parameters of the internal environment of the central nervous system (CNS). At the same time, it participates in the delivery of nutrients to CNS cells, removal of their metabolites and gas exchange. Finally, it protects the CNS from the harmful influence of a variety of compounds. It is important to note a certain kind of dualism presented by the BBB—it is both a barrier and transport structure. Barrier function is implemented on three planes: a physical barrier made up of endothelial cells [ECs] and tight junctions [TJs] that bond them, a metabolic barrier formed by specific enzymes produced by BBB components, and a transport barrier, realized by a variety of transporting proteins that remove certain substances from the territory of the CNS [1]. It is commonly known that both described functions of this structure are impaired in cases of neurodevelopmental and neurodegenerative disorders, such as Alzheimer’s Disease and amyotrophic lateral sclerosis [2][3]. According to recent studies, the loss of integrity of the BBB is a cause of neurodevelopmental disorders including autism spectrum disorder (ASD) [4] or schizophrenia [5]. Considering the importance of this structure, reliable models of BBB are urgently needed for both clinicians and researchers.
This crucial role of the BBB in nervous system functioning, and thus for the entire organism, have made this structure an object of numerous studies leading to an understanding of its properties. A purely clinical approach also sets a demand for such studies, since the BBB both forms an obstacle for various substances that could potentially be used in neurological disease therapy and is impaired in the progression of many diseases [6]. Juxtaposition of the demand for profound understanding of the BBB structure and function with increasing popularity and advancement of in vitro methods sets an obvious direction of development for this branch of neurobiology. To date, many models have been established to mimic the BBB. They are intended to reflect aspects of the BBB that previously demanded in vivo testing, but more importantly, they offer an opportunity to conduct studies in a rigorous, repeatable manner that allows for the comparison of results between independent research teams.
2. Transport through the Blood–Brain Barrier (BBB)
The BBB is responsible for maintaining proper conditions in the integral environment of the CNS, delivering nutrients and oxygen as well as removing metabolites and carbon dioxide. In addition to substances required for proper CNS functioning, other chemical compounds pass through the BBB as well, e.g., barbiturates and ethanol [1]. This section is dedicated to discussing five main paths of particle transport through the BBB, illustrated in Figure 1.
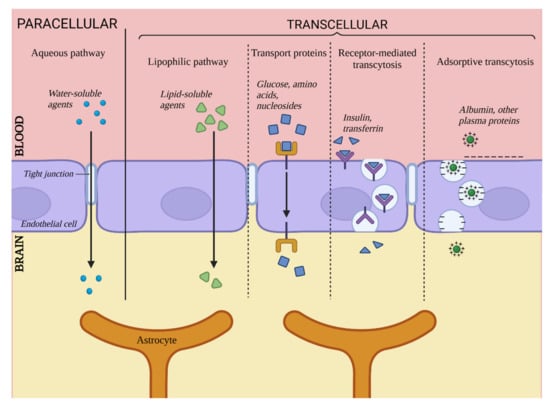
Figure 1. Transport via the blood–brain barrier. Created with BioRender.com (accessed on 22 February 2023).
First, transport through the BBB can be divided into paracellular and transcellular transport. Paracellular transport relates to particles passing through spaces between two adjacent ECs, and transcellular transport is a collective term for four pathways of transport directly via ECs.
2.1. Paracellular Transport
Unlike in ECs located in other parts of organisms, this particular pathway plays a relatively insignificant role in the context of whole transport through cell layers of the brain vasculature due to the presence of TJs. In physiological conditions, this pathway is limited to small hydrophilic particles and small ions. It has been determined that the maximum size of particles capable of passing through this pathway is approximately 10 Å [6]. Despite the limited role of paracellular transport in whole transport through the BBB, it is not to be overlooked during the search for potential carrying agents for substances targeted for the CNS. Since the low significance of this pathway is caused by the integrity of the TJs, it can become more prominent and available for larger particles in cases of pathological or artificially-induced integrity decrease [7].
2.2. Transcellular Transport
Transcellular transport includes four pathways leading directly through the EC body and is the primary means of transport through the BBB. It includes passive diffusion, transport via carrier proteins, receptor-related transcytosis and adsorption-related transcytosis [8].
2.2.1. Passive Diffusion
This mechanism is responsible for the transport of lipophilic and amphiphilic substances by diffusion through the cell body. A substance’s ability to penetrate the BBB through passive diffusion is directly proportional to its lipophilicity [9]. This ability also depends on factors such as particle size, electric charge or ability to create hydrogen bonds, but lipophilicity remains the most important determinant [8]. This pathway is somewhat limited by the presence of ABC transporters, for which many lipophilic substances are substrates [9]. Examples of such transporters are P-glycoproteins, which are associated with the mechanism of multidrug resistance. They are located on the surface of ECs on their luminal side, and they capture substances trying to pass through the BBB, preventing them from achieving high concentrations in the CNS [10].
2.2.2. Transport via Carrier Proteins
This pathway is responsible for transporting substances that exhibit a polar character and that are unable to diffuse via the cell membrane of ECs. Among other things, it takes care of components vital for the maintenance of proper CNS functioning, such as glucose, amino acids or nucleosides [9]. This takes place in the presence of carrier proteins secreted by ECs, many of which are highly specific for their designated carried substrate, e.g., GLUT1 (key carrier of glucose) [11]. Many others are less specific, recognizing certain functional groups of substances, e.g., carriers for LNAA (Large Neutral Amino Acids), which binds substances with carboxyl or α-amino groups [12]. Carrier proteins are members of two large protein families, solute carriers and ABCs [13]. It is believed that this pathway is most promising as a mean of targeted CNS therapy [8][9].
2.2.3. Receptor-Mediated Transcytosis
This pathway is responsible for the transport of particles such as large peptides and proteins (e.g., insulin [14] or transferrin [9]). It is associated with surface receptors of ECs that bind specific ligands. Binding leads to the emergence of a cavity in the cell membrane and the formation of transport vesicles. This pathway is also related to the barrier function of the BBB since it has been reported that once the vesicle enters the cell’s body, it can fuse with a lysosome, which leads to degradation of the contained substance [15]; thus, it can be considered a neutralization mechanism for substances undesirable for the CNS. This pathway can also be utilized for delivering pharmaceutics into the CNS under conditions of designing vectors specific for EC receptors and binding said pharmaceutic to them [14].
2.2.4. Adsorption-Related Transport
The fourth transport pathway described here is responsible for the transport of blood-derived proteins, e.g., albumin, that can undergo cationization - emergence of a positive electric charge that allows for interaction with the negatively charged cell membrane of ECs [16]. Next, similar to the previous path, the formation of a transport vesicle occurs, and the substance is transported inside the cell body. This pathway is less important for whole BBB transport since most cationized proteins are captured by the liver and excreted by the kidneys [9]. Nonetheless, it gives some hope for being used in potential CNS therapy by binding carried substances to a CPP marker (Cell Penetrating Protein). CPP markers possess a strong positive charge that activates transcytosis [17].
References
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53.
- Yuan, Y.; Sun, J.; Dong, Q.; Cui, M. Blood-brain barrier endothelial cells in neurodegenerative diseases: Signals from the “barrier”. Front. Neurosci. 2023, 24, 1047778.
- Lee, S.; Chung, M.; Lee, S.R.; Jeon, N. 3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro. Biotechnol. Bioeng. 2019, 10, 748–762.
- Memis, I.; Mittal, R.; Furar, E.; White, I.; Eshraghi, R.S.; Mittal, J.; Eshraghi, A.A. Altered Blood Brain Barrier Permeability and Oxidative Stress in Cntnap2 Knockout Rat Model. J. Clin. Med. 2022, 11, 2725.
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 83.
- Lazarovici, P.; Li, M.; Perets, A.; Mondrinos, M.J.; Lecht, S.; Koharski, C.D.; Bidez, P.R., III; Finck, C.M.; Lelkes, P.I. Intelligent Biomatrices and Engineered Tissue Constructs: In-Vitro Models for Drug Discovery and Toxicity Testing. In Drug Testing In Vitro Breakthroughs and Trends in Cell Culture Technology, 1st ed.; Marx, U., Sandig, V., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 1–39.
- Kopec, B.M.; Ulapane, K.R.; Moral, M.E.G.; Siahaan, T.J. Methods of Delivering Molecules Through the Blood-Brain Barrier for Brain Diagnostics and Therapeutics. In Blood Brain Barrier, 1st ed.; Barichello, T., Ed.; Humana Press: New York, NY, USA, 2019; pp. 9–44.
- Abbott, N.J.; Romero, I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 1996, 2, 106–113.
- Lalatsa, A.; Butt, A.M. Physiology of the Blood Brain Barrier and Mechanisms of Transport Across the BBB. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors, 1st ed.; Kesharwani, P., Gupta, U., Eds.; Academic Press: London, UK, 2018; pp. 49–66.
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194.
- Carruthers, A.; DeZutter, J.; Ganguly, A.; Devaskar, S.U. Will the original glucose transporter isoform please stand up! Am. J. Physiol. Endocrinol. Metab. 2009, 297, E836–E848.
- Wade, L.A.; Katzman, R. Synthetic amino acids and the nature of L-DOPA transport at the blood-brain barrier. J. Neurochem. 1975, 25, 837–842.
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers. AAPS J. 2017, 19, 1317–1331.
- Bickel, U.; Yoshikawa, T.; Pardridge, W.M. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev. 2001, 46, 247–279.
- Broadwell, R.D.; Balin, B.J.; Salcman, M. Transcytotic pathway for blood-borne protein through the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1988, 85, 632–636.
- Vorbrodt, A.W. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J. Neurocytol. 1989, 18, 359–368.
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
12 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




