Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamie A Maresca | -- | 1173 | 2023-05-09 15:27:20 | | | |
| 2 | Conner Chen | Meta information modification | 1173 | 2023-05-11 05:06:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maresca, J.A.; Demel, D.C.; Wagner, G.A.; Haase, C.; Geibel, J.P. Three-Dimensional Bioprinting Processes and Approaches. Encyclopedia. Available online: https://encyclopedia.pub/entry/44050 (accessed on 07 February 2026).
Maresca JA, Demel DC, Wagner GA, Haase C, Geibel JP. Three-Dimensional Bioprinting Processes and Approaches. Encyclopedia. Available at: https://encyclopedia.pub/entry/44050. Accessed February 07, 2026.
Maresca, Jamie A., Derek C. Demel, Grayson A. Wagner, Colin Haase, John P. Geibel. "Three-Dimensional Bioprinting Processes and Approaches" Encyclopedia, https://encyclopedia.pub/entry/44050 (accessed February 07, 2026).
Maresca, J.A., Demel, D.C., Wagner, G.A., Haase, C., & Geibel, J.P. (2023, May 09). Three-Dimensional Bioprinting Processes and Approaches. In Encyclopedia. https://encyclopedia.pub/entry/44050
Maresca, Jamie A., et al. "Three-Dimensional Bioprinting Processes and Approaches." Encyclopedia. Web. 09 May, 2023.
Copy Citation
Three-dimensional (3D) bioprinting describes the use of 3D additive manufacturing techniques aimed to integrate biological materials, such as cells, growth factors, and other biochemicals and biomaterials, into a multi-layer composite using high-precision printing technologies that can mimic the structures of target tissues.
hydrogels
bioink
osteoblast
1. Introduction
Three-dimensional (3D) bioprinting describes the use of 3D additive manufacturing techniques aimed to integrate biological materials, such as cells, growth factors, and other biochemicals and biomaterials, into a multi-layer composite using high-precision printing technologies that can mimic the structures of target tissues [1][2]. This allows for the reproducible automated production of functional living tissues. Bioprinting has garnered continued interest in the healthcare community for its ability to generate complex biological structures such as organs, blood vessels, and bones, leading to extraordinary advances in regenerative medicine [3]. Bioprinting technologies have allowed medical scientists and biomedical engineers to research new treatment modalities in cases where substantial portions of bone and tissue are removed or destroyed due to a traumatic injury or chemical or radiation exposure. The ability to repair bone using bioprinting techniques has especially gained attention due to its potential as a “game-changing” treatment to combat the limited availability of organ donors in the United States [3]. The complications surrounding “bone grafting”, the name given to procedures that use the transplanted bone to repair and rebuild diseased or damaged bones, have also garnished a large amount of attention from the biomedical community due to potentially reducing the risk of immunological responses. Some of the highlighted difficulties are around the problems of absorption and reabsorption of the graft material and the risk of infection associated with the procedures. To address these problems, different mixtures of cells, scaffold materials, and bioink materials have been proposed and are being studied further to produce mechanically strong, biocompatible, and durable graft properties capable of achieving the goals required for bone repair [1][4].
2. 3D Bioprinting Processes and Approaches
2.1. Bioink
What is bioink? Bioink is any material composed of cells that are suspended in a media containing additional material for cell growth, continued cell structure, and ink viscosity to allow for printing. The formulation and subsequent extrusion of a bioink are undoubtedly one of the most difficult characteristics of the 3D bioprinting process. A well-formulated ink should create the desired substrate to eventually develop structure while providing the printed cells with nutrients to survive. It is also important when developing bioink to assure that it can facilitate oxygen transport to the growing cells to allow for adhesion to the substrate and must be free of cytotoxic compounds to provide the optimal growing environment [5]. Along with these aspects, the “ink” must be able to absorb O2 and nutrients and extrude CO2 and waste products, thereby preventing changes in pH, along with fluid and electrolyte composition in the microenvironment, mimicking the native tissue in the normal physiological environment.
2.2. Extrusion-Based Bioprinting
Extrusion-based 3D bioprinting is the most common method employed within the field of 3D bioprinting at the present time. This method employs the release of material through a small needle or needle-like nozzle attached to a reservoir (typically a syringe of varying volumes) to generate a desired shape by utilization of a bioink, which suspends the printed cells in a media containing growth factors, cells, and other biochemicals essential to the printing process and that aids in cell viability before and after printing (Figure 1). Inkjet printing is a low-cost, high-efficiency, and moderately precise printing method, basic in its operation, where microdroplets of a liquid bioink are extruded onto specific areas of a substrate [6]. These printers can also be optimized using computer-aided design (CAD) programs to allow for highly precise printing, facilitating the ability to print highly specific shapes with a set amount of bioink material.
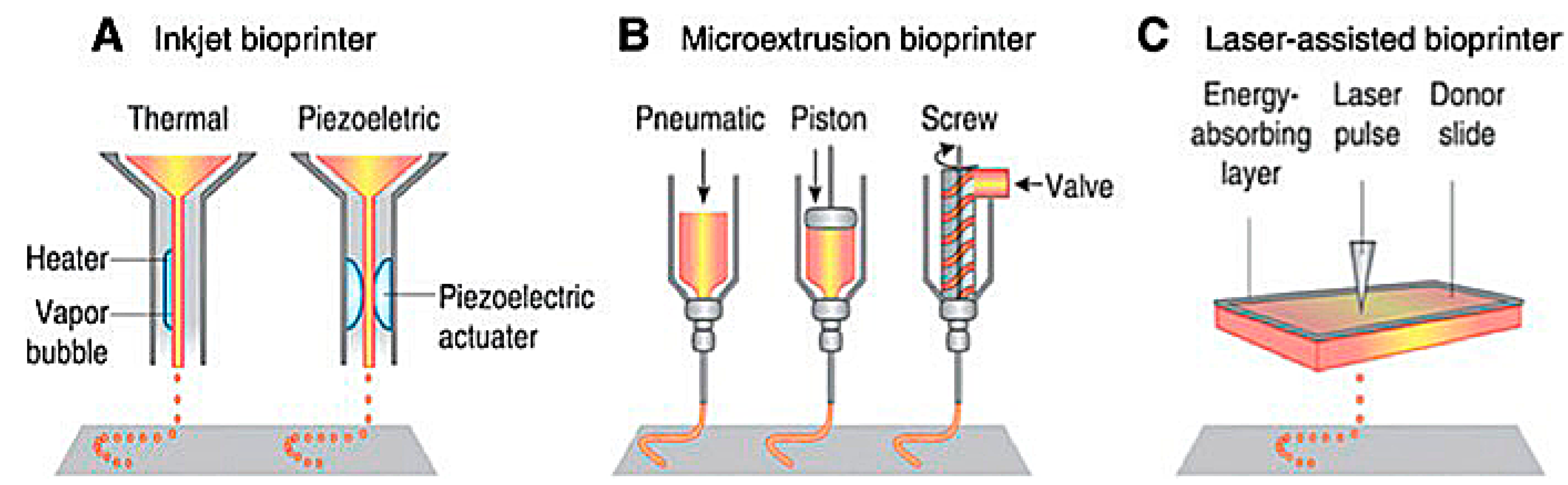
Figure 1. Schematic of present bioprinting techniques: (A) Inkjet bioprinters use a source of heat to create vapor in the print head. This vapor generates pressure within the nozzle that forces bioink from the tip. Piezoelectric print heads use pulses generated through ultrasound or piezoelectric pressure. (B) Extrusion printers use a mechanical dispensing system, requiring a screw, piston, or pneumatic pressure. (C) Laser bioprinting uses a laser pulse that strikes a point on a laser-absorbent surface; this surface generates a miniature burst of vapor that drives the biomaterial onto the substrate [6].
2.3. Laser-Assisted 3D Bioprinting
Laser-assisted bioprinting is one of the newest methods of 3D bioprinting. It involves the layering of a ribbon of glass oriented parallel to the collecting substrate, a ribbon of laser-focusing material such as gold, and a sheet of hydrogel, bioink, and biomaterials. The biomaterial is placed above a substrate, the glass and gold sheet are layered on top of this biomaterial, and once a laser pulse strikes a point on the gold ribbon, it creates a miniature burst of vapor that drives the biomaterial onto the substrate (Figure 1). Laser printing allows for the construction of more complex biological structures and complexes using laser technology, which removes any physical stress upon the cells suspended in the bioink [6][7]. This process allows for a higher resolution when printing, generating more precise shapes to model the design of the organ that is being repaired or potentially replaced. Laser-assisted techniques also lack a nozzle, meaning that the clogging issues found commonly in inkjet and extrusion printers are completely absent. However, laser-assisted bioprinting is expensive, making it an unfavorable candidate for daily research without spending a lot of funding. Except for the cost, laser-assisted techniques are vastly superior to inkjet and extrusion printers, and with more printers being deployed in the future, the costs may be reduced. While printing at a slightly slower rate, laser printers can extrude bioink with much more dense cell concentrations of up to 108 cells per mL, equating to around a single cell per microdroplet [6][7]. In addition to their ability to print a larger volume of cells, laser printers have the highest cell viability of all the printing techniques, with around 95% viability using this method.
2.4. Hydrogels as Scaffolding for 3D Bioprinting
Recent exploration in bioprinting technologies has focused on scaffold-free printing by further optimizing bioink used to provide the initial structural and mechanical support for printed cells. Hydrogels are an essential piece of 3D bioprinting as they provide a 3D structure and proper biochemical environment that can effectively simulate native bone tissue [8]. They are crosslinked hydrophilic polymers capable of absorbing substantial amounts of water or bioink without dissolving in them [9]. Hydrogel is widely used with many 3D bioprinting techniques, as it creates an adequate biological environment for cells to grow. The requirements for a suitable bioink are truly extensive due to biocompatibility issues across a wide range of cells and other tissue-specific properties, so hydrogels are now generally settled upon as the optimal substrate for bioprinting [8][10]. Though the dynamic biochemical characteristics of living tissue differ from the invariable conditions of hydrogels, it is part of this review to assess the development of hydrogels and discuss some of the modifications aimed at accommodating the potential flaws of the presently used hydrogel systems. The type of hydrogels used in bioprinting varies widely depending on the printing technique [10].
References
- Pavek, A.; Nartker, C.; Saleh, M.; Kirkham, M.; Pour, S.K.; Aghazadeh-Habashi, A.; Barrott, J.J. Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines 2021, 9, 551.
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.-J.; Atala, A.; Yoo, J.-J.; Lee, S.-J. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106.
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289.
- Daly, A.; Freeman, F.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater 2017, 6, 1700298.
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001.
- Maina, R.M.; Barahona, M.J.; Finotti, M.; Lysyy, T.; Geibel, P.; D’amico, F.; Mulligan, D.; Geibel, J.P. Generating vascular conduits: From tissue engineering to three-dimensional bioprinting. Innov. Surg. Sci. 2018, 3, 203–213.
- Ventura, R.D. An Overview of Laser-assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81.
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1155–1170.
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 1–9.
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
878
Revisions:
2 times
(View History)
Update Date:
11 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




