
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Da Sun | -- | 2963 | 2023-05-10 11:20:14 | | | |
| 2 | Lindsay Dong | Meta information modification | 2963 | 2023-05-10 11:30:17 | | |
Video Upload Options
Diabetic foot ulcers cause great suffering and are costly for the healthcare system. Normal wound healing involves hemostasis, inflammation, proliferation, and remodeling. However, the negative factors associated with diabetes, such as bacterial biofilms, persistent inflammation, impaired angiogenesis, inhibited cell proliferation, and pathological scarring, greatly interfere with the smooth progress of the entire healing process. It is this impaired wound healing that leads to diabetic foot ulcers and even amputations. Therefore, drug screening is challenging due to the complexity of damaged healing mechanisms. The establishment of a scientific and reasonable animal experimental model contributes significantly to the in-depth research of diabetic wound pathology, prevention, diagnosis, and treatment. In addition to the low cost and transparency of the embryo (for imaging transgene applications), zebrafish have a discrete wound healing process for the separate study of each stage, resulting in their potential as the ideal model animal for diabetic wound healing in the future.
1. Introduction
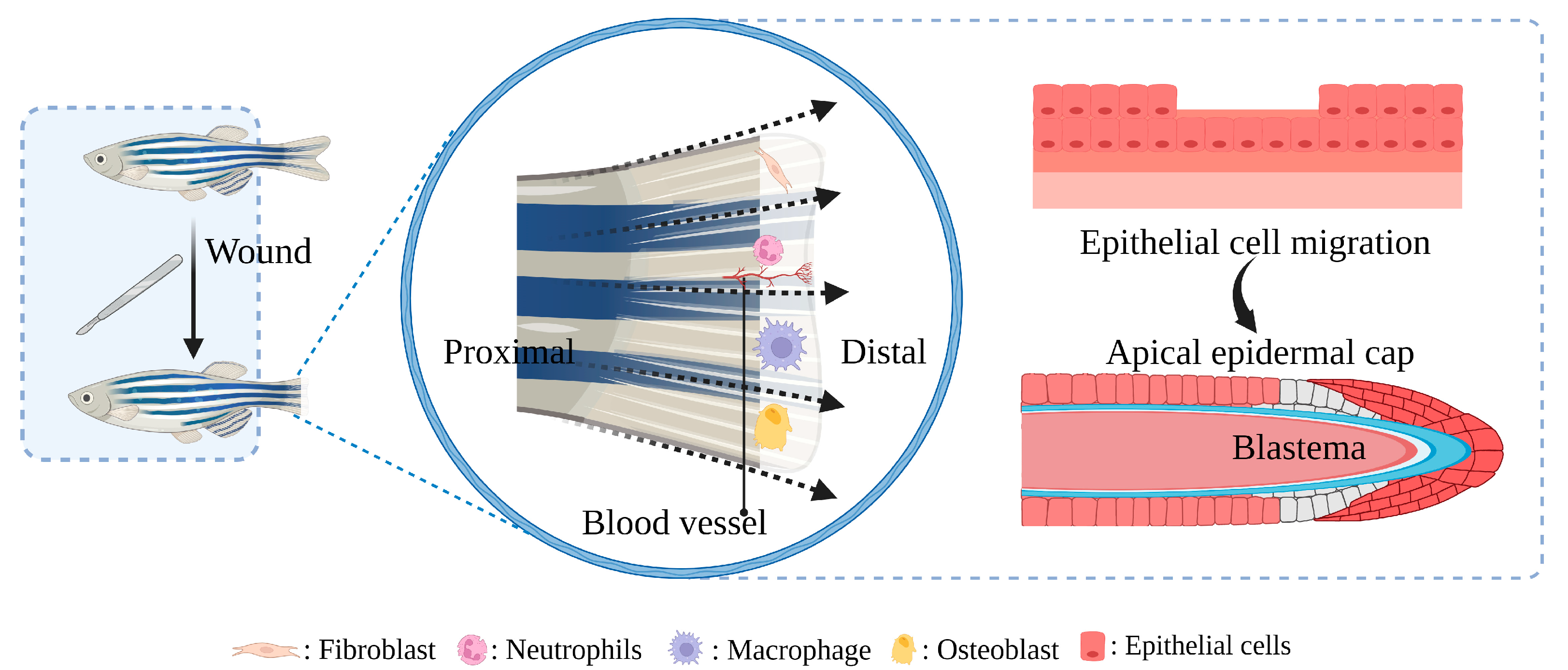
2. Mechanism of Delayed Healing of Diabetic Wounds

2.1. Immune Dysfunction
The inflammatory phase of the wound can last for weeks or even months under the influence of diabetes [31][32]. The repair process (bacterial defense, cell proliferation, and collagen synthesis) requires energy, and the strictly oxygen-dependent NADPH-linked oxygenase will produce ROS during this period. Many pathways (polyol pathway, hexosamine pathway, etc.) related to oxidative stress of AGEs are maladjusted, resulting in NADPH depletion, decreased glutathione production, activation of protein kinase C and NADPH oxidases, and excessive production of oxygen free radicals and ROS, which further magnify the inflammatory response under high glucose conditions [7][33]. The functional impairment of neutrophils and macrophages was manifested in the continuous activation of citrulline histone H3, NETosis, and irregular release of NETs in diabetic wounds. Macrophages were affected by increased secretion of prostaglandin E2/D2 and excessive accumulation of AGEs, resulting in weakening of burial and phagocytosis of polymorphonuclear leukocytes such as apoptotic neutrophils, which remained in the M1 phenotype for a long time [20][34][35].
2.2. Microbial Invasion
2.3. Impaired Cell Proliferation and Angiogenesis
2.4. Pathological Scar
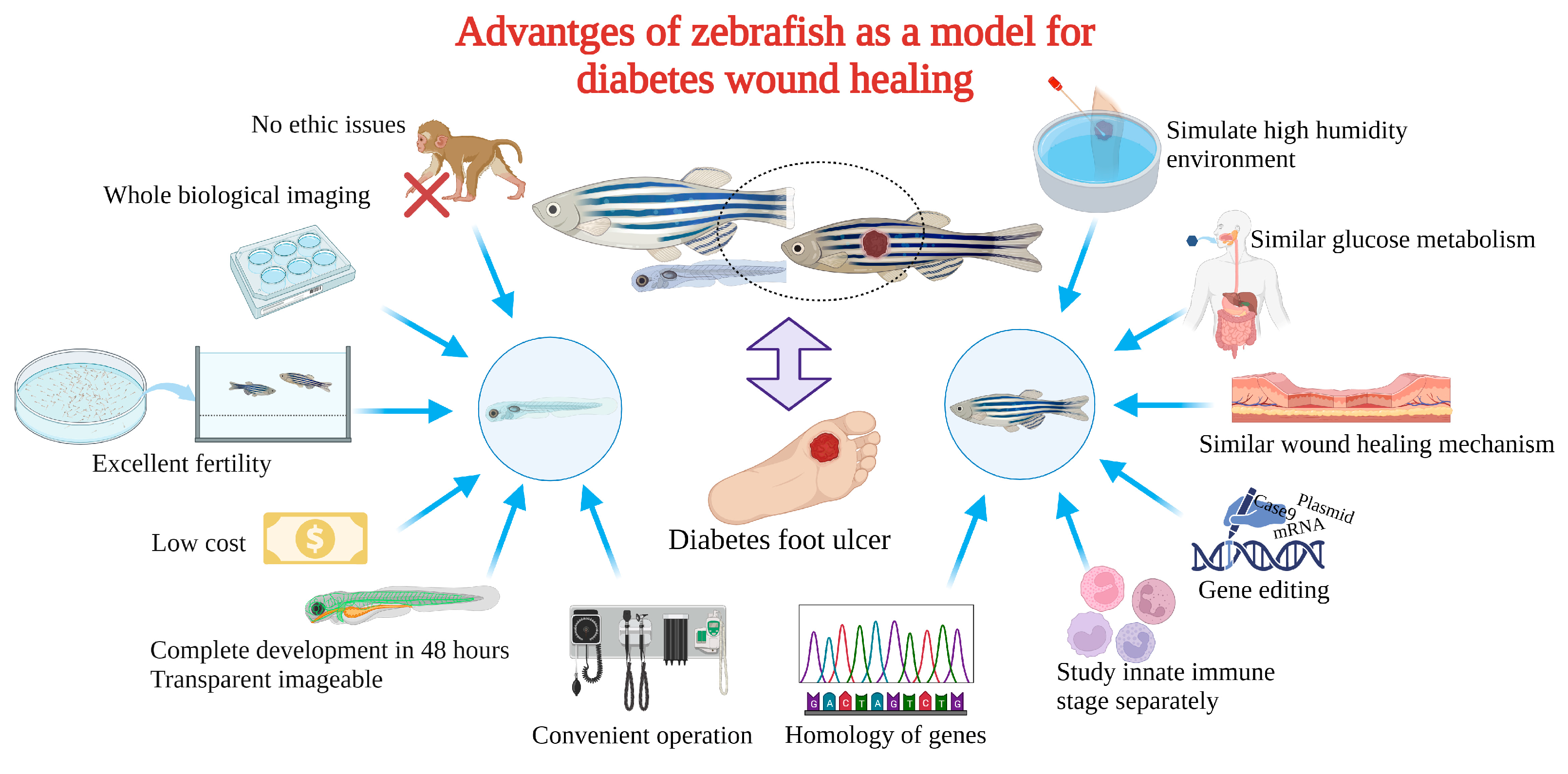
3. The Advantages of Zebrafish for Diabetic Wound Healing
4. Construction and Application of Zebrafish Diabetic Wound Model
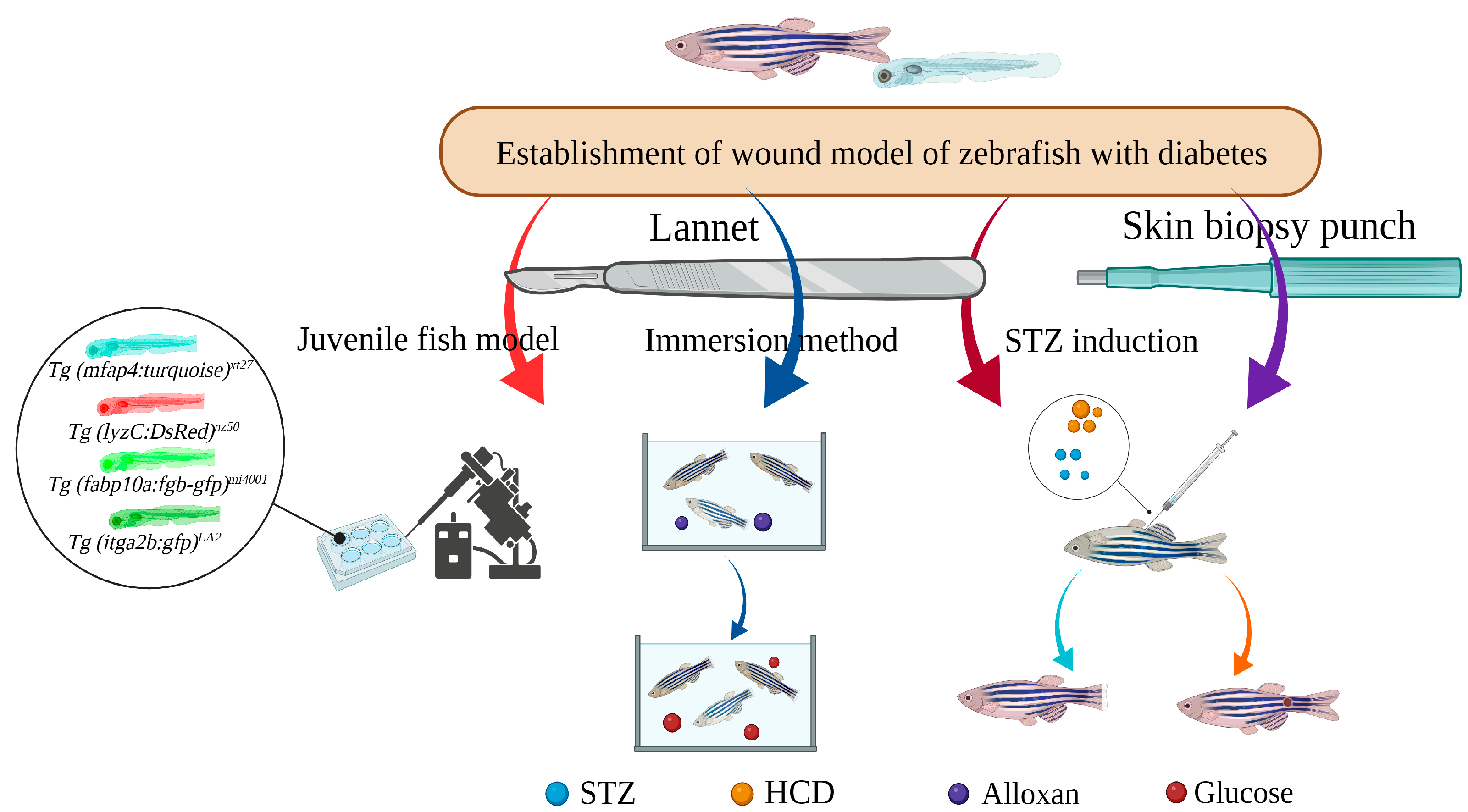
4.1. STZ-Induced Caudal Fin Regeneration Model of Zebrafish with Type 1 Diabetes
4.2. Caudal Fin Model of Type II Diabetes in Adult Zebrafish Induced by Alloxan and Glucose Combined with Aqueous Solution Exposure
4.3. Caudal Fin Regeneration Model of Zebrafish Juvenile Type II Diabetes Induced by Single Immersion or Injection of Glucose
4.4. Skin Wound Model of Adult Zebrafish Type I Diabetes Induced by STZ Injection
5. Conclusions
References
- Chen, W.; Mao, M.; Fang, J.; Xie, Y.; Rui, Y. Fracture Risk Assessment in Diabetes Mellitus. Front. Endocrinol. 2022, 13, 961761.
- Tsunoda, T.; Samadi, A.; Burade, S.; Mahmud, T. Complete Biosynthetic Pathway to the Antidiabetic Drug Acarbose. Nat. Commun. 2022, 13, 3455.
- Giugliano, D.; Longo, M.; Caruso, P.; Di Fraia, R.; Scappaticcio, L.; Gicchino, M.; Petrizzo, M.; Bellastella, G.; Maiorino, M.I.; Esposito, K. Feasibility of Simplification From a Basal-Bolus Insulin Regimen to a Fixed-Ratio Formulation of Basal Insulin Plus a GLP-1RA or to Basal Insulin Plus an SGLT2 Inhibitor: BEYOND, a Randomized, Pragmatic Trial. Diabetes Care 2021, 44, 1353–1360.
- Li, S.; Mohamedi, A.H.; Senkowsky, J.; Nair, A.; Tang, L. Imaging in Chronic Wound Diagnostics. Adv. Wound Care New Rochelle 2020, 9, 245–263.
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542.
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223.
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in Acute and Chronic Wound Healing: Oxygen in Wound Healing. Br. J. Dermatol. 2010, 163, 257–268.
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655.
- Zheng, Z.; Wan, Y.; Liu, Y.; Yang, Y.; Tang, J.; Huang, W.; Cheng, B. Sympathetic Denervation Accelerates Wound Contraction but Inhibits Reepithelialization and Pericyte Proliferation in Diabetic Mice. J. Diabetes Res. 2017, 2017, 7614685.
- Randi, A.M.; Laffan, M.A. Von Willebrand Factor and Angiogenesis: Basic and Applied Issues. J. Thromb. Haemost. 2017, 15, 13–20.
- Zindle, J.K.; Wolinsky, E.; Bogie, K.M. A Review of Animal Models from 2015 to 2020 for Preclinical Chronic Wounds Relevant to Human Health. J. Tissue Viability 2021, 30, 291–300.
- Chen, C.; Gu, Y.; Philippe, J.; Zhang, P.; Bachman, H.; Zhang, J.; Mai, J.; Rufo, J.; Rawls, J.F.; Davis, E.E.; et al. Acoustofluidic Rotational Tweezing Enables High-Speed Contactless Morphological Phenotyping of Zebrafish Larvae. Nat. Commun. 2021, 12, 1118.
- Leung, T.; Chen, H.; Stauffer, A.M.; Giger, K.E.; Sinha, S.; Horstick, E.J.; Humbert, J.E.; Hansen, C.A.; Robishaw, J.D. Zebrafish G Protein Gamma2 Is Required for VEGF Signaling during Angiogenesis. Blood 2006, 108, 160–166.
- Li, Y.-W.; Chiang, K.-Y.; Li, Y.-H.; Wu, S.-Y.; Liu, W.; Lin, C.-R.; Wu, J.-L. MiR-145 Mediates Zebrafish Hepatic Outgrowth through Progranulin A Signaling. PLoS ONE 2017, 12, e0177887.
- Zhao, S.; Xia, J.; Wu, X.; Zhang, L.; Wang, P.; Wang, H.; Li, H.; Wang, X.; Chen, Y.; Agnetti, J.; et al. Deficiency in Class III PI3-Kinase Confers Postnatal Lethality with IBD-like Features in Zebrafish. Nat. Commun. 2018, 9, 2639.
- Wilkinson, R.N.; Koudijs, M.J.; Patient, R.K.; Ingham, P.W.; Schulte-Merker, S.; van Eeden, F.J.M. Hedgehog Signalling via a Calcitonin Receptor-like Receptor Can Induce Arterial Differentiation Independently of VEGF Signalling in Zebrafish. Blood 2012, 120, 477–488.
- Berger, J.; Li, M.; Berger, S.; Meilak, M.; Rientjes, J.; Currie, P.D. Effect of Ataluren on Dystrophin Mutations. J. Cell Mol. Med. 2020, 24, 6680–6689.
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6.
- Wang, Y.; Chen, L.; Ren, D.-Y.; Feng, Z.-X.; Zhang, L.-Y.; Zhong, Y.-F.; Jin, M.-Y.; Xu, F.-W.; Feng, C.-Y.; Du, Y.-Z.; et al. Mussel-Inspired Collagen-Hyaluronic Acid Composite Scaffold with Excellent Antioxidant Properties and Sustained Release of a Growth Factor for Enhancing Diabetic Wound Healing. Mater. Today Bio 2022, 15, 100320.
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615.
- Wang, D.; Chen, H.; Lei, L.; Chen, J.; Gao, J.; Liu, J.; Li, Q.; Xie, Y.; Hu, Y.; Ni, Y. Biofabricated Macrophage and Fibroblast Membranes Synergistically Promote Skin Wound Healing. Bioeng. Transl. Med. 2022, 7, e10344.
- Ma, J.; Yong, L.; Lei, P.; Li, H.; Fang, Y.; Wang, L.; Chen, H.; Zhou, Q.; Wu, W.; Jin, L.; et al. Advances in MicroRNA from Adipose-Derived Mesenchymal Stem Cell-Derived Exosome: Focusing on Wound Healing. J. Mater. Chem. B 2022, 10, 9565–9577.
- Liu, J.; Carnero-Montoro, E.; van Dongen, J.; Lent, S.; Nedeljkovic, I.; Ligthart, S.; Tsai, P.-C.; Martin, T.C.; Mandaviya, P.R.; Jansen, R.; et al. An Integrative Cross-Omics Analysis of DNA Methylation Sites of Glucose and Insulin Homeostasis. Nat. Commun. 2019, 10, 2581.
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.S.; de Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.M.; Jackson, C.J.; de Azevedo Maia, G.L.; et al. Lupeol, a Dietary Triterpene, Enhances Wound Healing in Streptozotocin-Induced Hyperglycemic Rats with Modulatory Effects on Inflammation, Oxidative Stress, and Angiogenesis. Oxid. Med. Cell Longev. 2019, 2019, 3182627.
- Feng, W.; Zhang, C.; Yu, T.; Zhu, D. Quantitative Evaluation of Skin Disorders in Type 1 Diabetic Mice by in vivo Optical Imaging. Biomed. Opt. Express 2019, 10, 2996–3008.
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847.
- Brazel, C.B.; Simon, J.C.; Tuckermann, J.P.; Saalbach, A. Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing. J. Clin. Med. 2020, 9, 3878.
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Anuar, M.R.; Karim, S.; Ong, S.K.; Yusof, F.A.M.; Tan, W.-N.; Sulaiman, B.; Ooi, M.L.; et al. Homalomena Pineodora Essential Oil Nanoparticle Inhibits Diabetic Wound Pathogens. Sci. Rep. 2020, 10, 3307.
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF Loaded Electrospun Dual Porous Core-Shell Membrane for Diabetic Wound Healing. Int. J. Nanomed. 2019, 14, 8573–8588.
- Ma, J.; Lei, P.; Chen, H.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Jin, L.; Sun, D. Advances in LncRNAs from Stem Cell-Derived Exosome for the Treatment of Cardiovascular Diseases. Front. Pharmacol. 2022, 13, 986683.
- Jiang, T.; Liu, S.; Wu, Z.; Li, Q.; Ren, S.; Chen, J.; Xu, X.; Wang, C.; Lu, C.; Yang, X.; et al. Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio 2022, 16, 100365.
- Tilves, C.M.; Zmuda, J.M.; Kuipers, A.L.; Nestlerode, C.S.; Evans, R.W.; Bunker, C.H.; Patrick, A.L.; Miljkovic, I. Association of Lipopolysaccharide-Binding Protein with Aging-Related Adiposity Change and Prediabetes Among African Ancestry Men. Diabetes Care 2016, 39, 385.
- Ji, H.; Peng, R.; Jin, L.; Ma, J.; Yang, Q.; Sun, D.; Wu, W. Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications. Pharmaceutics 2021, 13, 1452.
- Eichelberger, K.R.; Goldman, W.E. Manipulating Neutrophil Degranulation as a Bacterial Virulence Strategy. PLoS Pathog. 2020, 16, e1009054.
- Zhang, J.; Dai, Y.; Wei, C.; Zhao, X.; Zhou, Q.; Xie, L. DNase I Improves Corneal Epithelial and Nerve Regeneration in Diabetic Mice. J. Cell Mol. Med. 2020, 24, 4547–4556.
- Moura, J.; Rodrigues, J.; Gonçalves, M.; Amaral, C.; Lima, M.; Carvalho, E. Impaired T-Cell Differentiation in Diabetic Foot Ulceration. Cell Mol. Immunol. 2017, 14, 758–769.
- Daeschlein, G. Antimicrobial and Antiseptic Strategies in Wound Management. Int. Wound J. 2013, 10 (Suppl. 1), 9–14.
- Tang, N.; Zheng, Y.; Jiang, X.; Zhou, C.; Jin, H.; Jin, K.; Wu, W.; Haick, H. Wearable Sensors and Systems for Wound Healing-Related PH and Temperature Detection. Micromachines 2021, 12, 430.
- Pang, J.; Urao, N.; Koh, T.J. Proliferation of Ly6C+ Monocytes/Macrophages Contributes to Their Accumulation in Mouse Skin Wounds. J. Leukoc. Biol. 2020, 107, 551–560.
- Icli, B.; Nabzdyk, C.S.; Lujan-Hernandez, J.; Cahill, M.; Auster, M.E.; Wara, A.; Sun, X.; Ozdemir, D.; Giatsidis, G.; Orgill, D.P.; et al. Regulation of Impaired Angiogenesis in Diabetic Dermal Wound Healing by MicroRNA-26a. J. Mol. Cell Cardiol. 2016, 91, 151–159.
- Chen, C.-Y.; Yin, H.; Chen, X.; Chen, T.-H.; Liu, H.-M.; Rao, S.-S.; Tan, Y.-J.; Qian, Y.-X.; Liu, Y.-W.; Hu, X.-K.; et al. Ångstrom-Scale Silver Particle–Embedded Carbomer Gel Promotes Wound Healing by Inhibiting Bacterial Colonization and Inflammation. Sci. Adv. 2020, 6, eaba0942.
- Chen, C.-Y.; Rao, S.-S.; Ren, L.; Hu, X.-K.; Tan, Y.-J.; Hu, Y.; Luo, J.; Liu, Y.-W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from Human Urine-Derived Stem Cells Facilitates Diabetic Wound Repair by Promoting Angiogenesis. Theranostics 2018, 8, 1607–1623.
- Seo, G.Y.; Ho, M.T.; Bui, N.T.; Kim, Y.M.; Koh, D.; Lim, Y.; Hyun, C.; Cho, M. Novel Naphthochalcone Derivative Accelerate Dermal Wound Healing through Induction of Epithelial-Mesenchymal Transition of Keratinocyte. J. Biomed. Sci. 2015, 22, 47.
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential Glucose Requirement in Skin Homeostasis and Injury Identifies a Therapeutic Target for Psoriasis. Nat. Med. 2018, 24, 617–627.
- Pedowitz, N.J.; Batt, A.R.; Darabedian, N.; Pratt, M.R. MYPT1 O-GlcNAc Modification Regulates Sphingosine-1-Phosphate Mediated Contraction. Nat. Chem. Biol. 2021, 17, 169–177.
- Hu, S.C.-S.; Lan, C.-C.E. High-Glucose Environment Disturbs the Physiologic Functions of Keratinocytes: Focusing on Diabetic Wound Healing. J. Dermatol. Sci. 2016, 84, 121–127.
- Nishikori, Y.; Shiota, N.; Okunishi, H. The Role of Mast Cells in Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Mice. Arch. Dermatol. Res. 2014, 306, 823–835.
- Park, K.H.; Han, S.H.; Hong, J.P.; Han, S.-K.; Lee, D.-H.; Kim, B.S.; Ahn, J.H.; Lee, J.W. Topical Epidermal Growth Factor Spray for the Treatment of Chronic Diabetic Foot Ulcers: A Phase III Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Diabetes Res. Clin. Pract. 2018, 142, 335–344.
- Huang, C.; Ogawa, R. Role of Inflammasomes in Keloids and Hypertrophic Scars—Lessons Learned from Chronic Diabetic Wounds and Skin Fibrosis. Int. J. Mol. Sci. 2022, 23, 6820.
- Maierdiyali, A.; Wang, L.; Luo, Y.; Li, Z. Effect of Tank Size on Zebrafish Behavior and Physiology. Animals 2020, 10, 2353.
- Andersson, O.; Adams, B.A.; Yoo, D.; Ellis, G.C.; Gut, P.; Anderson, R.M.; German, M.S.; Stainier, D.Y.R. Adenosine Signaling Promotes Regeneration of Pancreatic β-Cells in vivo. Cell Metab. 2012, 15, 885–894.
- Moustaqil, M.; Fontaine, F.; Overman, J.; McCann, A.; Bailey, T.L.; Soto, P.R.; Bhumkar, A.; Giles, N.; Hunter, D.J.B.; Gambin, Y.; et al. Homodimerization Regulates an Endothelial Specific Signature of the SOX18 Transcription Factor. Nucleic Acids Res. 2018, 46, 11381–11395.
- Soman, S.; Keatinge, M.; Moein, M.; Da Costa, M.; Mortiboys, H.; Skupin, A.; Sugunan, S.; Bazala, M.; Kuznicki, J.; Bandmann, O. Inhibition of the Mitochondrial Calcium Uniporter Rescues Dopaminergic Neurons in pink1−/− Zebrafish. Eur. J. Neurosci. 2017, 45, 528–535.
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665.
- LeBert, D.C.; Huttenlocher, A. Inflammation and Wound Repair. Semin. Immunol. 2014, 26, 315–320.
- Jörgens, K.; Hillebrands, J.-L.; Hammes, H.-P.; Kroll, J. Zebrafish: A Model for Understanding Diabetic Complications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 186–187.
- Martínez-Navarro, F.J.; Martínez-Morcillo, F.J.; de Oliveira, S.; Candel, S.; Cabas, I.; García-Ayala, A.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Mesa-del-Castillo, P.; Cayuela, M.L.; et al. Hydrogen Peroxide in Neutrophil Inflammation: Lesson from the Zebrafish. Dev. Comp. Immunol. 2020, 105, 103583.
- Yamamoto, D.; Sato, D.; Nakayama, H.; Nakagawa, Y.; Shimada, Y. ZF-Mapper: Simple and Complete Freeware for Fluorescence Quantification in Zebrafish Images. Zebrafish 2019, 16, 233–239.
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058.
- Cho, K.-H.; Kim, J.-H.; Nam, H.-S.; Kang, D.-J. Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients. Geriatrics 2022, 7, 45.
- Yoon, J.-H.; Cho, K.-H. A Point Mutant of Apolipoprotein A-I (V156K) Showed Enhancement of Cellular Insulin Secretion and Potent Activity of Facultative Regeneration in Zebrafish. Rejuvenation Res. 2012, 15, 313–321.
- Intine, R.V.; Olsen, A.S.; Sarras, M.P. A Zebrafish Model of Diabetes Mellitus and Metabolic Memory. J. Vis. Exp. 2013, 72, e50232.
- Sarras, M.P.; Leontovich, A.A.; Olsen, A.S.; Intine, R.V. Impaired Tissue Regeneration Corresponds with Altered Expression of Developmental Genes That Persists in the Metabolic Memory State of Diabetic Zebrafish. Wound Repair Regen 2013, 21, 320–328.
- Wibowo, I.; Utami, N.; Anggraeni, T.; Barlian, A.; Putra, R.E.; Indriani, A.D.; Masadah, R.; Ekawardhani, S. Propolis Can Improve Caudal Fin Regeneration in Zebrafish (Danio rerio) Induced by The Combined Administration of Alloxan and Glucose. Zebrafish 2021, 18, 274–281.
- Morris, S.; Cholan, P.M.; Britton, W.J.; Oehlers, S.H. Glucose Inhibits Haemostasis and Accelerates Diet-Induced Hyperlipidaemia in Zebrafish Larvae. Sci. Rep. 2021, 11, 19049.




